The role of peritoneal dialysis (PD) in the management of patients with acute kidney injury (AKI) is not well defined. In fact, a review published this year, discussing the dose of renal replacement therapy (RRT) for patients with AKI, makes no mention of PD as a treatment option (1). Major concerns about PD as a treatment modality for AKI were raised by a randomized trial conducted in Vietnam and published in the New England Journal of Medicine in 2002 (and discussed later in this article) (2). That study, suggesting a higher mortality rate in AKI patients treated with PD than in patients treated with continuous venovenous hemofiltration (CVVH), made clinicians rethink the role of PD for patients with AKI. And that rethinking occurred despite a paper from India published in Kidney International in the same year suggesting that, for patients with AKI, a mild-to-moderate hypercatabolic state, and relative hemodynamic stability, outcomes with both automated tidal PD and standard continuous ambulatory PD techniques were certainly acceptable (3).
The use of PD for AKI, then, became somewhat limited and did not receive much attention until about 2008. The Brazilian group from Sao Paolo recently rekindled interest in PD for AKI with a series of innovative publications in which they used a randomized trial design to confirm the efficacy of PD and to show that results with PD are at least as good as those with HD (4,5). The Brazilian findings are particularly important because attention is beginning again to be focused on the use of PD to treat AKI in low-income developing countries, as is clearly documented in the current issue of Peritoneal Dialysis International (PDI).
Acute kidney injury is an important cause of morbidity and mortality in low-income countries in which resources to recognize and treat AKI are lacking (6,7). The epidemiology of AKI in low-income countries differs from that in middle- and high-income countries (8,9). In most low-income countries, renal replacement programs exist in large cities and are available only to those who can afford to pay. Such programs are generally associated with units established for patients with end-stage renal disease, in which treatments are offered only to those few patients who can afford the therapy.
Acute PD offers significant advantages over hemodialysis (HD) in low-resource settings because it requires neither electricity nor machinery (with the associated ongoing maintenance requirements), and it is both technically simpler for health professionals to learn and less expensive if PD fluid can be obtained at a reasonable cost (10,11). Importantly, the relatively good clinical outcomes experienced at several programs discussed in the present issue of PDI suggest a promising future for acute PD in the setting of AKI in the developing world. Those good outcomes have been achieved despite the challenges faced in those settings, including late patient referral for treatment, limited supporting resources, and limited availability of pathology or specialized diagnostic or microbiologic testing in many of the centers where programs have been established.
What then does the literature suggest should be the role of PD to treat patients with AKI? In contrast to the large number of studies comparing continuous and intermittent RRT modalities for AKI, only nine studies have compared PD with extracorporeal RRT techniques. Most were observational; only three prospective randomized controlled trials have been conducted (Table 1). Those three studies were conducted primarily among critically ill patients (77% – 100% of cases), and sample sizes were modest, ranging from 50 to 120 patients. Notably, the mean patient age and proportion of septic patients in two of the three studies (2,12) were relatively lower than those seen in recent randomized trials of RRT dose in critically ill patients (13,14). Two studies compared PD with continuous RRT; the third used daily intermittent HD as the comparator. The results of the three studies were mixed.
TABLE 1.
Randomized Controlled Studies Comparing Peritoneal Dialysis (PD) and Extracorporeal Blood Purification (EPB) Techniques for Renal Replacement Therapy
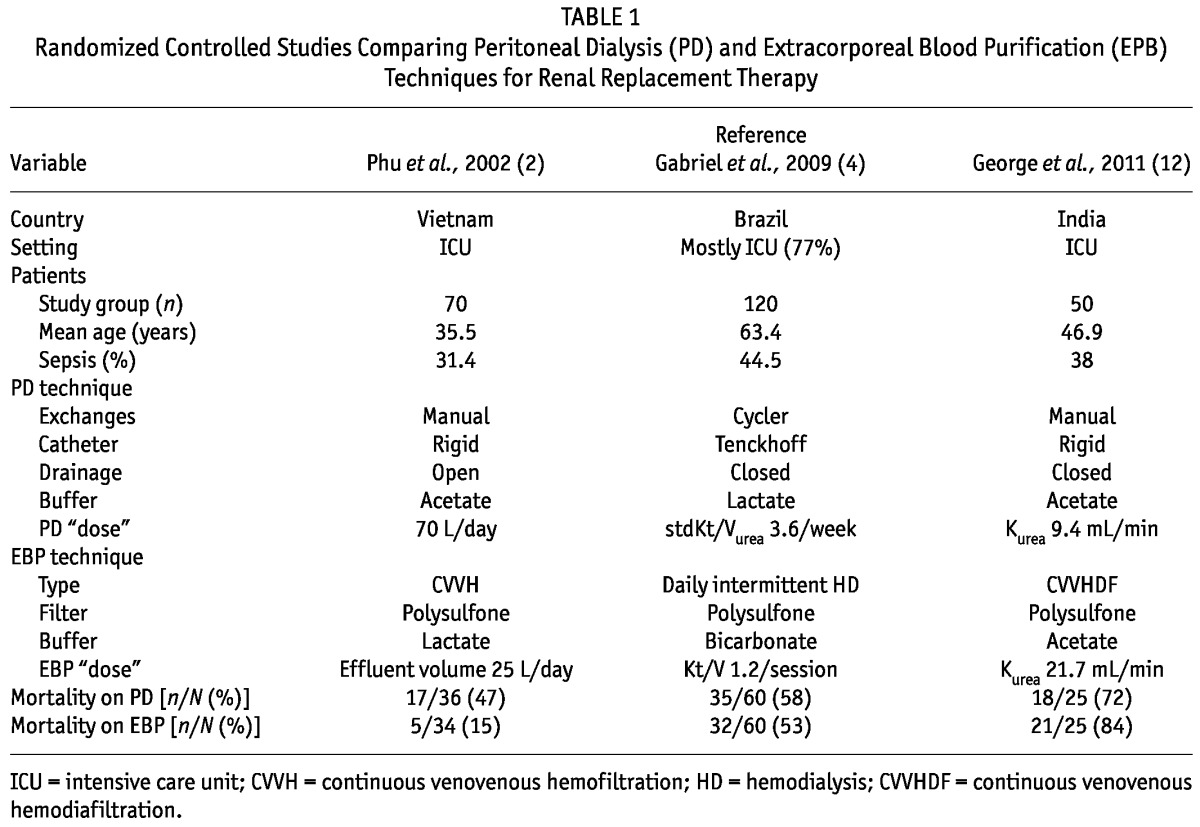
In the study by Phu et al., CVVH was strikingly superior to PD in terms of mortality (2). That difference was noted despite a relatively low dose (by current standards) of CVVH. However, the PD technique was also less than ideal, with the use of rigid catheters, manual exchanges, and open drainage. Although the peritonitis rate was reported as 2.8% (defined by positive peritoneal fluid cultures), cloudy dialysate was observed in 41.6% of patients. The causes of the cloudy fluid were not clear, but it is possible that the instances of cloudy fluid also represented peritonitis episodes with negative cultures related to culturing techniques or to the use of systemic antibiotics for other infections. If so, those instances could have contributed to poor outcomes in the PD group. The study was also unique in that the leading cause of AKI was severe falciparum malaria (68% of patients). The use of glucose-containing PD solutions may potentially be detrimental in specific clinical situations such as malaria. It has been postulated that the erythrocytic phase of malarial parasites can be accelerated by the high splanchnic glucose levels resulting from PD fluid, and that factor may also have contributed to the high mortality in the PD group in the study (15). However, two observational studies on PD for malaria-associated AKI reported lower mortality rates of 10% – 26% (16,17). The potential down side of PD in this specific population therefore remains unclear.
In the other two studies, mortality rates with PD and extracorporeal RRT techniques were similar. Gabriel et al. used modern PD techniques, with cyclers and flexible tunneled catheters, and compared the results with those achieved in patients receiving daily HD (5). This particular PD approach allowed for the use of relatively higher exchange volumes than were used in the other trials. The overall mortality in the study population was comparable to that reported in other studies looking at critically ill AKI patients requiring RRT, and it was similar for the PD and the intermittent HD groups (13,14,18,19). The authors also noted that duration of dialysis dependence was significantly less with PD than with HD (5.5 ± 2.7 days and 7.5 ± 3.1 days respectively, p = 0.02). That finding suggests earlier recovery of renal function with PD.
More recently, George et al. compared PD with continuous venovenous hemodiafiltration (CVVHDF), looking primarily at solute control (that is, correction of uremia, electrolyte, and acid–base disorders) as well as correction of fluid overload (12). As expected, urea and creatinine clearances were significantly higher with CVVHDF than with PD; control of fluid overload was also faster with CVVHDF. Correction of acidosis was faster with PD. There were no significant differences between PD and CVVHDF with respect to correction of hyperkalemia and hemodynamic instability. Furthermore, cost of disposables with PD was less than half that with CVVHDF. In the study, mortality was quite high—but similar—in both groups.
One of the major concerns with PD for AKI involves the issue of the “dose” of dialysis. Can PD provide sufficient dialysis for patients with AKI to permit recovery of both the patient and the kidney? Certainly, that issue was one of those raised by the randomized trial from Vietnam in 2002 (2). Was the significantly higher mortality rate observed in patients assigned to PD rather than to CVVH related to the dose of dialysis? The amount of PD prescribed in the study was actually quite high: 2-L exchanges were used with a 30-minute dwell time (a total of approximately 70 L daily). Interestingly, the paper from India in Kidney International, which indicated acceptable results with PD for patients with AKI and a mild-to-moderate hypercatabolic state, used both automated tidal PD and standard continuous ambulatory PD. The Kt/V urea achieved with continuous ambulatory PD was 1.80 ± 0.32 (range: 1.47 – 2.75); for tidal PD, it was 2.43 ± 0.87 (range: 1.11 – 3.49). Remember that patients with severe hypercatabolic conditions and those who were hemodynamically unstable were excluded, but they accounted for fewer than 18% of 113 patients. The Brazilian group from Sao Paolo used high-dose PD in their initial two studies, achieving a mean weekly Kt/V urea of 3.6 – 3.8 (4,20). Large amounts of PD solution were used: 36 – 44 L of fluid and 18 – 22 exchanges were used daily. Patients randomized to daily HD reached a Kt/V urea of 4.7 ± 0.6 (5). In a follow-up study, the Brazilian group randomized 61 patients to targeted high- and low-dose PD regimens, corresponding to a daily prescribed Kt/V of 0.8 and 0.5 respectively (21). Actual doses delivered in the study were slightly lower, with a weekly Kt/V urea of 4.13 ± 0.6 achieved in the high-dose group and 3.01 ± 0.5 achieved in the low dose group. These was no difference in the primary outcome (mortality) or in obvious metabolic control between the groups.
So, where does all that leave us?
We think that there are four important lessons to be learned from the papers in the present issue of PDI when taken together with earlier publications. First, the optimal treatment of AKI remains uncertain. Second, studies looking at various therapeutic approaches to AKI have demonstrated differing results, reflecting in part the severity of disease studied, patient selection, and patterns of RRT. Third, in terms of PD, the optimal dose of dialysis is unclear. High-dose PD (weekly Kt/V urea > 3) provide results comparable to those with HD. Whether lower doses in the range of 2.1, as suggested by some authors (22,23), provide results comparable to those achieved with the higher doses remains to be determined, but data suggest that such a result may in fact be true.
In the absence of precise data, the clinician needs to exercise practical judgment. Peritoneal dialysis needs to be considered a reasonable treatment for AKI, particularly in low-resource settings in which other forms of RRT are not available. It is clear from data, such as those presented in this issue of PDI, that results with PD can be perfectly acceptable. In full-resource settings, PD offers a viable alternative to HD for patients with AKI, particularly patients with only mild-to-moderate catabolic conditions.
The dose of PD that needs to be targeted for AKI remains uncertain and presents a challenge that is not different from the challenge presented in defining the optimal dose of HD or hemofiltration in the same situation. Of particular note is a recent review discussing the dose of RRT for patients with AKI (1). The authors of that review suggest that no firm guidelines have emerged concerning the dose of dialysis to be targeted with CVVH or slow, low-efficiency HD. The authors point out that recent clinical trials have not shown any advantage of increasing the dose of RRT above that obtained with alternate-day HD achieving a Kt/V urea of 1.2 per treatment. In practice, achieving that Kt/V of 1.2 usually means targeting a Kt/V of 1.4. That target would correspond to a standardized Kt/V of 2.1. Importantly, a Kt/V urea in that range can easily be reached with PD without the use of large volumes of solution. The authors emphasize the need to individualize therapy for each patient based on the clinical circumstances, severity of illness, hemodynamic stability, catabolic state, and so on.
DISCLOSURES
None of the authors has any financial conflicts of interest to disclose.
REFERENCES
- 1. Vijayan A, Palevsky PM. Dosing of renal replacement therapy in acute kidney injury Am J Kidney Dis 2012; 59:569–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Phu NH, Hien TT, Mai NT, Chau TT, Chuong LV, Loc PP, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med 2002; 347:895–902 [DOI] [PubMed] [Google Scholar]
- 3. Chitalia VC, Almeida AF, Rai H, Bapat M, Chitalia KV, Acharya VN, et al. Is peritoneal dialysis adequate for hypercatabolic acute renal failure in developing countries? Kidney Int 2002; 61:747–57 [DOI] [PubMed] [Google Scholar]
- 4. Gabriel DP, Caramori JT, Martin LC, Barretti P, Balbi AL. Continuous peritoneal dialysis compared with daily hemodialysis in patients with acute kidney injury. Perit Dial Int 2009; 29(Suppl 2):S62–71 [PubMed] [Google Scholar]
- 5. Gabriel DP, Caramori JT, Martim LC, Barretti P, Balbi AL. High volume peritoneal dialysis vs daily hemodialysis: a randomized, controlled trial in patients with acute kidney injury Kidney Int Suppl 2008; (108):S87–93 [DOI] [PubMed] [Google Scholar]
- 6. Cerdá J, Lameire N, Eggers P, Pannu N, Uchino S, Wang H, et al. Epidemiology of acute kidney injury. Clin J am Soc Nephrol 2008; 3:881–6 [DOI] [PubMed] [Google Scholar]
- 7. Lameire N, Van Biesen W, Vanholder R. Acute renal failure. lancet 2005; 365:417–30 [DOI] [PubMed] [Google Scholar]
- 8. Jha V, Malhotra HS, Sakhuja V, Chugh KS. Spectrum of hospital-acquired acute renal failure in the developing countries—Chandigarh study. Q J Med 1992; 83:497–505 [PubMed] [Google Scholar]
- 9. Lameire N, Van Biesen W, Vanholder R. The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol 2006; 2:364–77 [DOI] [PubMed] [Google Scholar]
- 10. Carter M, Kilonzo K, Odiit A, Kalyesubula R, Kotanko P, Levin NW, et al. Acute peritoneal dialysis treatment programs for countries of the East African community. Blood Purif 2012; 33:149–52 [DOI] [PubMed] [Google Scholar]
- 11. Callegari JG, Kilonzo KG, Yeates KE, Handelman GJ, Finkelstein FO, Kotanko P, et al. Peritoneal dialysis for acute kidney injury in sub-Saharan Africa: challenges faced and lessons learned at Kilimanjaro Christian Medical Centre. Kidney Int 2012; 81:331–3 [DOI] [PubMed] [Google Scholar]
- 12. George J, Varma S, Kumar S, Thomas J, Gopi S, Pisharody R. Comparing continuous venovenous hemodiafiltration and peritoneal dialysis in critically ill patients with acute kidney injury: a pilot study. Perit Dial Int 2011; 31:422–9 [DOI] [PubMed] [Google Scholar]
- 13. Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, et al. on behalf of the VA/NIH Acute Renal Failure Trial Network. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 2008; 359:7–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, et al. on behalf of the RENAL Replacement Therapy Study Investigators. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 2009; 361:1627–38 [DOI] [PubMed] [Google Scholar]
- 15. Daugirdas JT. Peritoneal dialysis in acute renal failure— why the bad outcome? N Engl J Med 2002; 347:933–5 [DOI] [PubMed] [Google Scholar]
- 16. Indraprasit S, Charoenpan P, Suvachittanont O, Mavichak V, Kiatboonsri S, Tanomsup S. Continuous peritoneal dialysis in acute renal failure from severe falciparum malaria. Clin Nephrol 1988; 29:137–43 [PubMed] [Google Scholar]
- 17. Trang TT, Phu NH, Vinh H, Hien TT, Cuong BM, Chau TT, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis 1992; 15:874–80 [DOI] [PubMed] [Google Scholar]
- 18. Vesconi S, Cruz DN, Fumagalli R, Kindgen–Milles D, Monti G, Marinho A, et al. on behalf of the Dose Response Multicentre International collaborative initiative (DO-RE-MI Study Group). Delivered dose of renal replacement therapy and mortality in critically ill patients with acute kidney injury. Crit Care 2009; 13:R57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Piccinni P, Cruz DN, Gramaticopolo S, Garzotto F, Dal Santo M, Aneloni G, et al. on behalf of the NEFROINT Investigators. Prospective multicenter study on epidemiology of acute kidney injury in the ICU: a critical care nephrology Italian collaborative effort (NEFROINT). Minerva anestesiol 2011; 77:1072–83 [PubMed] [Google Scholar]
- 20. Gabriel DP, Nascimento GV, Caramori JT, Martim LC, Barretti P, Balbi AL. High volume peritoneal dialysis for acute renal failure. Perit Dial Int 2007; 27:277–82 [PubMed] [Google Scholar]
- 21. Ponce D, Brito GA, Abrão JG, Balb AL. Different prescribed doses of high-volume peritoneal dialysis and outcome of patients with acute kidney injury. adv Perit Dial 2011; 27:118–24 [PubMed] [Google Scholar]
- 22. Ponce D, Berbel MN, Regina de Goes C, Almeida CT, Balbi AL. High-volume peritoneal dialysis in acute kidney injury: indications and limitations. Clin J am Soc Nephrol 2012;:[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23. Chionh CY, Ronco C, Finkelstein FO, Soni SS, Cruz DN. Acute peritoneal dialysis: what is the “adequate” dose for acute kidney injury? Nephrol Dial Transplant 2010; 25:3155–60 [DOI] [PubMed] [Google Scholar]


