Abstract
Acute kidney injury (AKI) is a common complication in children after surgery for congenital heart disease, and peritoneal dialysis (PD) is usually the renal replacement therapy (RRT) of choice, especially in very young children. The aim of the present study was to describe our experience of using PD to treat AKI after cardiac surgery.
We retrospectively analyzed children 1 week to 16 years of age undergoing cardiac surgery during 2000 – 2008 and found the incidence of AKI treated with PD to be 2.3%. In the 23 patients treated with PD (13 male; average age: 29 ± 48.4 months; weight: 9.1 ± 8.1 kg), the indications for PD initiation were oliguria (n = 13), anuria (n = 9), and acidosis (n = 1). The average time between cardiac surgery and AKI was 4.8 ± 16.8 hours, and between AKI and PD initiation, it was 12 ± 16.8 hours. Patients were treated for a mean of 4.8 ± 3.8 days. Two patients developed peritonitis, and mechanical dysfunction of the PD catheter occurred in 1 patient. In-hospital mortality was 43.4%. Patients treated with PD weighed less (p = 0.004) and had longer bypass time (p = 0.004), inotrope use (p = 0.000), and mechanical ventilation (p = 0.000). However, in a regression analysis, only cardiopulmonary bypass time (odds ratio: 1.021; 95% confidence interval: 0.998 to 1.027; p = 0.032) remained predictive of a subsequent need for PD.
We conclude that PD is an efficacious RRT for AKI in children undergoing cardiac surgery and that, in this setting, bypass time is the strongest predictor of a subsequent need for RRT.
Keywords: Acute kidney injury, cardiac surgery, prognosis
Children with congenital heart disease are at increased risk of acute kidney injury (AKI) after cardiac surgery. Several conditions increase vulnerability to this complication, including the acute inflammatory response to cardiopulmonary bypass and postoperative hemodynamic instability, ischemia or reperfusion injury, circulating endotoxins and myoglobin, postoperative hemolysis, septic complications, congestive heart failure, and baseline renal disease secondary to cyanotic congenital heart disease. The reported AKI incidence ranges between 1% and 17% (1,2), depending largely on the criteria used to define the condition, and the associated mortality is high (between 21% and 70%) (3).
General considerations about the treatment of postoperative AKI include correction or removal of the precipitating factor and management of volume status. However, when volume overload and oliguria or anuria supervenes, renal replacement therapy (RRT) should be considered. In this setting, peritoneal dialysis (PD) has long been advocated as the preferred technique because of better hemodynamic stability, no requirement for vascular access, simplicity, and low cost (4).
The aim of the present work was to describe our experience using PD after congenital heart disease surgery in children under 16 years of age and to identify risk factors associated with poor outcome.
METHODS
We retrospectively analyzed children who, from January 2000 to March 2008, required PD for the treatment of AKI after surgery for congenital heart disease. Data were collected from patient files and intensive care unit (ICU) registries; first-time and consecutive PD procedures were both included. Demographic and clinical data included gestational age, weight and age at time of surgery, type of cardiac disease, surgical procedure details, and Risk Adjustment in Congenital Heart Surgery (RACHS-1) score (5). The RACHS-1 score was developed to compare outcomes of congenital cardiac surgical care and is based on the complexity of the cardiac defects. It comprises 6 risk categories (1 being the least severe; 6 being the most severe) associated with increased in-hospital mortality. Pre- and postoperative renal function data, time to diagnosis of AKI and to PD initiation after surgery, cardiopulmonary and aorta cross-clamping time, and type and duration of inotropic support were obtained. The Paediatric Risk of Mortality (PRISM II) score (6), which uses data about the first 24 hours after admission to the ICU to predict patient outcome, was also calculated. In PRISM II, values above 15 are associated with a high mortality risk.
Acute kidney injury was diagnosed using the Acute Kidney Injury Network (AKIN) criteria (7), defined as a percentage increase in serum creatinine of 50% or more (1.5 times baseline) or a reduction in urine output of less than 0.5 mL/kg per hour for more than 6 hours. Two patients had renal failure before cardiac surgery; they were preoperatively treated with PD.
Catheters for acute dialysis were implanted surgically by experienced surgeons and under general anesthesia. In some patients, the PD catheter was inserted during cardiac surgery because of reduced urinary output throughout the surgical procedure or predictable development of AKI postoperatively. The PD catheters were of the Tenckhoff type (Fresenius Medical Care, Bad Homburg, Germany), pediatric size (25 cm), double-cuffed, and tunneled with a downward-oriented exit site.
A manual PD technique was performed because of its simplicity and lack of a requirement for more sophisticated equipment. To prevent leakage, the exchange volume of dialysate was restricted to 10 mL/kg at the beginning of PD in all patients. That volume was subsequently increased according to body surface area and clinical and laboratory parameters—namely, requirements for ultrafiltration and solute clearance.
Standard commercially available bicarbonate-based solutions with dextrose concentrations of 1.5%, 2.5%, and 4.25% (Fresenius Medical Care) were used. Before bicarbonate-based solutions became generally available, a 1.5% dextrose solution was prepared by the hospital pharmacy (700 mL 0.9% sodium chloride, 30 mL 30% dextrose, 30 mL 8.4% sodium bicarbonate, with distilled water to reach 1 L of solution). Most of the patients were initially prescribed the lowest osmolarity solutions. When clinically appropriate, the more hypertonic solutions were used to treat fluid overload. (In the solution prepared by the hospital pharmacy, hypertonic solutions were achieved by adding 100 mL instead of 30 mL 30% dextrose.)
When commercial solutions were used, a dialysate calcium concentration of 1.75 mmol/L was selected. Additives such as potassium chloride, heparin, or antibiotics were included in the solutions as necessary. No patient received intraperitoneal insulin.
Complications related to PD, including major infectious and noninfectious problems, were reported.
The statistical analysis was performed using the SPSS software application (version 17: SPSS, Chicago, IL, USA). Quantitative variables were compared using the Student t-test, and categorical variables, the chi-square test.
Considering the small sample size, logistic regression analyses to identify factors predictive of subsequent need for PD and of mortality after cardiac surgery were performed using the entire cohort of patients that developed AKI (n = 280). In the first model, we included preoperative and intraoperative variables (age, weight, RACHS-1 score ≥ 4, aortic cross-clamp time, cardiopulmonary bypass time, and urinary output) to identify factors that would be predictive of a need for PD. Subsequently, in the second model, we included preoperative, intraoperative, and postoperative variables [age, weight, RACHS-1 score ≥ 4, aortic cross-clamp time, cardiopulmonary bypass time, duration of inotrope use, length of mechanical ventilation, and treatment with PD (yes or no)] to find the independent predictors of mortality. In both models, the level of significance was defined as p < 0.05.
RESULTS
Between January 2000 and March 2008, 998 patients under 16 years of age underwent cardiac surgery for congenital heart disease. In that period, the incidence of AKI by AKIN criteria was 28.1% (280 patients), of which 63.6% were male, 67% were less than 12 months of age, and 4.2% had already undergone a cardiac surgery. Management of acute renal dysfunction required PD to be applied in 23 patients (2.3%). Of those 23 patients, 13 (56.5%) were male. The average age in the PD group was 29 ± 48.4 months (range: 7 days – 165 months), and the average weight was 9.1 ± 8.1 kg. Three infants (13%) were preterm (gestational ages of 30, 34, and 36 weeks).
Table 1 presents the cardiac diagnoses, types of surgical procedure, and RACHS-1 classifications for the patients that received PD. In 9 patients (39%), the RACHS-1 score was 4 or higher, and in 2 patients, RACHS-1 could not be used because of cardiac transplant. Average PRISM II score was 19.3 ± 6.
TABLE 1.
Age, Diagnosis, Surgery, and Risk Adjustment in Congenital Heart Surgery (RACHS-1) Scores for the Study Patients
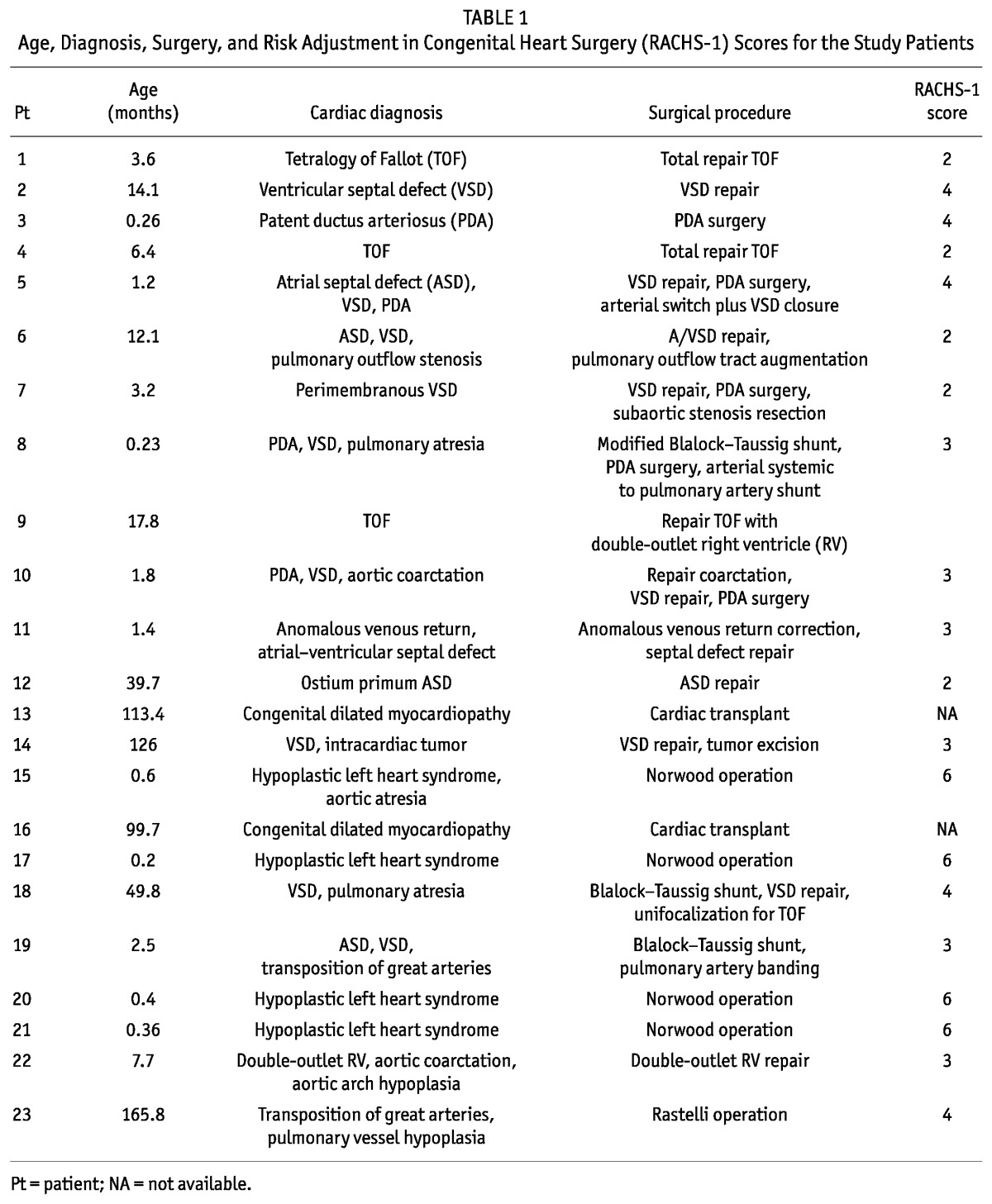
A previous history of AKI was present in 4 patients. In 2 patients with hypoplastic left heart syndrome and severe hemodynamic compromise, PD was begun preoperatively (2 and 51 days before surgery); PD was continued after the surgery until resolution of AKI. In the other 2 patients, 1 presented with AKI after therapy with angiotensin converting-enzyme inhibitor, and 1 developed AKI secondary to low cardiac output syndrome; in both, complete recovery of renal function was achieved at least 2 months before surgery. Additionally, in 3 patients, congenital urinary tract anomalies—bilateral pelviectasis, unilateral megaureter, and horseshoe kidney—had previously been identified, but none of those patients has a documented history of AKI.
In 14 patients, AKI occurred in the first 12 – 24 hours after surgery. The mean time between surgery and AKI diagnosis was 4.8 ± 16.8 hours (range: 0 – 48 hours), and between AKI diagnosis and PD initiation, it was 12 ± 16.8 hours (range: 0 – 72 hours). The median time between surgery and RRT was 2 days. In 8 patients (34.7%), including all the preterm infants, PD was started less than 24 hours after surgery; in 5, a PD catheter had already been inserted during the cardiac surgery. The indications for starting dialysis were oliguria (13 patients), anuria (9 patients), and severe acidosis (1 patient).
The mean duration of dialysis (censored for death) was 115.2 ± 91.2 hours [4.8 ± 3.8 days (range: 1 – 14 days)]. Among the patients that survived, the mean duration of dialysis was longer [5.1 ± 4.2 days (range: 1 – 14 days)].
The exchange volume of dialysate was 10 mL/kg at the beginning of PD. In 18 patients, this volume was progressively increased to 35 mL/kg (minimum: 25 mL; maximum: 1000 mL). Dwell time was steadily increased in 20 patients to 20 – 60 minutes. The daily ultrafiltration rate with PD ranged between 0 mL/kg and 165 mL/kg (mean: 23 ± 20 mL/kg). Hypertonic solutions were required in 13 patients (56.5%) to optimize ultrafiltration and to correct volume overload.
All patients who required PD, except 1, received inotropic support after cardiac surgery with at least 2 drugs. In 12 patients (52%), 3 inotropic agents were required. The average duration of inotrope use was 124.8 ± 40.8 hours (5.2 ± 1.7 days). Cardiopulmonary bypass was performed in 20 patients (86.9%). Mean bypass time was 126.6 ± 78.4 minutes, and aortic cross-clamp time was 55.7 ± 46.5 minutes. Temporary pacing was required in 9 patients (39%).
Complications related to PD occurred in 3 patients: 2 developed peritonitis, and 1 experienced mechanical dysfunction of the PD catheter. The episodes of peritonitis were diagnosed 2 and 6 days after PD initiation; both were attributed to Staphylococcus aureus. However, no systemic septic complications were evident, and in no case did the infection lead to discontinuation of PD. Episodes of peritonitis were treated with intraperitoneal antibiotics while PD was maintained; subsequently, a switch to systemic intravenous therapy was performed to complete the antimicrobial course. Catheter malposition was corrected by guidewire manipulation and subsequently confirmed by abdominal radiography. No patient required catheter removal because of these complications.
In-hospital mortality was 6.2% (n = 16) in patients with AKI and 43.4% (n = 10) in patients with AKI treated with PD. Eight patients (34.7%) died while on PD treatment. In the PD-treated patients, in-hospital mortality was attributable to cardiogenic shock in 4 patients (40%), cardiorespiratory arrest in 2 (20%), multi-organ failure in 2 (20%), disseminated intravascular coagulation in 1 (10%), and malignant arrhythmia in 1 (10%). Three deaths occurred in preterm infants (30%): 1 had a previous episode of AKI not treated with PD before surgery, and 1 had a documented episode of peritonitis. In long-term follow-up, 3 patients died: 2 from cardiac failure, and 1 from undetermined causes 5 years after surgery. All patients who survived completely recovered their renal function.
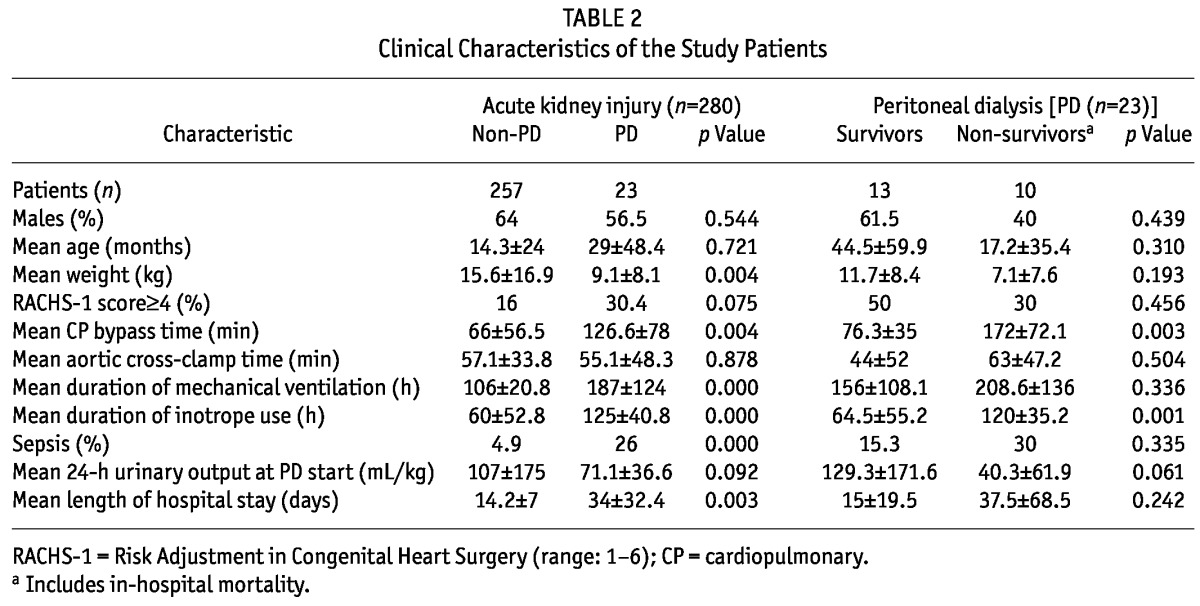
In the cohort of patients that developed AKI, those that required PD weighed less, but had longer bypass time, use of inotropic agents, mechanical ventilation, and hospital stay (Table 2). In this group, a trend was also observed toward reduced urinary output immediately after surgery, but no differences in RACHS-1 score were seen. In PD-treated patients who died, longer cardiopulmonary bypass time and duration of inotrope use was also observed (Table 2). In that group, a longer interval between AKI diagnosis and PD initiation was also documented (0.25 ± 0.45 days vs. 3.1 ± 7.9 days, p = 0.089), albeit not a statistically significant one. Inhospital mortality in patients treated with PD showed no differences according to RACHS-1 score (p = 0.456).
TABLE 2.
Clinical Characteristics of the Study Patients
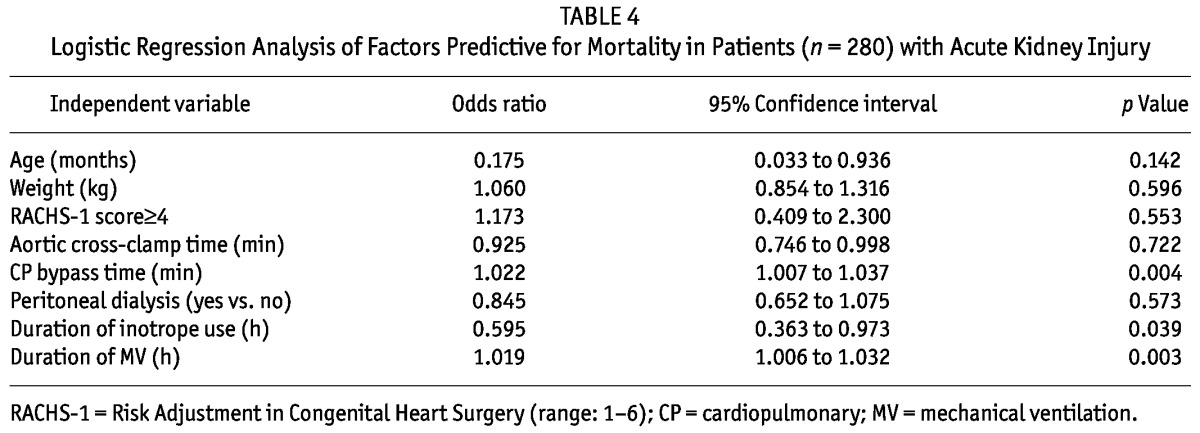
By logistic regression analysis, longer bypass time was the strongest predictor of subsequent need of PD after cardiac surgery (Table 3). However, when considering prognosis, the durations of cardiopulmonary bypass time, of mechanical ventilation time, and of inotrope use were associated with mortality (Table 4).
TABLE 3.
Logistic Regression Analysis of Factors Predictive for Peritoneal Dialysis in Patients (n = 280) with Acute Kidney Injury
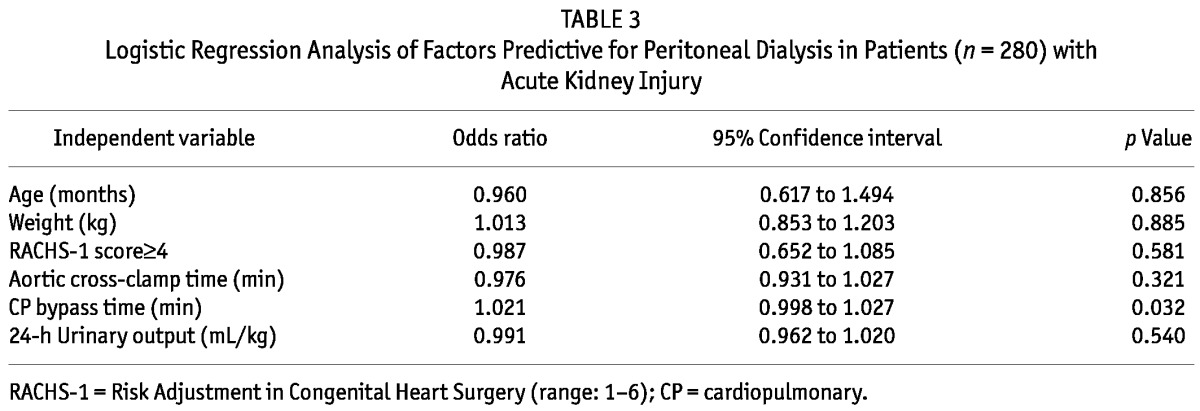
TABLE 4.
Logistic Regression Analysis of Factors Predictive for Mortality in Patients (n = 280) with Acute Kidney Injury
DISCUSSION
Children undergoing surgery for congenital heart disease are especially prone to AKI. However, it is difficult to appreciate the true incidence of this complication because, in the literature, great variability is seen in the criteria used for the diagnosis. The condition has been defined as an increase in serum creatinine 30% above basal levels or a reduction in diuresis (8); a 100% rise in serum creatinine or oliguria with urinary output reduction varying according to age and weight (3); or a tripling of baseline serum creatinine, or oliguria or anuria, or both (2). One of the largest series published to date (4) considered only patients with AKI who required PD, which inevitably underestimates the overall occurrence of this complication. For our patients, we used the recently published AKIN criteria, which resulted in an incidence of 28.1%. That value is higher than the incidence found in the literature, which usually ranges between 1% and 10.8% (1,2), and might be related to the greater sensitivity of AKIN, especially for milder forms of AKI.
It has been reported that 1% – 17% of patients with AKI require RRT (3). In this setting, PD or continuous venovenous hemodiafiltration can be offered to pediatric patients. Some authors suggest that PD may not be as effective as continuous venovenous hemodiafiltration in hypercatabolic patients or in those with severe fluid overload requiring rapid net ultrafiltration. However, several prospective and retrospective studies have compared clinical outcomes with different RRT techniques, and no technique has been shown to be superior (3,4). In smaller children, PD may have some advantages because it obviates the need for a vascular catheter and is well tolerated in patients with hemodynamic instability (9). In most instances, however, the option for one technique relates more to the cost and the availability and experience of the ICU staff (9,10,11). In our hospital, only PD has been used in the treatment of severe AKI after surgery for congenital heart disease. The percentage of patients with AKI treated with PD was 2.3%, a value in the range previously published (8,12). The indications for starting RRT were similar to those described in other reports (4,8), namely oliguria and anuria, but we also included metabolic acidosis unresponsive to conservative measures, which is an indication not usually found in the literature.
Acute kidney injury is an early complication after cardiac surgery. Some centers, anticipating this event, place a dialysis catheter in the operating room immediately after cardiac surgery (2), and RRT is usually prescribed in the first days after the operation. In our experience, we observed a median of 2 days between surgery and PD commencement; other series report similar values (3,8). Nonetheless, pre-emptive placement of the dialysis catheter may affect the time to initiation of dialysis: in our series, we observed that 5 of 8 patients that already had a PD catheter began RRT on the day of surgery.
Various authors have demonstrated that an earlier initiation of dialysis is associated with a lower mortality rate (3,13–15). In our series, patients who were treated with PD and who died presented a trend toward a longer time between AKI diagnosis and PD initiation; however, that trend was not statistically significant, probably because of the small sample size. However, those results may suggest that, in seriously ill children, rapid correction of metabolic disturbances and hypervolemia is associated with better outcomes. Moreover, an earlier start of RRT may also be related to recovery from AKI (3), although in our series, we could not demonstrate such an association, because all surviving patients recovered their renal function.
Once correction of the low output syndrome is achieved, rapid improvement of renal function is usually the rule. In our series, the rate of recovery of renal function was 100% among surviving patients, but in the literature, the recovery rate is variable, ranging from 29.5% (16) to 50% (3,17) or higher [94% (18)]. The differences are probably related to the diverse patient populations and varying degrees of cardiac disease complexity described, or to in-center practices related to criteria for initiating RRT.
With regard to complications, PD is generally a safe method. Even though cardiothoracic surgery may constitute a relative contraindication to PD for the risk of peritoneopleural diaphragmatic communication, the technique is widely used with a low rate of complications. In our series, only 3 major complications occurred, although minor problems, such as leakage, might have been overlooked because of negligible clinical impact and the retrospective nature of the analysis. Nonetheless, minor complications are the most frequently reported problems, but difficult to evaluate in the present context. One of the largest series on PD after surgery for congenital cardiac problems found an overall rate of complications of 21%, of which 6.2% were major complications; the remaining problems were minor (4). Complexity of cardiac lesions and duration of PD were among the factors associated with an increased risk for PD problems (4).
The overall mortality rates, both in the hospital and over the long term, were comparable to values found in the literature (3). In this setting, mortality relates more to the underlying primary cardiac disease than to other medical conditions. Even if the presence of AKI is usually associated with an increased rate of complications, longer hospital stay, and worse prognosis in this setting, the association between AKI and mortality must take into account the overall risk of death because of the underlying cardiac condition (19). In low-risk patients, the correlation between AKI and mortality was strong; in those with higher risk, the association was weaker, because death was attributable not only to AKI but also to other organ failure, especially cardiac failure (4). As in other series (19), we also found that longer bypass time was a predictive factor for a subsequent need for PD, although in our cohort, the severity of the cardiac disease did not correlate with the likelihood of RRT or mortality.
Limitations of our retrospective analysis should be addressed. In addition to the aforementioned shortcomings concerning minor problems related to PD (which might not be recorded because the clinical impact was perceived to be negligible), we were also unable to determine indexes of dialysis efficacy and evaluate their impact on prognosis in this setting.
CONCLUSIONS
It is expected that the incidence of AKI after surgery for congenital heart disease will continue to rise. Factors contributing to this increase include earlier and better accuracy in diagnosis, leading to more complex surgeries at younger ages. Thus, attention to improving the technique of PD and the identification of factors associated with poor prognosis are crucial to improving the care of these patients.
DISCLOSURES
The authors have no grant supports or financial conflicts of interest to declare.
REFERENCES
- 1. Ramage IJ, Beattle TJ. Acute renal failure following cardiac surgery. Nephrol Dial Transplant 1999; 14:2777 [DOI] [PubMed] [Google Scholar]
- 2. Skippen PW, Krahn GE. Acute renal failure in children undergoing cardiopulmonary bypass. Crit Care Resusc 2005; 7:286–91 [PubMed] [Google Scholar]
- 3. Jander A, Tkaczyk M, Pagowska–Klimek I, Pietrzykowski W, Moll J, Krajewski W, et al. Continuous veno-venous hemodiafiltration in children after cardiac surgery. Eur J Cardiothorac Surg 2007; 31:1022–8 [DOI] [PubMed] [Google Scholar]
- 4. Pederson KR, Hjortdal VE, Christensen S, Pederson J, Hjortholm, Larsen S, et al. Clinical outcome in children with acute renal failure treated with peritoneal dialysis after surgery for congenital heart disease. Kidney Int Suppl 2008; (108):S81–6 [DOI] [PubMed] [Google Scholar]
- 5. Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg 2002; 123:110–18 [DOI] [PubMed] [Google Scholar]
- 6. Pollack MM, Ruttimann UE, Getson PR. Pediatric risk of mortality (PRISM) score. Crit Care Med 1988; 16:1110–16 [DOI] [PubMed] [Google Scholar]
- 7. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. on behalf of the Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Romao JE, Jr, Fuzissima MG, Vidonho AF, Jr, Noronha IL, Quintaes PS, Abensur H, et al. Outcome of acute renal failure associated with cardiac surgery in infants. Arq Bras Cardiol 2000; 75:313–21 [DOI] [PubMed] [Google Scholar]
- 9. Bonilla–Félix M. Peritoneal dialysis in the pediatric intensive care unit setting. Perit Dial Int 2009; 29(Suppl 2):S183–5 [PubMed] [Google Scholar]
- 10. Walters S, Porter C, Brophy PD. Dialysis and pediatric acute kidney injury: choice of renal support modality. Pediatr Nephrol 2009; 24:37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reznik VM, Randolph G, Collins CM, Peterson BM, Lemire JM, Mendoza SA. Cost analysis of dialysis modalities for pediatric acute renal failure. Perit Dial Int 1993; 13:311–13 [PubMed] [Google Scholar]
- 12. Plötz FB, Bouma AB, van Wijk JA, Kneyber MC, Bökenkamp A. Pediatric acute kidney injury in the ICU: an independent evaluation of pRIFLE criteria. Intensive Care Med 2008; 34:1713–17 [DOI] [PubMed] [Google Scholar]
- 13. Werner HA, Wensley DF, Lirenman DS, LeBlanc JG. Peritoneal dialysis in children after cardiopulmonary bypass. J Thorac Cardiovasc Surg 1997; 113:64–70 [DOI] [PubMed] [Google Scholar]
- 14. Elahi MM, Lim MY, Joseph RN, Dhannapuneni RR, Spyt TJ. Early hemofiltration improves survival in post-cardiotomy patients with acute renal failure. Eur J Cardiothorac Surg 2004; 26:1027–31 [DOI] [PubMed] [Google Scholar]
- 15. Dittrich S, Dähnert I, Vogel M, Stiller B, Haas NA, Alexi–Meskishvili V, et al. Peritoneal dialysis after infant open heart surgery: observations in 27 patients. Thorac Surg 1999; 68:160–3 [DOI] [PubMed] [Google Scholar]
- 16. Picca S, Principato F, Mazzera E, Corona R, Ferrigno L, Marcelletti C, et al. Risks of acute renal failure after cardiopulmonary bypass surgery in children: a retrospective 10-year case–control study. Nephrol Dial Transplant 1995; 10:630–6 [PubMed] [Google Scholar]
- 17. Baxter P, Rigby ML, Jones OD, Lincoln C, Shinebourne EA. Acute renal failure following cardiopulmonary bypass in children: results of treatment. Int J Cardiol 1985; 7:235–43 [DOI] [PubMed] [Google Scholar]
- 18. Giuffre RM, Tam KH, Williams WW, Freedom RM. Acute renal failure complicating pediatric cardiac surgery: a comparison of survivors and nonsurvivors following acute peritoneal dialysis. Pediatr Cardiol 1992; 13:208–13 [DOI] [PubMed] [Google Scholar]
- 19. Chan KL, Ip P, Chiu CS, Cheung YF. Peritoneal dialysis after surgery for congenital heart disease in infants and young children. Ann Thorac Surg 2003; 76:1443–9 [DOI] [PubMed] [Google Scholar]


