Abstract
♦ Background: Morphology changes of the peritoneal membrane after long-term peritoneal dialysis (PD) consist of denudation of peritoneal mesothelial cells, interstitial sclerosis, and hyalinizing vasculopathy. Those changes are considered to be the result of uremia and bioincompatible effects of conventional acidic lactate-buffered dialysate with glucose degradation products (GDPs). In the last decade, biocompatible dialysate with neutral pH and low GDPs has become widely used. Clinical practice has been modified in Japan, especially for anuric patients, and now includes the use of hybrid therapy. The impact on peritoneal morphology has not been well reported.
♦ Objective: The aim of the present study was to investigate the long-term effect on peritoneal morphology and function of biocompatible fluid use and current clinical practice in Japan, including hybrid dialysis therapy.
♦ Methods: We evaluated peritoneal biopsy specimens from patients who had undergone PD for more than 3 years. We used the average peritoneal thickness (APT) of the submesothelial compact zone as a marker of interstitial sclerosis and the lumen/vessel diameter ratio (L/V ratio) at postcapillary venules as a marker of hyalinizing vasculopathy. Demography and other data for the patients, including dialysate-to-plasma (D/P) ratio of creatinine, were obtained at baseline and every 6 months by peritoneal equilibration test.
♦ Results: Between 2002 and 2009, 110 patients started PD therapy with biocompatible dialysate at Tokyo University Hospital. Among them, 11 patients (8 men, 3 women; age: 54.2 ± 11.8 years; 1 with diabetes mellitus) were enrolled into this morphology study. The mean duration of PD in this group was 61 ± 11.3 months, and the mean time to peritoneal biopsy was 58 ± 15.1 months. The median APT was 180 μm (96 – 1424 μm), and the median L/V ratio was 0.66 (0.46 – 0.74). No obvious correlations between APT, L/V ratio, and PD duration were detected. The D/P creatinine of the 11 patients was maintained at a favorably low value, comparable with that of the other 99 patients.
♦ Conclusions: Peritoneal dialysis therapy using biocompatible dialysate in conjunction with modification of clinical practice may minimize the progression of peritoneal interstitial sclerosis and hyalinizing vasculopathy, preserving favorable peritoneal function for more than 3 years.
Keywords: Peritoneal membrane morphology, peritoneal solute transport
Loss of peritoneal function is a major factor leading to failure of peritoneal dialysis (PD) treatment (1–3). Although the precise biologic mechanisms responsible for these changes have not been confirmed, it is widely assumed that alteration in peritoneal function is related to structural changes in the peritoneal membrane.
In 2002, Williams et al. (4) reported on the morphology changes of the peritoneal membrane in patients on PD. Those authors found that the thickness of the submesothelial compact zone increased with the duration of PD therapy. They also evaluated vasculopathy in the PD patients, using a semiquantitative grading system to assess the thickness of the subendothelial hyaline material and luminal distortion, narrowing, and obliteration. Thereafter, Honda et al. (5) reported that the “average peritoneal thickness” (APT) at 5 randomly selected points in the peritoneum and the “lumen/vessel diameter ratio” (L/V ratio) at the postcapillary venule (PCV) are useful parameters to quantify the severity of vasculopathy.
The cause of these changes, called peritoneal sclerosis, has been considered to be mainly the effect on the peritoneal membrane of bioincompatible conventional dialysate, with its high osmolarity, low pH, high glucose concentrations, glucose degradation products (GDPs), and advanced glycation end-products (AGEs), acting through various biologic mechanisms, including degenerative damage to tissue components and abnormal biologic responses (6). Accumulating evidence suggests that long-term exposure to bioincompatible dialysate components, together with repeated episodes of bacterial peritonitis, plays a major role in long-term changes in peritoneal function (2,7–9).
Since the year 2000, use of biocompatible dialysate has become widespread around the world, and some studies have demonstrated favorable effects of biocompatible dialysate on survival and residual renal function in PD patients (10,11). In addition, non-negligible progress has also occurred in the policy of PD therapy in Japan, including hybrid therapy combining PD with hemodialysis (HD) (12,13).
However, the impact on peritoneal sclerosis resulting form these changes is still unclear. We conducted the present retrospective study using a quantitative method of measuring the thickness of the submesothelial compact zone and L/V ratio at PCVs to evaluate morphology changes in the peritoneal membrane of patients who had undergone long-term PD therapy with biocompatible fluid.
METHODS
PATIENTS
Between 2002 and 2009, 110 patients started PD with biocompatible dialysate at Tokyo University Hospital. Peritoneal biopsy specimens were obtained from 11 of these Japanese patients who continued to receive PD with biocompatible dialysate over a period of at least 3 years. All patients were treated with lactate-buffered (40 mEq/L), neutral-pH (6.3 – 7.3), low-GDP dialysate containing either 1.35% or 2.5% glucose (Midperiq L135 or L250: Terumo, Tokyo, Japan), using twin-bag technology. The biopsies were performed as part of normal clinical patient care at the time of removal of the PD catheter for PD-related problems (for example, inadequate fluid management, catheter malposition), at the time of PD catheter reinsertion after complete recovery from PD-associated peritonitis, or at the time of removal of the PD catheter because of the patient’s intention to switch to HD or undergo transplantation. The characteristics and clinical courses of these 11 patients—including cause of renal failure, duration of PD, episodes of peritonitis, time from the start of PD to hybrid therapy, time from the start of PD to peritoneal biopsy, reason for peritoneal biopsy, reason for discontinuing PD, and presence of diabetes mellitus—were obtained from their records. Net ultrafiltration, peritoneal and renal weekly Kt/V urea, and dialysate-to-plasma (D/P) ratio of creatinine (obtained in peritoneal equilibration tests, which were performed every 6 months) were identified from clinical records. Approval for the study was obtained from the local ethics committees, and all patients gave written informed consent.
PROCESSING OF BIOPSY SAMPLES
Samples of parietal peritoneum were biopsied in the usual manner. Briefly, the peritoneal tissue sample (approximately 1 cm in size and up to 5 mm in depth) was excised by scalpel at least several centimeters from the incisional wound. The tissue sample was immediately fixed in 20% buffered formalin. After overnight fixation at room temperature, the samples were routinely processed for microscopy and embedded in paraffin. Sections of 4-μm were cut and stained with periodic acid–Schiff, azan, and elastica–van Gieson.
SAMPLE ANALYSIS METHODS
The biopsy samples were assessed by microscopy using the standardized method described in the subsections that follow. Two experienced examiners—that is, one pathologist (YT) and one nephrologist (NA), who were unaware of the clinical backgrounds of the patients— evaluated the samples independently.
Adequacy of Specimens for Histologic Evaluation: The adequacy of a specimen for histologic evaluation of peritoneal thickness and vasculopathy was determined independently. For evaluation of peritoneal thickness, each specimen was assessed in terms of size, site, and direction of the specimen and classified as being adequate or inadequate. An adequate specimen had sufficient sample size and contained several layers of peritoneum (mesothelial, submesothelial, and adipose tissue layers). The direction of embedding was almost vertical, so that the thickness of the submesothelial layer could be measured properly. For evaluation of vasculopathy, an adequate specimen must contain a PCV with an external diameter of 25 – 50 μm.
Evaluation of Peritoneal Fibrosis: The extent of peritoneal fibrosis was determined by the thickness of the submesothelial interstitial layer (submesothelial compact zone) between the basal border of the surface mesothelial cells and the upper border of the peritoneal adipose tissue. The thickness of the mesothelial cell layer, when present on the peritoneal surface, was excluded from the measurement. When the submesothelial interstitium was continuous with the underlying dense connective tissue (abdominal fascia) without peritoneal adipose tissue, the peritoneal thickness could not be measured. Five portions were randomly selected for measurement of submesothelial thickness [Figure 1(A)]. The thickness was measured using a micrometer on the microscope lens or an image analyzer. The APT was then calculated. Areas where the peritoneum looked severely fibrotic as a result of tangential embedding or miscellaneous inflammatory reactions were excluded from measurement. The mean value of the 2 APTs determined by the 2 examiners was taken as the representative APT for each patient.
Figure 1.
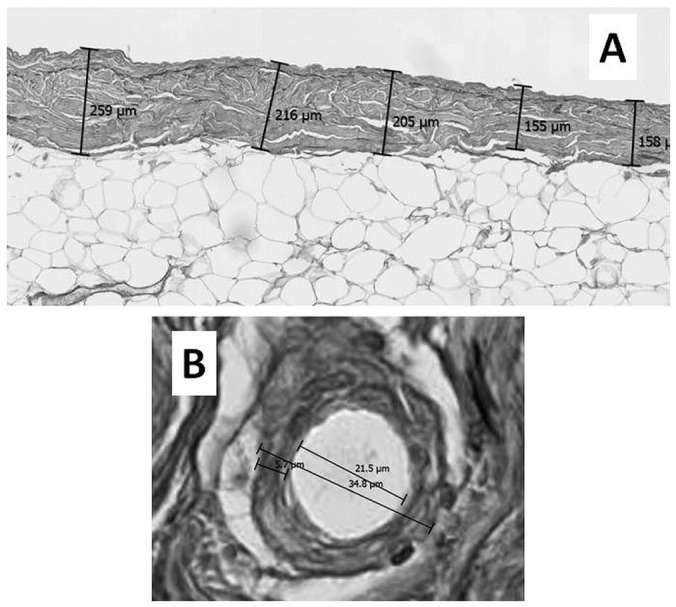
— Evaluation methods for peritoneal sclerosis. (A) Average peritoneal thickness (APT) by the 5-point measurement method. Peritoneal thickness is measured at 5 randomly selected points, and the APT is then calculated. In this peritoneal dialysis patient (number 10), peritoneal thicknesses ranged from 155 μm to 259 μm. The APT was calculated to be 198.6 μm. The average of 2 APT values determined by 2 examiners was taken as the representative APT for this patient. (B) Quantitative evaluation of vasculopathy at a postcapillary venule (PCV). Severity of luminal narrowing was determined using the ratio of luminal diameter to vessel diameter (L/V), representing the patency of the blood vessel. A PCV whose diameter was in the range 25 – 50 μm was selected for measurement. The short axis was measured, with the most severely affected vessel being chosen for the measurement. The average of 2 L/V values determined by 2 examiners was taken as the representative L/V. In this peritoneal dialysis patient (number 8), the luminal and vessel diameters were 21.5 μm and 34.8 μm respectively. Thus, the final L/V ratio was 0.62.
Evaluation of Vasculopathy: The extent of vasculopathy was determined by the presence of hyalinosis, thickness of the vascular wall, and severity of luminal narrowing at the level of the PCV. For evaluation of the severity of luminal narrowing, vascular wall thickness (VWT) was measured, and the L/V ratio (luminal diameter to vessel external diameter), which represents the patency of the blood vessel, was determined [Figure 1(B)]. In general, hyalinizing vasculopathy is usually observed at the PCV or capillary level in PD patients; we therefore selected for morphologic measurements a PCV whose diameter ranged from 25 μm to 50 μm, because the L/V ratio is influenced by the level of the blood vessel examined. The measurement was taken along the short axis to avoid the artificial effect of elongated distance as a result of tangential cutting of the vessel during histology preparation. When the severity of vasculopathy varied from vessel to vessel, the most severely affected vessel in each specimen was chosen for measurement. The average of 2 VWTs and 2 L/V ratios measured by 2 examiners was taken as the representative values for the patient.
POLICY OF TREATMENT
We believe that adequate PD consists of
sufficient total small-solute clearance, for a minimum Kt/V of 1.7;
strict fluid control;
control of phosphate and calcium levels;
good nutrition; and
an absence of inflammation and anemia.
Fluid, phosphate, and calcium control and good nutrition are achieved with patient education. When malnutrition, inflammation, anemia, or fluid retention is suspected, we increased the dose of PD or switched the patient to hybrid therapy (a combination of PD 5 or 6 days each week and HD once each week), now a common clinical practice in Japan (12,13), especially for oliguric or anuric patients. We recommend that patients switch to HD when their D/P creatinine continuously exceeds 0.7, when their peritoneal mesothelial cells show hypertrophy or severe atypia, or when their compliance with prescribed treatment become severely poor and uncorrectable.
STATISTICAL ANALYSIS
Normally distributed variables are expressed as mean ± standard deviation, and non-normally dis tributed variables are expressed as median and range. We performed one-way repeated-measures analysis of variance (ANOVA) with a post hoc Tukey–Kramer HSD (“honestly significant difference”) test in analyses of a single parameter measured under different conditions in the same subjects. A value of p < 0.05 was considered significant.
RESULTS
CHARACTERISTICS AND CLINICAL COURSES
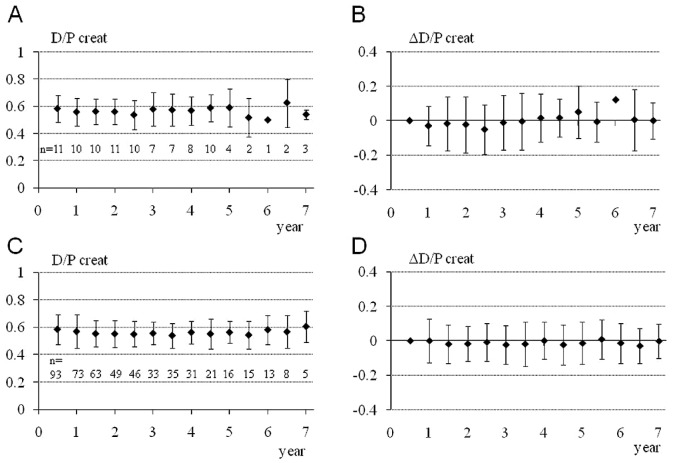
Tables 1 and 2 show the characteristics and clinical courses of the 11 study subjects. The D/P creatinine of these patients was maintained at a low value [Figure 2(A)] throughout their long-term PD therapy and was similar to the D/P creatinine of the other 99 patients [Figure 2(C)]. A plot of the change in D/P creatinine from baseline in the 11 patients was favorably flat [Figure 2(B)], as it also was in the other 99 patients [Figure 2(D)]. The one-way repeated-measures ANOVA and post hoc Tukey–Kramer HSD test revealed no significant time-course changes in each variable in each group.
TABLE 1.
Baseline Dataa for the Study Patients
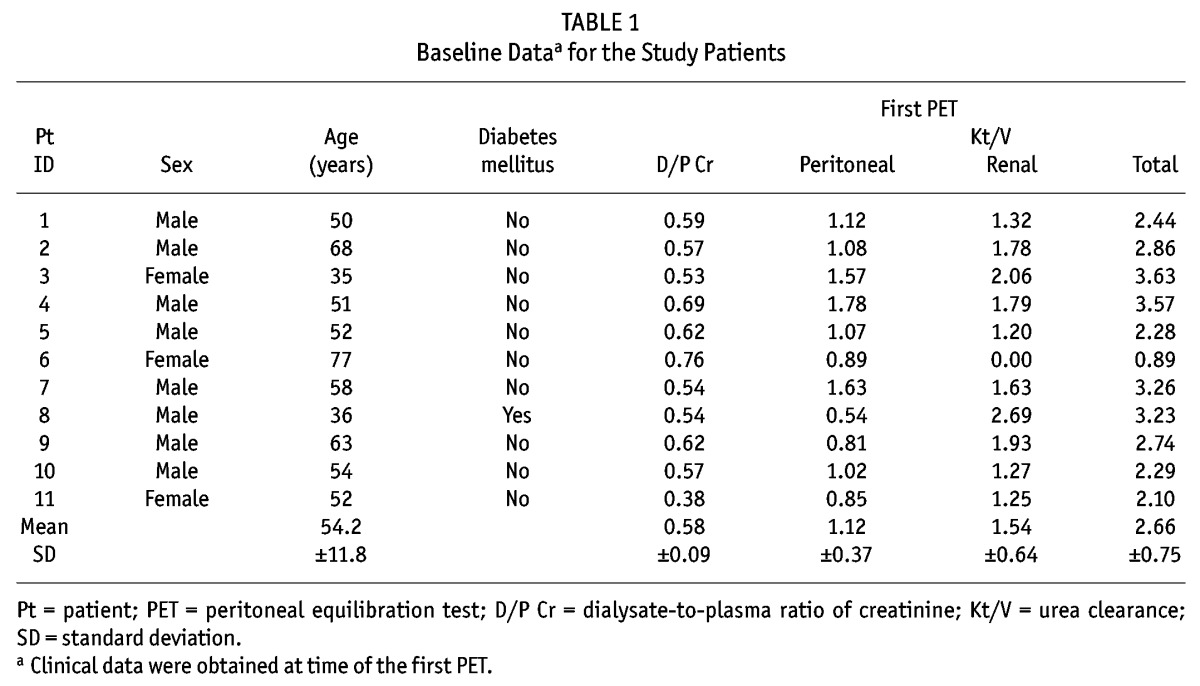
TABLE 2.
Clinical Course of the Patients
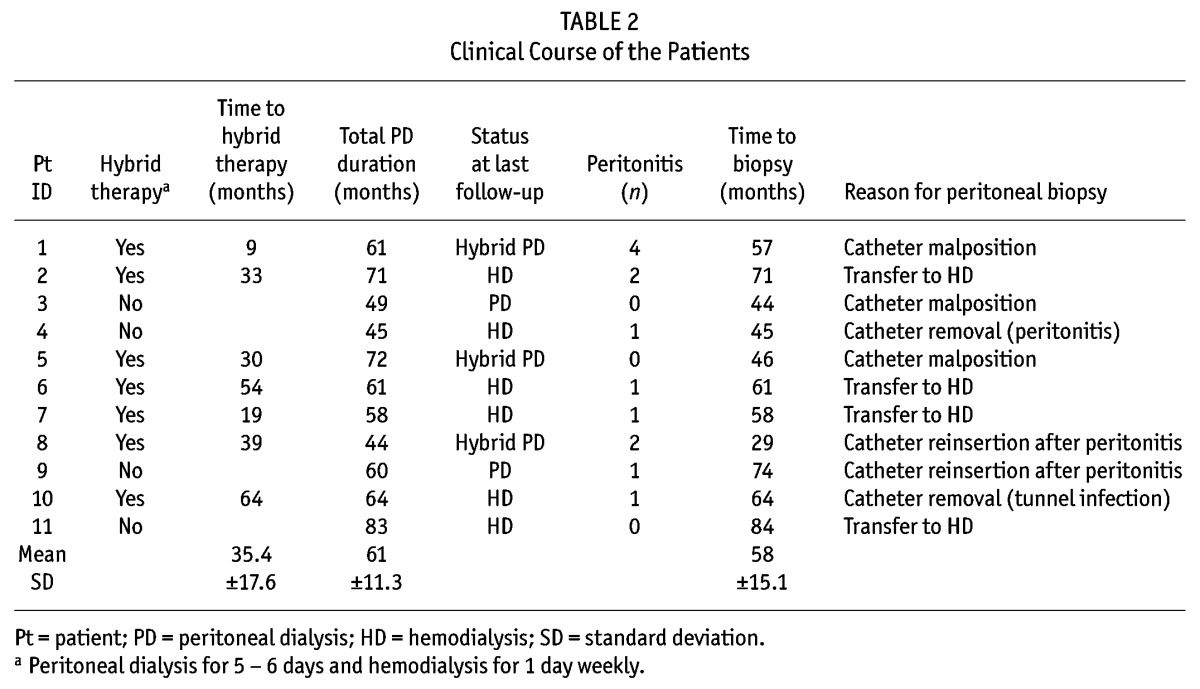
Figure 2.
— Time course of the dialysate-to-plasma ratio of creatinine (D/P creat). (A) The D/P creat and (B) the changes from baseline in D/P creat are shown for the 11 peritoneal dialysis (PD) patients who continued PD therapy using biocompatible dialysate for more than 3 years and underwent peritoneal biopsy in the present study. (C) The D/P creat and (B) the changes from baseline in D/P creat are shown for the 99 patients who started PD therapy using biocompatible dialysate at Tokyo University Hospital between 2002 and 2009. Values are mean ± standard error of the mean. One-way repeated-measures analysis of variance and post hoc Tukey–Kramer HSD (“honestly significant difference”) test found no significant time course changes in each variable in each group.
PERITONEAL FIBROSIS AND VASCULOPATHY
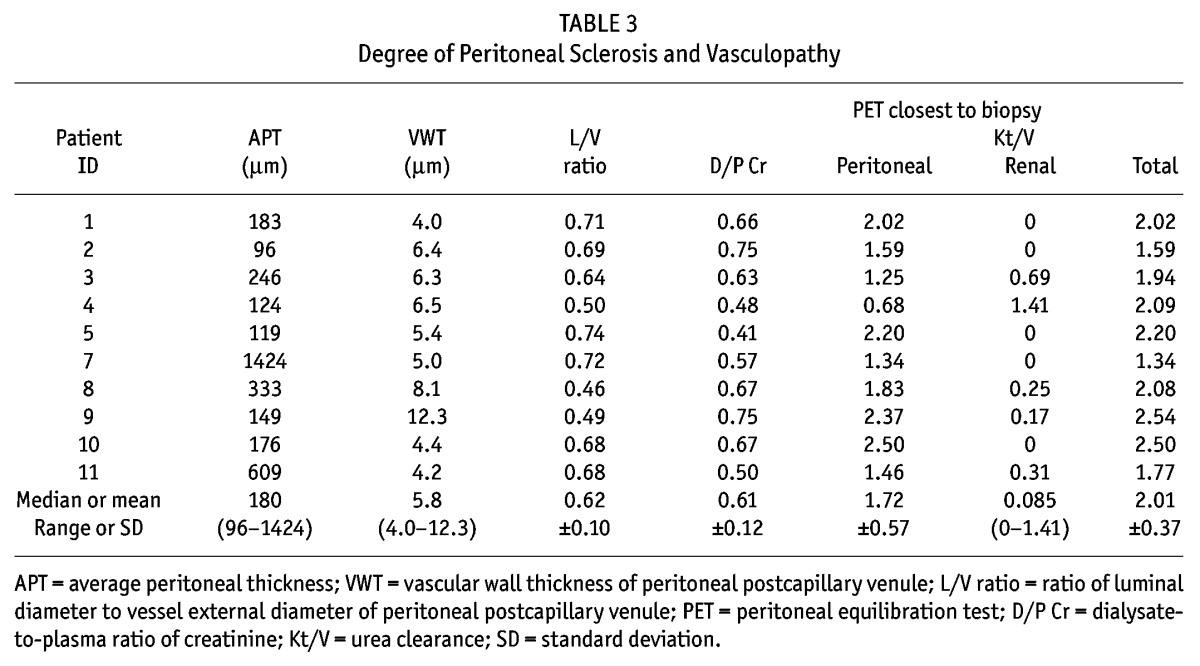
The specimen obtained from patient 6 was not adequate for the study because the direction of embedding was not vertical, and patient 6 was excluded from further evaluation. Table 3 shows the quantitative parameters of peritoneal fibrosis and vasculopathy in the specimens of the remaining 10 patients. For those 10 patients, the median APT was 180 μm (96 – 1424 μm); the median VWT, 5.8 μm (4.0 – 12.3 μm); and the median L/V ratio, 0.66 (0.46 – 0.74). The APT remained thin and the L/V ratio was maintained at a low level for more than 3 years. These values seemed not to correlate with PD duration, although we could not perform a statistical analysis because of the limited number of patients in the study cohort. Only in patient 7, who remained noncompliant, with poor fluid management (using only 2.5% glucose dialysate), was the peritoneal membrane considerably thickened (APT: 1424 μm), standing out from the others. No patient showed evidence of encapsulating peritoneal sclerosis.
TABLE 3.
Degree of Peritoneal Sclerosis and Vasculopathy
DISCUSSION
In the present study, we for the first time used quantitative methods to evaluate peritoneal fibrosis and vasculopathy and to describe the effect on peritoneal morphology of biocompatible dialysate and current clinical practice in Japan.
It has been well-reported that, in biopsy samples obtained from patients who undergo PD with conventional bioincompatible dialysate, the thickness of the peritoneal compact zone progressively increases with the PD duration. Williams et al. (4) reported that the median thickness of the submesothelial compact collagenous zone in 130 patients undergoing PD with conventional bioincompatible dialysate fluid increased significantly with the duration of PD therapy: 180 μm in those who underwent PD for 0 – 24 months, 240 μm for 25 – 48 months of PD, 300 μm for 49 – 72 months; 750 μm for 73 – 96 months, and 700 μm for 97 months or more. A similar result was reported in a Japanese study of 80 patients undergoing PD with conventional dialysate, in which the APT at 5 randomly selected points in the peritoneum was 166 μm in patients who had undergone PD for fewer than 4 years, 261 μm in patients who had undergone PD for 4 – 8 years, and 466 μm in patients who had undergone PD for more than 8 years (5). In our study, interstitial sclerosis did not progress during more than 3 years, remaining mild as has been observed in the early years of PD, which seems meaningfully different from the results of the two studies already mentioned.
On the other hand, several studies reported that the peritoneal vasculopathy in biopsy samples obtained from patients who had undergone PD with conventional bioincompatible dialysate demonstrated significant progression with the duration of PD. Williams et al. (4) used a semiquantitative grading system to demonstrate that the prevalence of vasculopathy in the peritonea of PD patients increased significantly with PD duration. Honda et al. (5) reported observing an inverse correlation between L/V ratio at PCV and PD duration. They also reported a significant correlation between the L/V ratio and the D/P creatinine, which confirms the idea that D/P creatinine might be a marker of peritoneal vasculopathy. In contrast to those reports, the peritoneal vasculopathy of the PD patients in the present study did not advance for more than 3 years, remaining favorably mild throughout their long-term PD therapy. Their D/P creatinine was also maintained at a favorably low level throughout their long-term PD treatment.
Accumulating evidence suggests that several factors contribute to peritoneal sclerosis in PD patients, including uremia, peritonitis, the presence of the catheter, and instillation of the dialysate itself. Various components of dialysate—including buffer, low pH, glucose concentration, and GDPs generated during heat sterilization—can influence peritoneal sclerosis (14). In rat models, the presence of the catheter partly induces PD-related morphology changes (15,16). Moreover, pressure is itself an inflammatory trigger. In rat models, Zareie et al. (16,17) showed that instillation of lactate buffer without glucose or GDPs resulted in increased cell influx, mesothelial regeneration, angiogenesis, and an increased number of milky spots, although fibrosis was not significantly enhanced. The addition of glucose to the buffer enhances angiogenesis and mesothelial regeneration and induces fibrosis and cell influx (16–18).
The presence of GDPs further enhances all of the foregoing peritoneal changes except for cell influx and mesothelial regeneration (16,17,19–21). In addition to GDPs, AGEs formed when glucose is heated are considered to contribute to the toxicity of peritoneal dialysate (22). The GDPs and AGEs may damage peritoneal cells and proteins through various mechanisms (22–28), leading to peritoneal damage. It has become increasingly clear that, in animal models of PD, the more-biocompatible peritoneal dialysates (bicarbonate/lactate buffer, low GDPs) induce less damage and less impairment of ultrafiltration (16,19,29,30). In addition, supplementing peritoneal dialysate with aminoguanidine, which scavenges GDPs and prevents AGE formation, results in less mesothelial denudation (31), fibrosis, and angiogenesis in omentum and parietal peritoneum (32).
A retrospective study suggested that the use of biocompatible solutions conferred a survival advantage among PD patients (33). Additionally, a crossover study (10) and a prospective randomized clinical trial (11) demonstrated the beneficial effects of biocompatible dialysate on residual renal function. However, the observation periods in those latter studies tended to be limited (<3 years), and no study has ever succeeded in demonstrating a benefit of biocompatible dialysate for peritoneal function. A prospective study by Fan et al. failed to detect a beneficial effect on residual renal function (34). In addition, the prevention of morphology change in the peritonea of PD patients with the use of biocompatible fluid has never been reported, especially with long-term use. The minimal changes in peritoneal morphology observed in patients in the present study might have resulted from the favorable effect of biocompatible dialysate and therefore might be additional in vivo evidence supporting the findings already mentioned. However, we cannot simply compare the favorable outcomes in the present study with those in the studies using conventional bioincompatible dialysate fluid, because in this decade, significant progress has also been made in the policy on managing dialysis modality (35), including hybrid therapy in Japan (12,13). These advances might partially contribute to the minimized peritoneal sclerosis seen in the present study.
The question of whether the use of biocompatible dialysate with neutral pH and low GDP concentrations preserves peritoneal solute transport and ultrafiltration remains controversial. Using assessment of unrestricted pore area over unit diffusion path length [“area parameter” (for small-solute transport: A0/Dx)], Rippe et al. (36,37) demonstrated no significant differences in peritoneal transport characteristics between patients treated with conventional peritoneal dialysates and those treated with low-GDP dialysates. Choi et al. (38) also found no statistically significant differences in D/P creatinine or in the change of D/P creatinine between patients treated with conventional dialysates and those treated with biocompatible dialysates with neutral pH and a very low concentration of GDPs. However, the Euro-Balance trial group reported that D/P creatinine was higher in patients treated with low-GDP PD solution (10). In the present study, D/P creatinine and its change from baseline were maintained at relatively low levels throughout the observation period, a result superior to that in longitudinal observations of PD with conventional dialysates (39).
The present study has some limitations. First, because of the lack of a control group using conventional bioincompatible dialysate or a group observed before the widespread introduction of hybrid therapy with added HD, we could not draw a definitive conclusion on the superiority of neutral-pH, low-GDP glucose solutions or the impact of hybrid HD therapy on peritoneal morphology. Second, the sample size was relatively small. We could not perform correlation analyses for APT, L/V ratio, PD duration, and D/P creatinine. The failure to detect an obvious correlation between APT and L/V ratio, which was reported in earlier studies (5), might also be explained by the limited number of patients. Further long-term and larger-scale studies are needed. However, values of D/P creatinine and its change from baseline in the 11 patients included in the present study were similar to those of the other 99 patients who received PD therapy with biocompatible dialysate at our institution. Considering that D/P creatinine is a marker of peritoneal vasculopathy, we believe that the morphology changes in the 11 study patients might represent the long-term effect on the peritoneum in all patients of biocompatible dialysate use and current clinical practice in Japan, including hybrid therapy.
CONCLUSIONS
Morphology changes in the peritoneal membrane were minimal, and small-solute transport was kept at favorably low values in patients who underwent long-term PD with biocompatible fluid under contemporaneous Japanese clinical management polices, including hybrid dialysis therapy.
DISCLOSURES
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1. Churchill DN, Thorpe KE, Nolph KD, Keshaviah PR, Oreopoulos DG, Pagé D. Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. The Canada–USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol 1998; 9:1285–92 [DOI] [PubMed] [Google Scholar]
- 2. Davies SJ, Bryan J, Phillips L, Russell GI. Longitudinal changes in peritoneal kinetics: the effects of peritoneal dialysis and peritonitis. Nephrol Dial Transplant 1996; 11:498–506 [PubMed] [Google Scholar]
- 3. Davies SJ, Phillips L, Griffiths AM, Russell LH, Naish PF, Russell GI. What really happens to people on long-term peritoneal dialysis? Kidney Int 1998; 54:2207–17 [DOI] [PubMed] [Google Scholar]
- 4. Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, et al. on behalf of the Peritoneal Biopsy Study Group. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 2002; 13:470–9 [DOI] [PubMed] [Google Scholar]
- 5. Honda K, Hamada C, Nakayama M, Miyazaki M, Sherif AM, Harada T, et al. on behalf of the Peritoneal Biopsy Study Group of the Japanese Society for Peritoneal Dialysis. Impact of uremia, diabetes, and peritoneal dialysis itself on the pathogenesis of peritoneal sclerosis: a quantitative study of peritoneal membrane morphology. Clin J Am Soc Nephrol 2008; 3:720–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Margetts PJ, Bonniaud P. Basic mechanisms and clinical implications of peritoneal fibrosis. Perit Dial Int 2003; 23:530–41 [PubMed] [Google Scholar]
- 7. Davies SJ, Phillips L, Naish PF, Russell GI. Peritoneal glucose exposure and changes in membrane solute transport with time on peritoneal dialysis. J Am Soc Nephrol 2001; 12:1046–51 [DOI] [PubMed] [Google Scholar]
- 8. Hendriks PM, Ho-dac-Pannekeet MM, van Gulik TM, Struijk DG, Phoa SS, Sie L, et al. Peritoneal sclerosis in chronic peritoneal dialysis patients: analysis of clinical presentation, risk factors, and peritoneal transport kinetics. Perit Dial Int 1997; 17:136–43 [PubMed] [Google Scholar]
- 9. Selgas R, Fernandez–Reyes MJ, Bosque E, Bajo MA, Borrego F, Jimenez C, et al. Functional longevity of the human peritoneum: how long is continuous peritoneal dialysis possible? Results of a prospective medium long-term study. Am J Kidney Dis 1994; 23:64–73 [DOI] [PubMed] [Google Scholar]
- 10. Williams JD, Topley N, Craig KJ, Mackenzie RK, Pischetsrieder M, Lage C, et al. on behalf of the Euro Balance Trial Group. The Euro-Balance Trial: the effect of a new biocompatible peritoneal dialysis fluid (Balance) on the peritoneal membrane. Kidney Int 2004; 66:408–18 [DOI] [PubMed] [Google Scholar]
- 11. Kim S, Oh J, Kim S, Chung W, Ahn C, Kim SG, et al. Benefits of biocompatible PD fluid for preservation of residual renal function in incident CAPD patients: a 1-year study. Nephrol Dial Transplant 2009; 24:2899–908 [DOI] [PubMed] [Google Scholar]
- 12. Kawanishi H, Hashimoto Y, Nakamoto H, Nakayama M, Tranæus A. Combination therapy with peritoneal dialysis and hemodialysis. Perit Dial Int 2006; 26:150–4 [PubMed] [Google Scholar]
- 13. Working Group Committee for Preparation of Guidelines for Peritoneal Dialysis, Japanese Society for Dialysis Therapy; Japanese Society for Dialysis Therapy. 2009 Japanese Society for Dialysis Therapy guidelines for peritoneal dialysis. Ther Apher Dial 2010; 14:489–504 [DOI] [PubMed] [Google Scholar]
- 14. Jörres A, Bender TO, Finn A, Witowski J, Fröhlich S, Gahl GM, et al. Biocompatibility and buffers: effect of bicarbonate-buffered peritoneal dialysis fluids on peritoneal cell function. Kidney Int 1998; 54:2184–93 [DOI] [PubMed] [Google Scholar]
- 15. Flessner MF, Credit K, Henderson K, Vanpelt HM, Potter R, He Z, et al. Peritoneal changes after exposure to sterile solutions by catheter. J Am Soc Nephrol 2007; 18:2294–302 [DOI] [PubMed] [Google Scholar]
- 16. Zareie M, Keuning ED, ter Wee PM, Schalkwijk CG, Beelen RH, van den Born J. Improved biocompatibility of bicarbonate/lactate–buffered PDF is not related to pH. Nephrol Dial Transplant 2006; 21:208–16 [DOI] [PubMed] [Google Scholar]
- 17. Zareie M, Hekking LH, Welten AG, Driesprong BA, Schadee–Eestermans IL, Faict D, et al. Contribution of lactate buffer, glucose and glucose degradation products to peritoneal injury in vivo. Nephrol Dial Transplant 2003; 18:2629–37 [DOI] [PubMed] [Google Scholar]
- 18. Chan TM, Yung S. Studying the effects of new peritoneal dialysis solutions on the peritoneum. Perit Dial Int 2007; 27(Suppl 2):S87–93 [PubMed] [Google Scholar]
- 19. Mortier S, Faict D, Lameire NH, De Vriese AS. Benefits of switching from a conventional to a low-GDP bicarbonate/lactate–buffered dialysis solution in a rat model. Kidney Int 2005; 67:1559–65 [DOI] [PubMed] [Google Scholar]
- 20. Musi B, Braide M, Carlsson O, Wieslander A, Albrektsson A, Ketteler M, et al. Biocompatibility of peritoneal dialysis fluids: long-term exposure of nonuremic rats. Perit Dial Int 2004; 24:37–47 [PubMed] [Google Scholar]
- 21. Wieczorowska–Tobis K, Polubinska A, Schaub TP, Schilling H, Wisniewska J, Witowski J, et al. Influence of neutral-pH dialysis solutions on the peritoneal membrane: a long-term investigation in rats. Perit Dial Int 2001; 21(Suppl 3):S108–13 [PubMed] [Google Scholar]
- 22. Honda K, Nitta K, Horita S, Yumura W, Nihei H, Nagai R, et al. Accumulation of advanced glycation end products in the peritoneal vasculature of continuous ambulatory peritoneal dialysis patients with low ultra-filtration. Nephrol Dial Transplant 1999; 14:1541–9 [DOI] [PubMed] [Google Scholar]
- 23. Morgan LW, Wieslander A, Davies M, Horiuchi T, Ohta Y, Beavis MJ, et al. Glucose degradation products (GDP) retard remesothelialization independently of d-glucose concentration. Kidney Int 2003; 64:1854–66 [DOI] [PubMed] [Google Scholar]
- 24. Mortier S, Faict D, Schalkwijk CG, Lameire NH, De Vriese AS. Long-term exposure to new peritoneal dialysis solutions: effects on the peritoneal membrane. Kidney Int 2004; 66:1257–65 [DOI] [PubMed] [Google Scholar]
- 25. Schwenger V, Morath C, Salava A, Amann K, Seregin Y, Deppisch R, et al. Damage to the peritoneal membrane by glucose degradation products is mediated by the receptor for advanced glycation end-products. J Am Soc Nephrol 2006; 17:199–207 [DOI] [PubMed] [Google Scholar]
- 26. Tauer A, Knerr T, Niwa T, Schaub TP, Lage C, Passlick–Deetjen J, et al. In vitro formation of Nε-(carboxymethyl) lysine and imidazolones under conditions similar to continuous ambulatory peritoneal dialysis. Biochem Biophys Res Commun 2001; 280:1408–14 [DOI] [PubMed] [Google Scholar]
- 27. Tauer A, Zhang X, Schaub TP, Zimmeck T, Niwa T, Passlick–Deetjen J, et al. Formation of advanced glycation end products during CAPD. Am J Kidney Dis 2003; 41(Suppl 1):S57–60 [DOI] [PubMed] [Google Scholar]
- 28. Zeier M, Schwenger V, Deppisch R, Haug U, Weigel K, Bahner U, et al. Glucose degradation products in PD fluids: do they disappear from the peritoneal cavity and enter the systemic circulation? Kidney Int 2003; 63:298–305 [DOI] [PubMed] [Google Scholar]
- 29. Hekking LH, Zareie M, Driesprong BA, Faict D, Welten AG, de Greeuw I, et al. Better preservation of peritoneal morphologic features and defense in rats after long-term exposure to a bicarbonate/lactate-buffered solution. J Am Soc Nephrol 2001; 12:2775–86 [DOI] [PubMed] [Google Scholar]
- 30. Kim CD, Kwon HM, Park SH, Oh EJ, Kim MH, Choi SY, et al. Effects of low glucose degradation products peritoneal dialysis fluid on the peritoneal fibrosis and vascularization in a chronic rat model. Ther Apher Dial 2007; 11:56–64 [DOI] [PubMed] [Google Scholar]
- 31. Lee EA, Oh JH, Lee HA, Kim SI, Park EW, Park KB, et al. Structural and functional alterations of the peritoneum after prolonged exposure to dialysis solutions: role of aminoguanidine. Perit Dial Int 2001; 21:245–53 [PubMed] [Google Scholar]
- 32. Zareie M, Tangelder GJ, ter Wee PM, Hekking LH, van Lambalgen AA, Keuning ED, et al. Beneficial effects of aminoguanidine on peritoneal microcirculation and tissue remodelling in a rat model of PD. Nephrol Dial Transplant 2005; 20:2783–92 [DOI] [PubMed] [Google Scholar]
- 33. Lee HY, Park HC, Seo BJ, Do JY, Yun SR, Song HY, et al. Superior patient survival for continuous ambulatory peritoneal dialysis patients treated with a peritoneal dialysis fluid with neutral pH and low glucose degradation product concentration (Balance). Perit Dial Int 2005; 25:248–55 [PubMed] [Google Scholar]
- 34. Fan SL, Pile T, Punzalan S, Raftery MJ, Yaqoob MM. Randomized controlled study of biocompatible peritoneal dialysis solutions: effect on residual renal function. Kidney Int 2008; 73:200–6 [DOI] [PubMed] [Google Scholar]
- 35. Lo WK, Bargman JM, Burkart J, Krediet RT, Pollock C, Kawanishi H, et al. on behalf of the ISPD Adequacy of Peritoneal Dialysis Working Group. Guideline on targets for solute and fluid removal in adult patients on chronic peritoneal dialysis. Perit Dial Int 2006; 26:520–2 [PubMed] [Google Scholar]
- 36. Rippe B, Simonsen O, Heimbürger O, Christensson A, Haraldsson B, Stelin G, et al. Long-term clinical effects of a peritoneal dialysis fluid with less glucose degradation products. Kidney Int 2001; 59:348–57 [DOI] [PubMed] [Google Scholar]
- 37. Rippe B, Wieslander A, Musi B. Long-term results with low glucose degradation product content in peritoneal dialysis fluids. Contrib Nephrol 2003; (140):47–55 [DOI] [PubMed] [Google Scholar]
- 38. Choi HY, Kim DK, Lee TH, Moon SJ, Han SH, Lee JE, et al. The clinical usefulness of peritoneal dialysis fluids with neutral pH and low glucose degradation product concentration: an open randomized prospective trial. Perit Dial Int 2008; 28:174–82 [PubMed] [Google Scholar]
- 39. Davies SJ. Longitudinal relationship between solute transport and ultrafiltration capacity in peritoneal dialysis patients. Kidney Int 2004; 66:2437–45 [DOI] [PubMed] [Google Scholar]