Abstract
♦ Background and Objectives: The extent to which hemoglobin (Hb) cycling occurs in peritoneal dialysis (PD) patients is unclear. It is also uncertain whether different types of erythropoiesis-stimulating agents (ESAs) affect such cycling. We performed a retrospective cohort study of our PD population before and after the entire program was switched from epoetin beta (NeoRecormon: Hoffman–LaRoche, Basel, Switzerland) to continuous erythropoietin receptor activator [CERA (Mircera: Hoffman–LaRoche)].
♦ Design, Setting, Participants, and Measurements: The study included 79 patients receiving PD for end-stage renal failure and being treated with an ESA. Hemoglobin concentrations were measured monthly, and each study period ran for 12 months. Patient demographics and details of intercurrent illness and hospital admission were collected.
♦ Results: There was a trend to fewer patients on CERA (26 patients, 68.4%) than on epoetin beta (36 patients, 87.8%, p = 0.054) experiencing Hb excursions. The CERA group also required fewer dose changes. However, there was no difference in the proportion of patients experiencing complete Hb cycles. On logistic regression, the factors associated with Hb cycling were ESA dose increase or decrease and hospital admission. We also observed a positive correlation between the delta ESA dose and the amplitude of Hb excursion, suggesting that the dose changes were causal, rather than reactive.
♦ Conclusions: Hemoglobin cycling occurs in PD patients and is largely a consequence of current practice in ESA dosing, plus the effects of intercurrent illness. The longer half life of CERA may offer a small advantage in reducing the degree of Hb variability, possibly because of fewer dose changes per patient.
Keywords: Hemoglobin, erythropoietin, cycling, continuous erythropoietin receptor activator
Cyclical fluctuations in serum hemoglobin (Hb) are well recognized in hemodialysis (HD) patients treated with erythropoiesis-stimulating agents (ESAs). Several associated factors have been described, some relating to anemia treatment (such as ESA dose adjustment or intravenous iron dosing), and others, to intercurrent illness, especially infections and hospital admission (1–3). Large retrospective analyses have shown an association between the degree of Hb variation and mortality, with some data suggesting that downward Hb fluctuations in particular are associated with an increased risk of death (4,5). It has been suggested that fluctuations in Hb may lead to variability in oxygen delivery to tissues, thereby resulting in end-organ damage; however, no prospective studies have been undertaken to demonstrated a causative link between Hb cycling and poor patient outcomes. Nevertheless, large fluctuations in Hb levels are not desirable. Clinically, low Hb levels contribute to lesser quality of life for patients, and very high levels may be associated with mortality (6,7). Practically, treatment adjustments may require more clinician time, and higher ESA doses have cost implications.
To date, almost all of the published research on this topic has focused on HD patients; very limited data are available regarding patients on peritoneal dialysis (PD) (8,9). Furthermore, there has been very little study on whether different ESA types affect Hb stability. Continuous erythropoietin receptor activator [CERA (Mircera: Hoffman–LaRoche, Basel, Switzerland)] has a much longer mode of action than the first-generation recombinant epoetins and allows for successful anemia treatment with prolonged dosing intervals (10–12). Because ESA dose changes have been related to fluctuations in Hb, the lower number of dose changes with CERA may be beneficial (10). Although phase II and III trials have suggested “stable” Hb after a switch from epoetin, the degree of Hb variability with CERA has not been specifically studied, nor have any direct comparisons been made in this regard between various ESAs.
Because our PD program made a wholesale switch from epoetin beta (NeoRecormon: Hoffman–LaRoche) to CERA in 2008, we had the opportunity to study and compare the degree of Hb variability with each type of ESA. We also sought to examine the degree of Hb cycling in patients treated exclusively with PD and to identify key factors associated with such cycling.
METHODS
PATIENTS
All patients on continuous ambulatory PD (CAPD) or automated PD (APD) who were treated with an ESA at our center were screened for inclusion in a historical cohort study. We excluded those who had been established on dialysis for fewer than 3 months at the start of each study period and those who had received blood transfusions, had experienced bleeding episodes, or had received temporary HD. During the first study period, October 2006 to October 2007, epoetin beta was the sole ESA used. The program was converted to CERA in 2008. To allow for a period of stabilization and to avoid carryover effects, the second study period was defined as January 2009 to January 2010.
STUDY PROTOCOL
In all patients, serum Hb, ferritin levels, and transferrin saturation were measured monthly. The frequency of blood testing was identical during each study period. Dose adjustments of ESA occurred in response to defined Hb levels and were made by an anemia nurse specialist in discussion with a consultant nephrologist. All patient results were reviewed monthly. During both study periods, the unit’s anemia treatment policy remained unchanged. If serum Hb was 12.6 g/dL – 14 g/dL, then the ESA dose was reduced by 10%; if it was above 14.0 g/dL, then ESA was held for 2 weeks, and the dose was reduced by 25%. No change to ESA dose was made if serum Hb was 11 g/dL – 12.5 g/dL. The ESA dose was increased by 20% if serum Hb was 10 g/dL – 10.9 g/dL, by 30% if serum Hb was 9 g/dL – 9.9 g/dL, and by 40% if serum Hb was less than 9 g/dL. Short courses of intravenous iron sucrose (200 mg weekly for 5 weeks) were administered when ferritin was less than 200 ng/mL or when transferrin saturation was below 20%. No patients received iron on a maintenance basis. Any sudden drops in Hb or any level less than 9 g/dL triggered a physician review.
All patients had undergone urea kinetic modeling using PD Adequest 2.0 (Baxter Healthcare, Norfolk, UK) within 3 months of the start of the particular study period. We also retrieved data regarding intercurrent illness in the patients—in particular, hospital admissions and infections.
DEFINITIONS
To allow for comparisons with published data for HD patients, we used pre-existing definitions to describe Hb variability (3). A significant Hb excursion was defined as a change in serum Hb of more than 1.5 g/dL lasting for 4 or more weeks. One complete Hb cycle was defined to consist of 2 sequential excursions in opposite directions. The amplitude of an excursion was defined as the difference between the serum Hb concentration at the start and at the end of the excursion.
STATISTICAL ANALYSIS
Results are expressed as mean ± standard deviation unless otherwise stated. After demonstration of a normal distribution, a two-tailed unpaired Student t-test was used to compare the distribution of quantitative variables, and a Fisher exact test was used for categorical variables (paired tests were used to compare subgroups of patients within both study groups). Univariate analysis was performed to identify potential determinants of Hb cycling. Significant associations were then tested by binomial logistic regression analysis. An alpha error at p < 0.05 was judged to be significant.
RESULTS
The study included 79 patients (41 treated with epoetin beta, 38 treated with CERA), with 15 patients being common to both study groups. Baseline patient characteristics (Table 1) were not significantly different between the groups, except for mean time on dialysis, which was shorter in the CERA group (27.8 ± 21 months vs 40.5 ± 23 months in the epoetin group, p = 0.0066). In patients treated with epoetin beta, the average ESA dose was 5299 ± 3446 IU weekly; in those treated with CERA, the mean dose was 83.5 ± 71 μg monthly.
TABLE 1.
Baseline Characteristics of Patients Treated with Continuous Erythropoietin Receptor Activator (CERA) and with Epoetin Beta
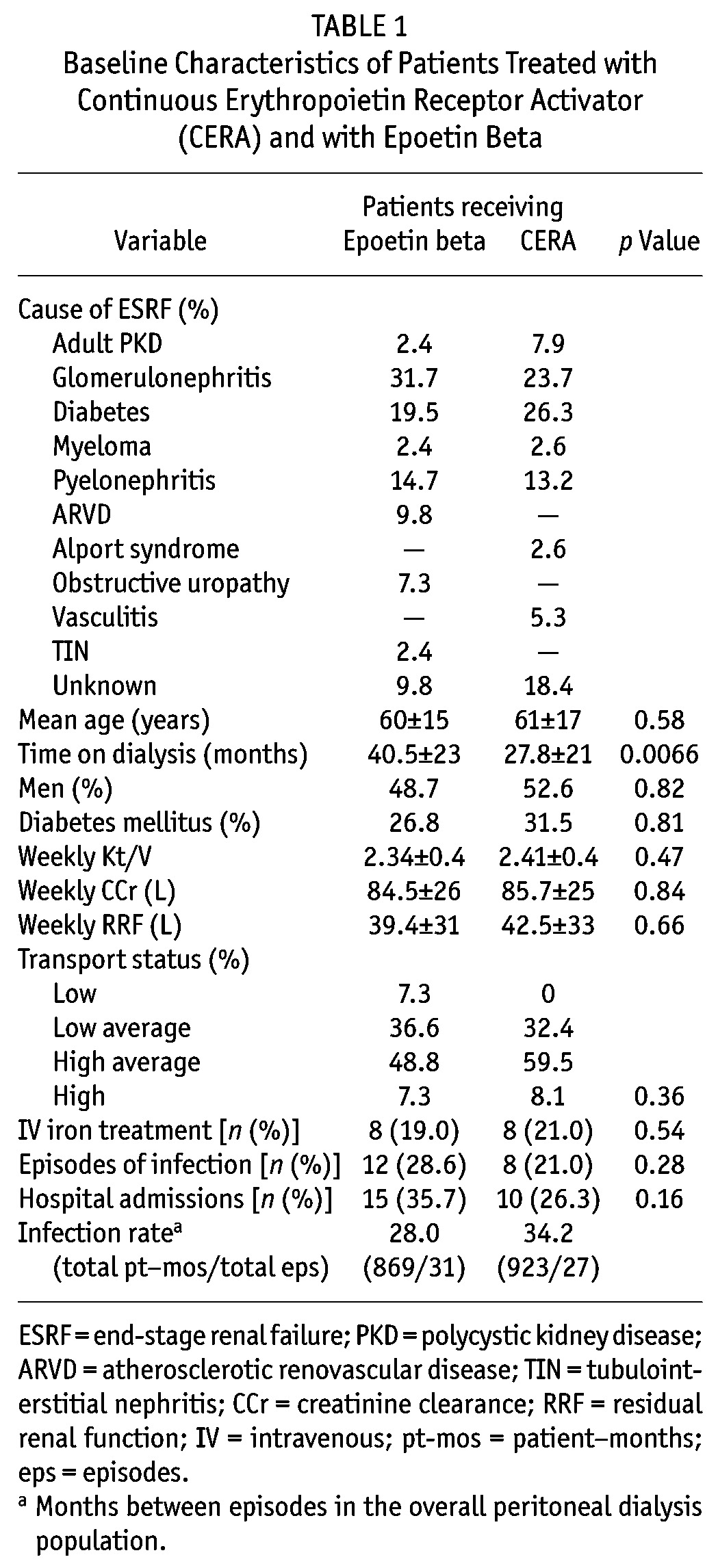
In the epoetin beta group, 24 patients (58.5%) experienced at least 1 Hb cycle during the 12-month period; in the CERA group, 16 patients (42.1%) experienced at least 1 cycle (p = 0.5). Patients treated with CERA showed a trend toward fewer instances of at least 1 Hb excursion: 26 patients (68.4%) compared with 36 patients (87.8%) among those receiving epoetin beta (p = 0.054). By extension, 12 patients in the CERA group (31.6%) and 5 patients in the epoetin beta group (12.2%) had stable serum Hb, with no fluctuations during the study period. However, the mean number of excursions per patient was not significantly different between the groups (1.9 ± 1.3 with epoetin beta vs 1.7 ± 1.6 with CERA, p = 0.78). Of all excursions, 96% with epoetin beta and 98% with CERA were outside the current UK Renal Association guideline range of 10.5 g/dL – 12.5 g/dL (p = 0.65).
The mean number of ESA dose changes per patient over the study period was significantly less in the CERA group (0.81 ± 0.7 compared with 1.9 ± 1.7 in the epoetin beta group, p = 0.0007). In both groups, the excursion amplitude was positively correlated with the delta ESA dose change (epoetin beta: r2 = 0.4, p < 0.0001; CERA: r2 = 0.37, p < 0.0001; Figure 1). The excursion amplitude was similar in both groups (epoetin beta: 2.81 ± 1.4 g/dL; CERA: 2.48 ± 0.89 g/dL; p = 0.76), although the mean duration of excursions was shorter with CERA (8.9 ± 5.2 weeks vs 10.6 ± 1.4 weeks, p = 0.045).
Figure 1.
— Correlation of hemoglobin excursion amplitude with change in dose of erythropoiesis-stimulating agents. (A) Epoetin beta (Epo), r2 = 0.4, p < 0.001. (B) Continuous erythropoietin receptor activator (CERA), r2 = 0.37, p < 0.001.
We also analyzed the small subgroup of patients who were included in both study periods. Of those 15 patients, 12 (80%) had at least 1 Hb excursion during epoetin beta treatment, and 11 (73%), during CERA treatment (p = 0.99). When on epoetin beta, 11 patients (73%) experienced at least 1 complete cycle; when on CERA, 9 (60%) did (p = 0.69). Equally, we observed no difference in the mean number of excursions per patient (1.9 ± 1.3 with epoetin beta vs 2.0 ± 1.8 with CERA, p = 0.91). As seen in the overall population, this subgroup trended toward fewer ESA dose changes while on CERA (1.0 ± 0.65 per patient vs 1.9 ± 1.8 per patient while on epoetin beta, p = 0.055).
To identify important associations with Hb variability, we assessed the group as a whole. Comparing the patients who did and did not experience Hb cycling, we observed no differences in demographics, dialysis vintage, dialysis adequacy, or prevalence of diabetes (Table 2). However, the mean annual number of ESA dose changes in patients who experienced cycling was 1.7 ± 1.5 compared with 1.0 ± 1.3 in patients who did not (p = 0.033). We also observed a nonsignificant trend toward a higher mean ESA dose in the patients who experienced cycling (Table 2).
TABLE 2.
Characteristics of Patients Who Did and Did Not Experience At Least 1 Hemoglobin Cycle
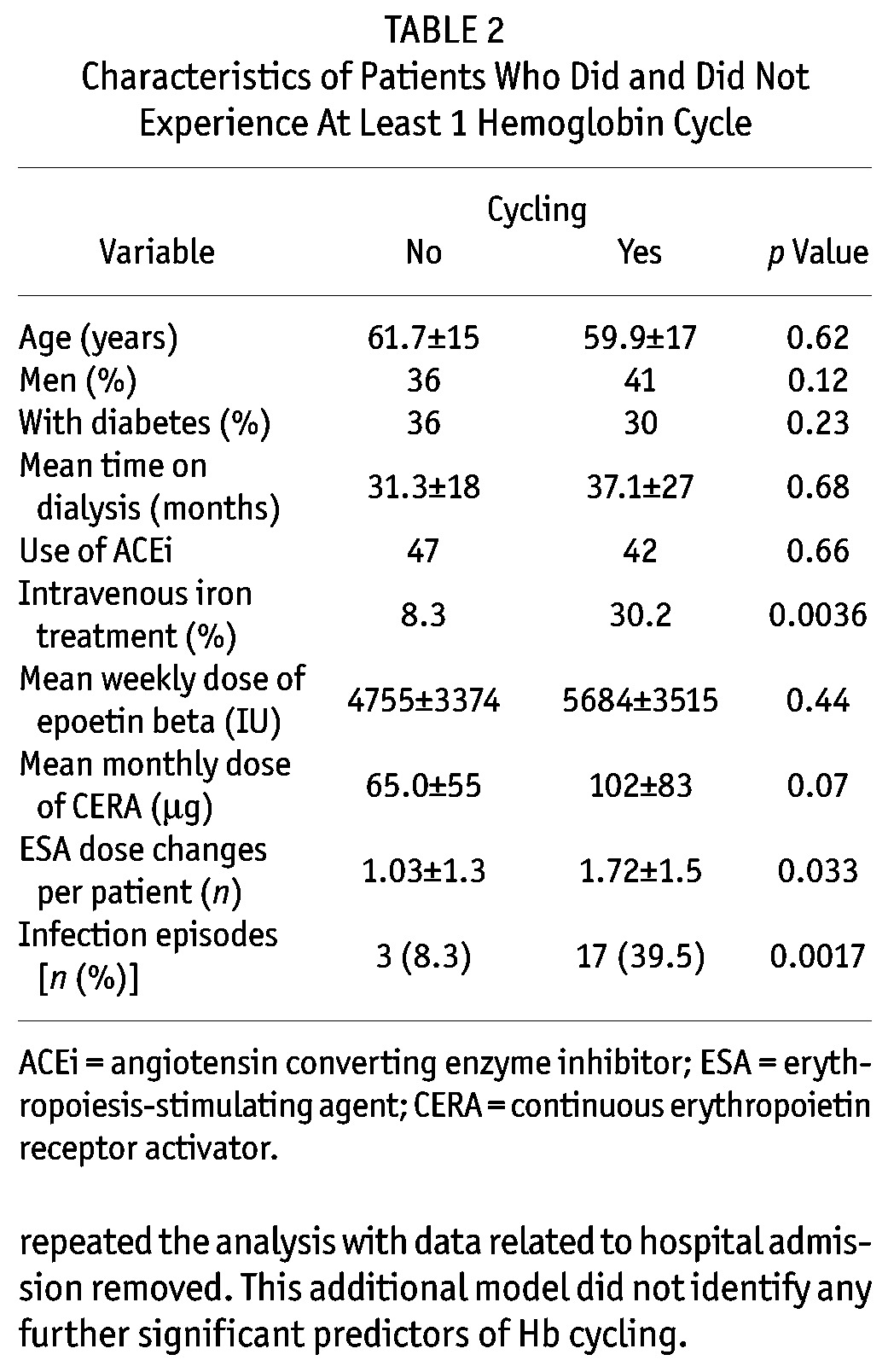
On univariate analysis, we found that ESA dose increases (r2 = 0.11, p = 0.0032) and decreases (r2 = 0.21, p < 0.0001) were associated with Hb cycles. Other associated variables were hospital admission (r2 = 0.34, p < 0.0001), infection (r2 = 0.13, p = 0.0012), and intravenous iron dosing (r2 = 0.1, p = 0.01). Weekly residual renal function was also significantly higher in the patients who did not experience Hb cycling: 48.7 ± 34 L compared with 34.4 ± 28 L, p = 0.009. No effect of ESA type or transport status on Hb variability was observed.
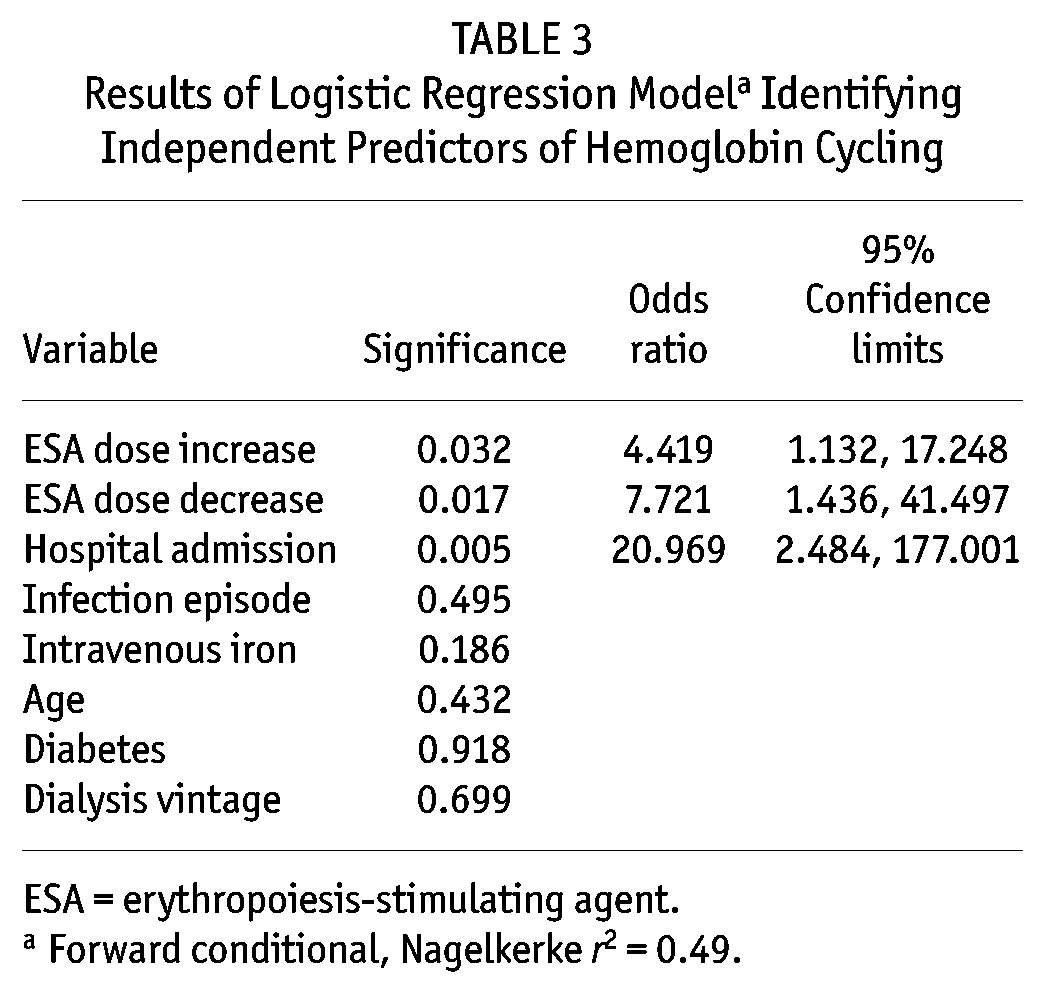
Binomial logistic regression analysis identified ESA dose increase [odds ratio (OR): 4.4; 95% confidence interval (CI): 1.13 to 17.2; p = 0.03], ESA dose decrease (OR: 7.7; 95% CI: 1.4 to 41.5; p = 0.017), and hospital admission (OR: 20.9; 95% CI: 2.5 to 177; p = 0.005) as independent determinants of Hb cycling (Nagelkerke r2 = 0.490). Table 3 shows the model results. Because hospital admission was such a strong predictor of Hb cycling, we repeated the analysis with data related to hospital admission removed. This additional model did not identify any further significant predictors of Hb cycling.
TABLE 3.
Results of Logistic Regression Modela Identifying Independent Predictors of Hemoglobin Cycling
DISCUSSION
Our study is the first to specifically compare the effect of ESA type on the variability of serum Hb levels and to examine this effect in patients exclusively on PD. It is well recognized that cyclical changes in serum Hb occur in patients on HD. Indeed, several studies suggest that approximately 90% of HD patients experience significant fluctuations in Hb levels over time; HD patients achieving a stable Hb within target range are in the minority (1–3). We observed a significant degree of Hb variability in a population of PD patients, albeit to a somewhat lesser extent. Other published data on Hb stability in PD patients are scarce. One study included 12 patients, but grouped them for analysis with patients on HD (8). The only other available data come from a registry report that lacks detailed resolution of individual patient details (9).
Fluctuations in serum Hb have been linked to poor patient outcomes, being associated with increased risks of hospitalization (1) and mortality (5,13). Persistently or transiently low Hb levels have also been shown to be associated with hospitalization and death (1,4,5,14), as have downward Hb excursions (4). However, it is important to note that no available data suggest that Hb cycling is causally related to poor outcomes, and it is certainly possible that Hb fluctuation is an indicator rather than a cause of intercurrent illness. Such an understanding would be supported by the strong links between Hb cycling and both infection and hospitalization (1,3).
Although direct effects of Hb variation on patient outcome are not known, it remains clear that large or frequent fluctuations are undesirable. Low Hb levels have a negative impact on symptoms and quality of life for patients; they also increase the requirement for blood transfusions. Hemoglobin levels above current target ranges may be associated with worse cardiovascular outcomes (6,7), and higher Hb levels maintained with higher ESA doses have a significant cost implication. It is also possible to hypothesize about other adverse effects of Hb cycling—for example, an effect on residual renal function that would be of great importance to patients on PD, although such an effect remains purely speculative at present. Finally, more frequent Hb fluctuations outside of target ranges necessitate more clinician time to determine response in terms of ESA dose adjustment or of intravenous iron dosing. It is therefore important to note that not only was variability in Hb common in our PD population, but that almost all Hb excursions reached levels outside the current UK Renal Association target range of 10.5 g/dL – 12.5 g/dL (15).
In comparing the two ESA types, there was a suggestion of benefit during the period when patients were treated with CERA, because a trend to fewer Hb excursions of shorter duration was observed. However, this possible difference did not reach statistical significance, and the benefit was slight, with no difference observed in the number of Hb cycles with CERA and with epoetin beta. One possible explanation for fewer excursions with CERA is the longer mode of action, leading to our observation of fewer dose changes. This propensity to a smaller number of dose changes has also been noted by others (10) and may have implications for dose adjustment strategies when CERA is used. However, when interpreting these results, it must be recognized that the present study was not a randomized controlled trial, and the historical nature of the comparison introduces the possibility of exposure bias. One factor that may be important in this regard is the difference in dialysis vintage between the two study populations. Conversely, the study groups had otherwise similar baseline characteristics, and apart from the change in ESA type, no other significant changes in practice or protocols occurred at our center between the two periods.
In the present study, we observed two major determinants of Hb fluctuation. The first was change in ESA dose. A positive correlation was observed between ESA dose change and amplitude of Hb excursion, suggesting that dose changes were causal, rather than reactive. That finding has also been reported by others (3) and firmly implicates current dosing strategies and anemia management protocols in the pathogenesis of Hb cycling. It was also interesting to note that, compared with dose increases, dose reductions appeared to be a stronger predictor of cycling. Recognizing the importance of ESA dose changes, attempts have been made to improve anemia management strategies by using computer-based functional data analysis systems (16). Traditionally, uniform anemia management protocols have been based on negative feedback, and changes in ESA dose are often made only when Hb reaches or exceeds the target range. A method using functional data analysis attempts to model the trajectory of Hb change at an individual level and introduces ESA dose changes at an earlier stage to smooth any oscillation—ideally before Hb strays outside the target range. On a more simplistic level, a recognition that ESA dose changes are at least in part responsible for Hb fluctuations, with an accompanying reflection of this understanding in individual practice or unit protocols may also help, in particular by anticipating the stronger influence of dose reductions.
As previously described in HD patients, we also observed that hospital admission was a very strong independent determinant of Hb cycling (1,3). Several factors probably contribute to this finding. Hospital admission is often associated with infectious and inflammatory conditions that increase resistance to the effect of ESAs, and blood loss may occur with surgical procedures, gastrointestinal bleeding, or frequent phlebotomy. In addition, emergency admission may sometimes lead to missed ESA doses. Conceptually, it seems more likely that the factors surrounding hospital admission with an acute illness lead to a fluctuation in Hb rather than the converse, in which the fall in Hb is the trigger for concurrent illness and subsequent admission. However, that statement remains to be tested in a prospective study, as does any methodology on how best to adjust the ESA dose during an acute admission—and even whether an adjustment should be made at all. The lack of data in this area is highlighted by a recent study in a different patient group, in which high-dose ESA after myocardial infarction was shown to confer no benefit in infarct size, but did increase cardiovascular event rates (17).
The present study has some weaknesses. Most importantly, the design was retrospective, which precludes a determination of causality between Hb fluctuations and most of the associated factors. In addition, some of the nonsignificant comparisons may have been the result of type 2 statistical error. Finally, we cannot exclude potential confounders that result from the time differences between the two study periods.
CONCLUSIONS
Our study shows that Hb fluctuations do occur in ESA-treated patients on PD and are largely a consequence of current practice in ESA dosing, plus the effects of intercurrent illness. The longer half life of CERA may offer a small advantage in reducing the degree of Hb variability, possibly because of fewer dose changes per patient.
DISCLOSURES
The results presented in this paper have not been published previously in whole or in part. RJF and MWT have received an unrestricted educational grant and also honoraria and sponsorship for travel to scientific meetings from Roche.
REFERENCES
- 1. Ebben JP, Gilbertson DT, Foley RN, Collins AJ. Hemoglobin level variability: associations with comorbidity, intercurrent events, and hospitalizations. Clin J am Soc Nephrol 2006; 1:1205–10 [DOI] [PubMed] [Google Scholar]
- 2. Lacson E, Jr, Ofsthun N, Lazarus JM. Effect of variability in anemia management on hemoglobin outcomes in ESRD. Am J Kidney Dis 2003; 41:111–24 [DOI] [PubMed] [Google Scholar]
- 3. Fishbane S, Berns JS. Hemoglobin cycling in hemodialysis patients treated with recombinant human erythropoietin. Kidney Int 2005; 68:1337–43 [DOI] [PubMed] [Google Scholar]
- 4. Regidor DL, Kopple JD, Kovesdy CP, Kilpatrick RD, McAllister CJ, Aronovitz J, et al. Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J am Soc Nephrol 2006; 17:1181–91 [DOI] [PubMed] [Google Scholar]
- 5. Gilbertson DT, Ebben JP, Foley RN, Weinhandl ED, Bradbury BD, Collins AJ. Hemoglobin level variability: associations with mortality. Clin J am Soc Nephrol 2008; 3:133–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339:584–90 [DOI] [PubMed] [Google Scholar]
- 7. Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–98 [DOI] [PubMed] [Google Scholar]
- 8. van der Putten K, van der Baan FH, Schellekens H, Gaillard CA. Hemoglobin variability in patients with chronic kidney disease in the Netherlands. Int J artif organs 2009; 32:787–93 [DOI] [PubMed] [Google Scholar]
- 9. Walker R, Pussell BA. on behalf of the Australian Renal Anaemia Group. Fluctuations in haemoglobin levels in haemodialysis, pre-dialysis and peritoneal dialysis patients receiving epoetin alpha or darbepoetin alpha. Nephrology (carlton) 2009; 14:689–95 [DOI] [PubMed] [Google Scholar]
- 10. Besarab A, Salifu MO, Lunde NM, Bansal V, Fishbane S, Dougherty FC, et al. Efficacy and tolerability of intravenous continuous erythropoietin receptor activator: a 19-week, phase II, multicenter, randomized, open-label, dose-finding study with a 12-month extension phase in patients with chronic renal disease. Clin Ther 2007; 29:626–39 [DOI] [PubMed] [Google Scholar]
- 11. Spinowitz B, Coyne DW, Lok CE, Fraticelli M, Azer M, Dalal S, et al. CERA maintains stable control of hemoglobin in patients with chronic kidney disease on dialysis when administered once every two weeks. Am J Nephrol 2008; 28:280–9 [DOI] [PubMed] [Google Scholar]
- 12. Sulowicz W, Locatelli F, Ryckelynck JP, Balla J, Csiky B, Harris K, et al. Once-monthly subcutaneous CERA maintains stable hemoglobin control in patients with chronic kidney disease on dialysis and converted directly from epoetin one to three times weekly. Clin J am Soc Nephrol 2007; 2:637–46 [DOI] [PubMed] [Google Scholar]
- 13. Brunelli SM, Joffe MM, Israni RK, Yang W, Fishbane S, Berns JS, et al. History-adjusted marginal structural analysis of the association between hemoglobin variability and mortality among chronic hemodialysis patients. Clin J am Soc Nephrol 2008; 3:777–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishani A, Solid CA, Weinhandl ED, Gilbertson DT, Foley RN, Collins AJ. Association between number of months below K/DOQI haemoglobin target and risk of hospitalization and death. Nephrol Dial Transplant 2008; 23:1682–9 [DOI] [PubMed] [Google Scholar]
- 15. UK Renal Association, Standards and Audit Subcommittee. Treatment of adults and children with Renal Failure: Standards and audit Measures. Guideline 2—Complications of CKD London, UK: Royal College of Physicians of London and UK Renal Association; 2007. [Google Scholar]
- 16. West RM, Harris K, Gilthorpe MS, Tolman C, Will EJ. Functional data analysis applied to a randomized controlled clinical trial in hemodialysis patients describes the variability of patient responses in the control of renal anemia. J am Soc Nephrol 2007; 18:2371–6 [DOI] [PubMed] [Google Scholar]
- 17. Najjar SS, Rao SV, Melloni C, Raman SV, Povsic TJ, Melton L, et al. Intravenous erythropoietin in patients with ST-segment elevation myocardial infarction: REVEAL: a randomized controlled trial. JAMA 2011; 305:1863–72 [DOI] [PMC free article] [PubMed] [Google Scholar]



