Abstract
♦ Background: The choices for renal replacement therapy (RRT) in childhood acute kidney injury (AKI) are limited in low-resource settings. Peritoneal dialysis (PD) appears to be the most practical modality for RRT in young children with AKI in such settings. Data from sub-Saharan Africa on the use of PD in childhood AKI are few.
♦ Methods: We performed a retrospective study of children who underwent PD for AKI at a tertiary-care hospital in southwest Nigeria from February 2004 to March 2011 (85 months).
♦ Results: The study included 27 children (55.6% female). Mean age was 3.1 ± 2.6 years, with the youngest being 7 days, and the oldest, 9 years. The causes of AKI were intravascular hemolysis (n = 11), septicemia (n = 8), acute glomerulonephritis (n = 3), gastroenteritis (n = 3), and hemolytic uremic syndrome (n = 2). Peritoneal dialysis was performed manually using percutaneous or adapted catheters. Duration of PD ranged from 6 hours to 12 days (mean: 5.0 ± 3.3 days). The main complications were peritonitis (n = 10), pericatheter leakage (n = 9), and catheter outflow obstruction (n = 5). Of the 27 patients, 19 (70%) survived till discharge.
♦ Conclusions: In low-resource settings, PD can be successfully performed for the management of childhood AKI. In our hospital, the use of adapted catheters may have contributed to the high complication rates. Peritoneal dialysis should be promoted for the management of childhood AKI in low-resource settings, and access to percutaneous or Tenckhoff catheters, dialysis fluid, and automated PD should be increased.
Keywords: Children, acute kidney injury, low-resource setting, peritoneal dialysis catheters, outcome, survival, Nigeria
In developed countries, the choice of modalities for renal replacement therapy (RRT) in pediatric acute kidney injury (AKI) is broad and includes peritoneal dialysis (PD), intermittent hemodialysis (HD), and continuous RRT (1). By contrast, options for RRT in pediatric AKI in many parts of sub-Saharan Africa are limited (1–4). Although continuous RRT is commonly used in the management of pediatric AKI in resource-rich countries, and although it may have some advantages in the hemodynamically unstable patient, it is rarely used in the management of AKI in many parts of sub-Saharan Africa because it is expensive and requires both sophisticated technology and consumables that are generally unavailable (5). Furthermore, in young children, not only is HD technically challenging, but the appropriate pediatric catheters and blood lines are also scarce in sub-Saharan Africa (2). On the other hand, the technique for PD is relatively simple. Peritoneal dialysis therefore appears to be the most practical modality for RRT in young children who develop AKI in sub-Saharan Africa (4).
Few studies have evaluated outcomes in patients who undergo PD for AKI in sub-Saharan Africa, and no studies have emerged from southwest Nigeria (4). We postulated that patient outcomes in this geographic area may be influenced by the causes of AKI, by late presentation for medical care, and by poor socio-economic conditions. A knowledge of the outcomes of PD and the associated challenges in resource-limited settings are crucial in identifying modifiable risk factors for adverse outcomes and in developing strategies to improve the care of children with AKI in Nigeria. Our objective was therefore to evaluate outcomes of all pediatric patients who underwent PD for AKI in a single tertiary-care center in southwest Nigeria.
METHODS
The University College Hospital Ibadan in southwest Nigeria is an 800-bed tertiary-care hospital with 120 pediatric beds. The hospital is located in Ibadan, the capital city of Oyo State. The immediate catchment area of the hospital, Oyo State, has 5 million inhabitants, 2 million of them being children. About 64% of the population live below the international poverty line of $1.25 per day (6). The patients usually pay out-of-pocket for medical treatment. The national health insurance scheme is not widely in use yet and does not cover fees for dialysis.
We performed a retrospective study of consecutive patients with AKI whose management included PD. The period of study was February 2004 to March 2011 (85 months). Over the study period, 41 patients received PD for AKI. Of those patients, 9 seen during October – November 2008 received PD for AKI secondary to diethyl glycol poisoning during an epidemic of pediatric AKI that followed ingestion of a tainted teething mixture (7). Those children were excluded from the present study because PD is not the optimal RRT in the management of AKI secondary to diethylene glycol poisoning (8,9). In another 5 patients who also received PD for AKI, detailed records of patient management were not available. Those patients were therefore also excluded from the analysis.
The case records of the study patients were reviewed. We collected information on baseline demographics, causes of AKI, serum urea and creatinine levels at presentation, indications for dialysis, and duration of PD. Primary outcomes were defined as death and need for dialysis at discharge. Secondary outcomes were PD-related complications and length of hospital admission. Peritonitis was defined as the presence of cloudy effluent with or without fever or abdominal tenderness. Pericatheter leakage was defined as dialysate moisture around the PD catheter. All patients were followed during the period of hospital admission to discharge.
Peritoneal dialysis was performed manually (Figure 1) using commercially available continuous ambulatory PD solutions with 1.5% and 2.5% glucose (Fresenius Medical Care, Bad Homburg, Germany). The solutions were donated free of charge to the pediatric nephrology unit by a benefactor and were administered at no cost to the patients. We added heparin 500 IU/L and gentamycin 4 mg/L to each bag of dialysis fluid. We used stylet percutaneous PD catheters (Medionics International, Markham, Ontario, Canada; Figure 2) or adapted catheters (nasogastric tubes; Figure 3).
Figure 1.
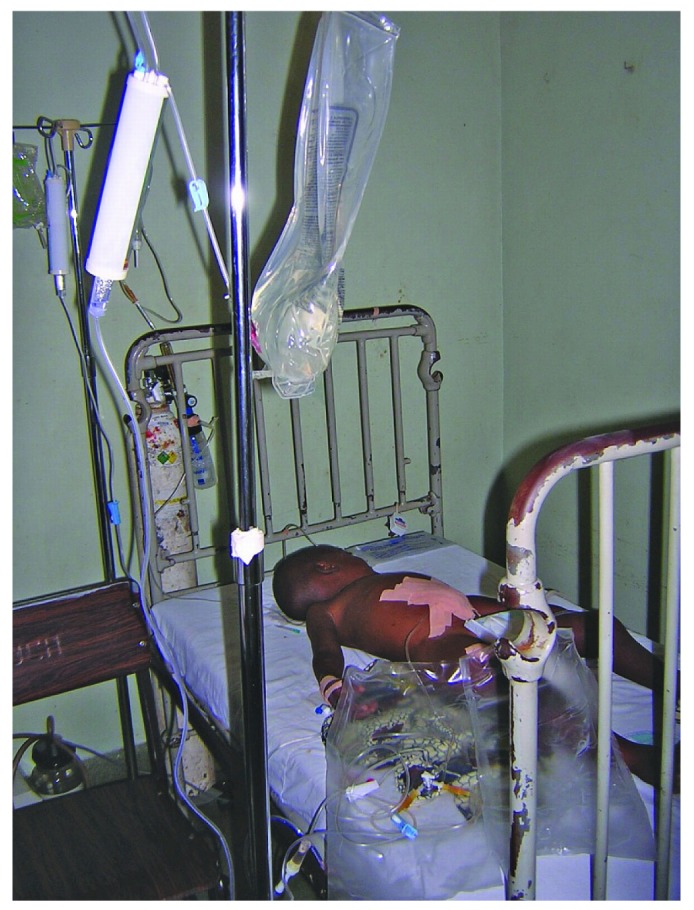
— A child undergoing peritoneal dialysis for acute kidney injury.
Figure 2.
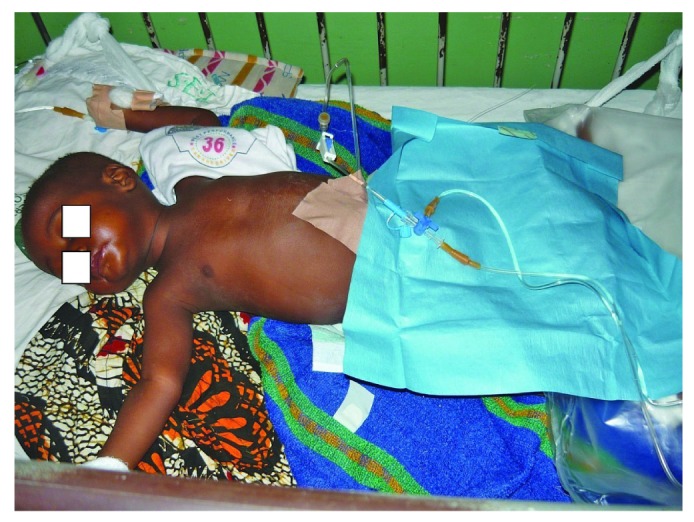
— A patient undergoes peritoneal dialysis using a percutaneous catheter.
Figure 3.
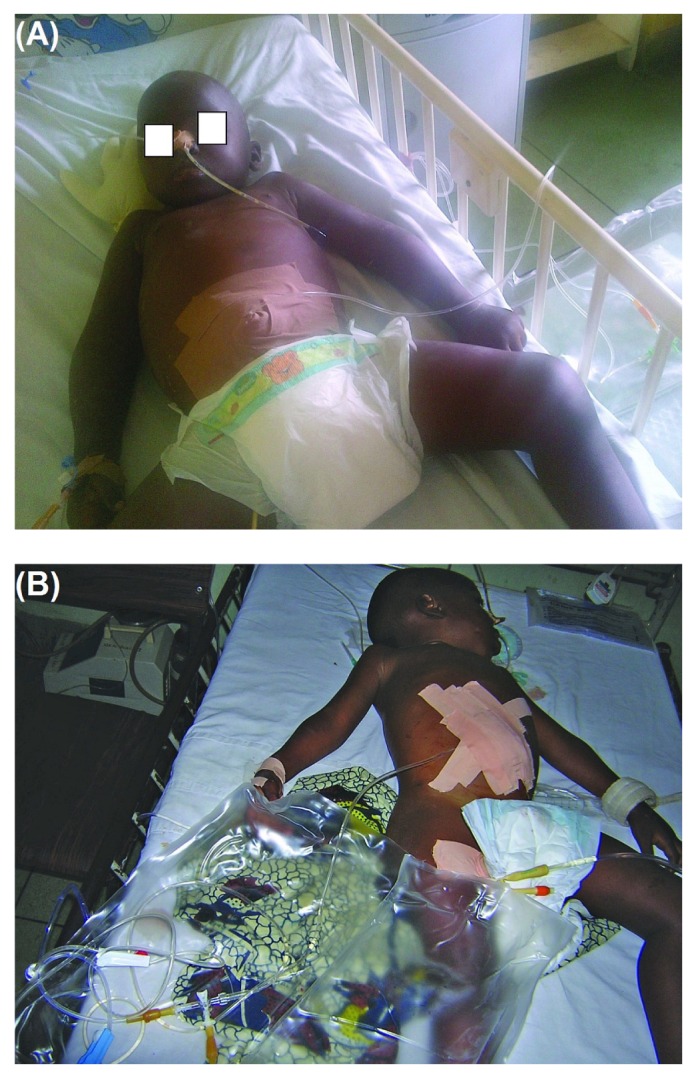
— (A,B) Children undergoing peritoneal dialysis using an adapted peritoneal dialysis catheter (nasogastric tube).
The catheters were inserted by the pediatric senior resident through a skin incision midline in the anterior abdominal wall, about 2 – 3 cm below the umbilicus. When nasogastric tubes were adapted as PD catheters, additional fenestrations were made at the distal end of the French 10G or 12G tube. A rigid probe was threaded down the nasogastric tube, through a fenestration 6 – 8 cm from the terminal end of the tube, serving as a stylet for insertion into the peritoneal cavity. The bladder was catheterized before the procedure. The abdomen was initially filled with 10 – 20 mL/kg dialysate, an amount that was gradually increased to 30 – 40 mL/kg. A burette was used to measure the volume of PD fluid delivered into the peritoneal cavity. Fluid cycles ranged from hourly to 2-hourly. Manual exchanges were performed by the physicians on duty. A PD data sheet was used to accurately record time, volume of PD fluid delivered into the peritoneal cavity, volume of PD fluid drained from the cavity, and dwell time. All the patients received intravenous cephalosporin during the procedure. Reasons to discontinue PD in patients included one or more of improvement in renal function, complications of PD, or exhaustion of the supplies of PD fluid.
Demographic and baseline clinical characteristics are reported as means and standard deviations, medians with interquartile ranges (IQRs), or proportions as appropriate. The statistical analyses were performed using the software application Statistical Package for the Social Sciences (version 17: SPSS, Chicago, IL, USA).
RESULTS
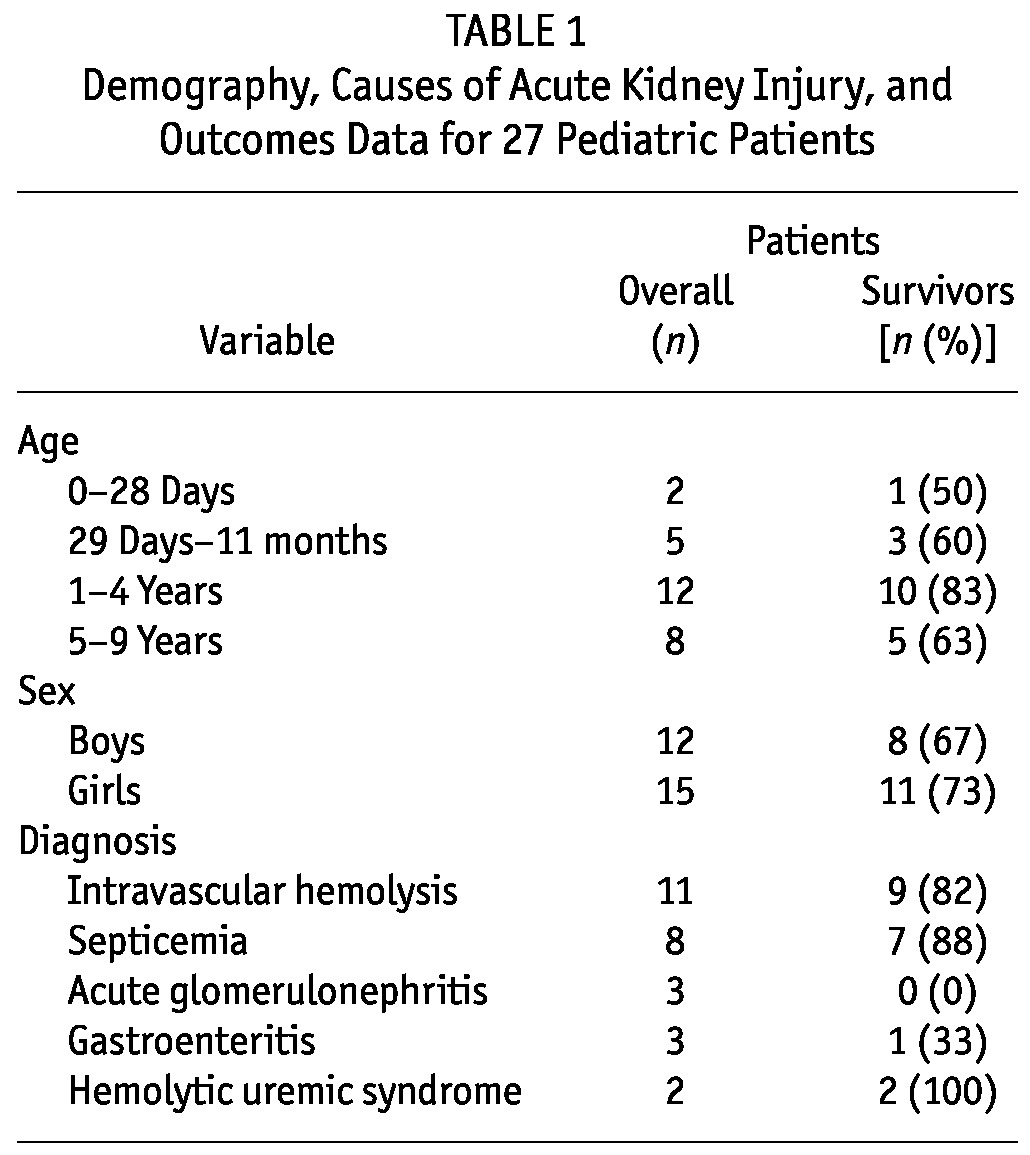
The study included 27 children, of whom 15 (55.6%) were female. The ages of the children ranged from 7 days to 9 years [mean: 3.1 ± 2.6 years; median: 4.0 years (IQR: 0.9 – 5.0 years)]. Table 1 sets out the sex and age distribution of the patients. The children weighed 1.6 – 24.2 kg (mean: 12.3 ± 5.9 kg). The median length or height of the children was 96.0 cm (IQR: 74.3 – 104.8 cm). Median serum urea and creatinine levels at presentation were 271 mg/dL (IQR: 184 – 314 mg/dL) and 6.7 mg/dL (IQR: 4.5 – 9.4 mg/dL) respectively.
TABLE 1.
Demography, Causes of Acute Kidney Injury, and Outcomes Data for 27 Pediatric Patients
CAUSES OF ACUTE KIDNEY INJURY
Table 1 shows the causes of AKI in the study patients. Intravascular hemolysis was the cause of AKI in 11 patients (41%), and in 3 of those 11, the malaria parasite was found in peripheral blood smears. In the other patients, the cause of intravascular hemolysis was not known. In 8 patients (30%), AKI was secondary to septicemia. Of the patients with septicemia, 3 had bronchopneumonia as a comorbidity, and 1 of those 3 children was also in heart failure. In 4 other children with septicemia, the comorbidities of perinatal asphyxia, cerebral palsy, hepatitis, and sickle cell anemia were present in 1 patient each. Of 3 patients with gastroenteritis, 2 presented in hypovolemic shock (the only ones with that condition), and both of those patients also went into cardiopulmonary arrest before commencement of PD. Acute glomerulonephritis (AGN) occurred in 3 other patients (12%). Renal biopsy was not performed in any of the patients with AGN, and none received immunosuppressives.
INDICATIONS FOR DIALYSIS
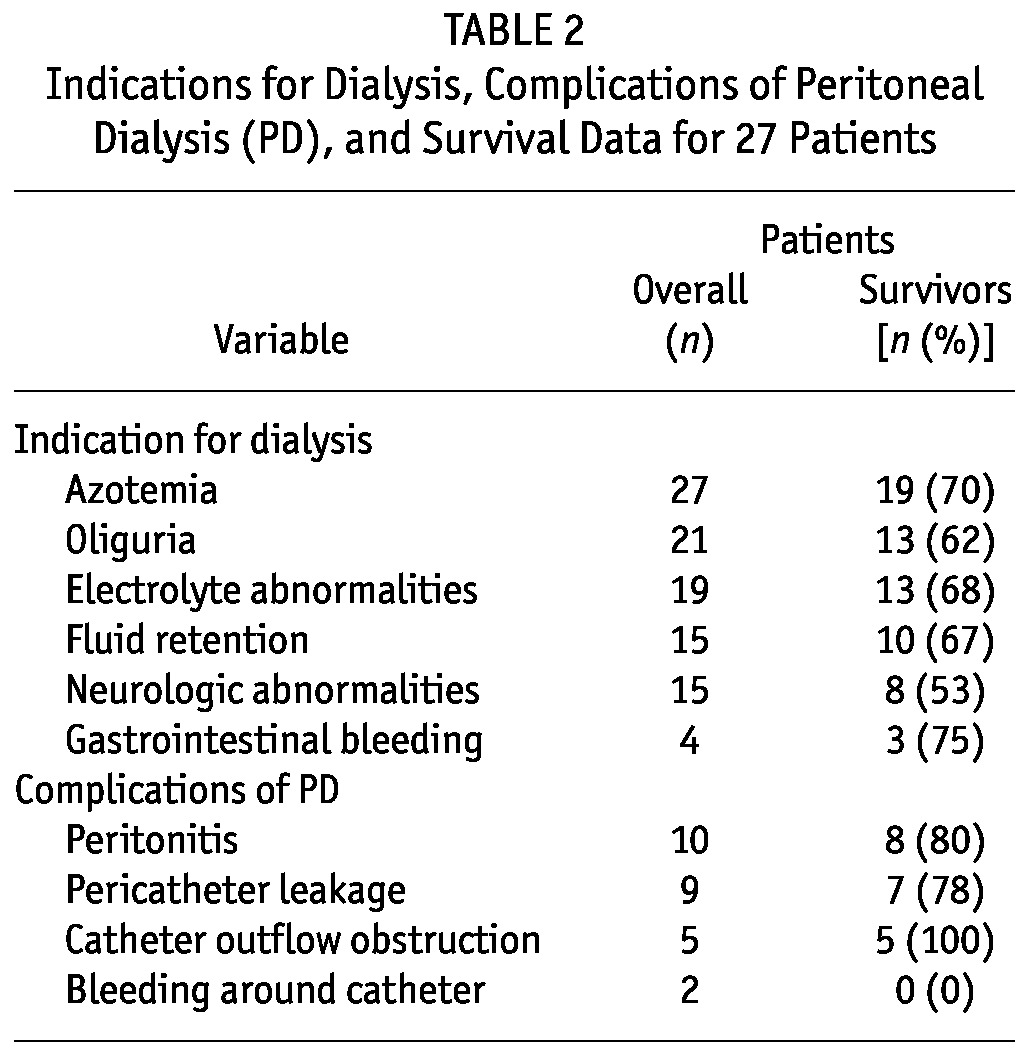
Azotemia and oliguria were the indications for dialysis in 100% and 78% of the children respectively. In 19 children (70%), electrolyte abnormalities were the indication for dialysis. Neurologic abnormalities secondary to uremic encephalopathy was an indication for dialysis in 52% of the children. Seizures occurred in 13 patients, and 10 patients experienced altered sensorium. Fluid retention was present in 15 children (56%), and in 4 patients (15%), gastrointestinal tract bleeding with melena in association with azotemia were indications for dialysis (Table 2).
TABLE 2.
Indications for Dialysis, Complications of Peritoneal Dialysis (PD), and Survival Data for 27 Patients
PERITONEAL DIALYSIS
The median duration of PD in the children was 5 days (IQR: 2 – 7 days), and the median number of PD cycles was 63 (IQR: 24 – 93 cycles).
COMPLICATIONS
Peritoneal dialysis was complicated by peritonitis in 10 children (37%) and by pericatheter leakage in 9 (33%). Outflow obstruction of the PD catheter occurred in 5 children (18.5%) and required manipulation or a change of catheter (Table 2).
OUTCOMES
The median length of hospital stay was 25 days (IQR: 17 – 35 days). Of the 27 patients, 19 (70%) survived till discharge. Among the patients with septicemia and hemolysis, survival was 88% and 82% respectively. At discharge, 2 children needed dialysis, both of whom had AKI secondary to septicemia. Both patients were taken home from hospital by their parents against medical advice. Two patients with hemolytic uremic syndrome both survived, and the two patients with AKI and hypovolemic shock died. All patients with acute glomerulonephritis died after PD was discontinued.
Of children in the age group 1 year to less than 5 years, 83% survived till discharge. Based on indications for dialysis, 57% and 61% of patients with, respectively, neurologic abnormalities and fluid retention were discharged home. Of the 10 patients with episodes of peritonitis complicating PD, 8 were discharged home (Tables 1 and 2).
DISCUSSION
Our study highlights the challenges and outcomes of childhood acute PD in southwest Nigeria. Overall survival in our cohort was 70%. Complication rates were higher than would have been expected if standard PD catheters had been used.
In resource-poor settings, continuous RRT is unavailable because of a lack of age-appropriate vascular catheters, pediatric blood lines, and dialyzers, combined with the cost of treatment, challenges in obtaining vascular access, and a need for specialized staff. Intermittent HD is also technology-dependent, and in our country, age- and size-appropriate blood lines, dialyzers, and catheters for infants and young children are also scarce. Furthermore, young children may not tolerate the volume shifts that occur during HD (10). The most readily available modality of RRT for young children with AKI in resource-limited countries is PD (11–13). Our study provides valuable data from a region having a paucity of data on PD in AKI (4).
In the present study, the patterns of causes and comorbidities of AKI are different from those observed in developed countries. In developed countries, AKI occurs mainly as a consequence of advancements in open-heart surgery and in bone-marrow and solid-organ transplantation for the management of oncologic disorders (14–17). In developed countries, AKI therefore commonly occurs in the setting of multiorgan dysfunction syndrome, with hemodynamic instability and a need for vasopressors (18). In our center, as in many parts of sub-Saharan Africa, such advancements in medical care are not routinely available. Intravascular hemolysis (presumably secondary to malaria) and septicemia were the major causes of AKI in our study (19). Based on the pattern of AKI causes seen in our study, many of our patients may potentially benefit from PD.
However, the provision of PD in our subregion is challenging with respect to cost and the poor availability of PD fluid and catheters. Catheters for PD are expensive and typically scarce—even more so for Tenckhoff catheters. That scarcity necessitated the use of adapted PD catheters in most patients. The percutaneous catheters used in some of the patients were obtained through a benefactor; patients did not have to pay for them. Peritoneal dialysis fluid is also expensive, and the fluid used in the management of our patients was also donated to our unit; patients received the dialysis fluid at no cost.
The complications of PD in our patients were catheter-related complications and infections. The frequency of those complications was higher than that reported in a study from the United States (20). The high rate of infections, pericatheter leakage, and catheter blockage may be related to the use of adapted PD catheters in most patients because of the restricted resources in our center. Access to tunneled Tenckhoff catheters and automated PD systems may have reduced our complication rates. Placement of a tunneled Tenckhoff catheter is preferred to prevent technical complications such as leaks and blocked catheters. Tunneled Tenckhoff catheters are also associated with a reduced rate of peritonitis, and would be ideal if it is anticipated that PD will be needed for more than 72 hours (20–23). In unstable children who cannot tolerate surgery, use of percutaneous catheters is a viable option. The peritonitis rate can be further reduced through the use of closed PD delivery systems and PD cyclers (12,23,24). With better access to proper PD catheters, cyclers, and closed PD delivery systems, our complication rates will decline.
The 70% overall survival of the patients in our study was higher than that seen in reports from other countries (11,20,25–27). Despite limited resources, the relatively good outcomes were likely related to the pattern of AKI causes, with its predominance of reversible causes. In addition, poor prognostic features such as multiorgan dysfunction syndrome with hypotension was not a prominent finding in our patients (18,28). Patients with AGN as the underlying cause of AKI in our study most likely had rapidly progressive glomerulonephritis and were less likely to recover renal function. Poor outcomes among the patients with AGN were related to our limited ability to offer long-term RRT (29). Additionally, patients who had hypotension and episodes of cardiopulmonary arrest before commencement of PD also did poorly. Despite our limitations, we were able to use PD to provide successful RRT in most patients.
Pediatric nephrologists should advocate for the expansion and improvement of PD services in sub-Saharan Africa. Governments of countries in sub-Saharan Africa should put health insurance programs into place to enable children with AKI to access PD. For instance, the Nigerian government should expand the national health insurance scheme to cover the cost of PD in children with AKI. The International Society for Peritoneal Dialysis, the International Pediatric Nephrology Association, and the International Society of Nephrology should continue to advocate for the promotion, expansion, and improvement of PD services in sub-Saharan Africa and in Nigeria. Clinicians in developing countries and those in resource-rich countries should collaborate, with the goal of setting up PD programs in developing countries for the management of childhood AKI.
CONCLUSIONS
We evaluated PD among children with AKI in a low-resource setting. In that setting, PD was performed using percutaneous catheters or adapted nasogastric tubes. The use of PD was associated with relatively high survival despite poor socio-economic conditions, but complication rates were high. Increased access to percutaneous or Tenckhoff catheters, closed PD delivery systems, and cyclers for automated PD may improve outcomes. In low-resource settings, PD should be promoted in the treatment of childhood AKI.
DISCLOSURES
The authors declare that no financial conflict of interest exists.
Acknowledgments
We appreciate the support and encouragement that we received from Professor Wendy Hoy, Susan Mott, and the Centre for Chronic Disease Management, University of Queensland School of Medicine, Royal Brisbane Hospital, Herston, Australia. We are grateful to Professor Wendy Hoy and to Dr. Susan Samuel, Alberta Children’s Hospital, Calgary, Alberta, Canada, for reviewing the manuscript.
REFERENCES
- 1. Walters S, Porter C, Brophy PD. Dialysis and pediatric acute kidney injury: choice of renal support modality. Pediatr Nephrol 2009; 24:37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Olowu WA. Renal failure in Nigerian children: factors limiting access to dialysis. Pediatr Nephrol 2003; 18:1249–54 [DOI] [PubMed] [Google Scholar]
- 3. Olowu WA, Adelusola KA. Pediatric acute renal failure in southwestern Nigeria. Kidney Int 2004; 66:1541–8 [DOI] [PubMed] [Google Scholar]
- 4. Anochie IC, Eke FU. Paediatric acute peritoneal dialysis in southern Nigeria. Postgrad Med J 2006; 82:228–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Symons JM, Chua AN, Somers MJ, Baum MA, Bunchman TE, Benfield MR, et al. Demographic characteristics of pediatric continuous renal replacement therapy: a report of the prospective pediatric continuous renal replacement therapy registry. Clin J am Soc Nephrol 2007; 2:732–8 [DOI] [PubMed] [Google Scholar]
- 6. UNICEF. At a glance: Nigeria statistics. [Available online at: http://www.unicef.org/infobycountry/nigeria_statistics.html; accessed 30 October 2011]
- 7. Centers for Disease Control and Prevention. Fatal poisoning among young children from diethylene glycol-contaminated acetaminophen—Nigeria, 2008–2009. MMWR Morb Mortal Wkly Rep 2009; 58:1345–7 [PubMed] [Google Scholar]
- 8. Singh J, Dutta AK, Khare S, Dubey NK, Harit AK, Jain NK, et al. Diethylene glycol poisoning in Gurgaon, India, 1998. Bull World Health Organ 2001; 79:88–95 [PMC free article] [PubMed] [Google Scholar]
- 9. Schep LJ, Slaughter RJ, Temple WA, Beasley DM. Diethylene glycol poisoning. Clin Toxicol (Phila) 2009; 47:525–35 [Erratum in: Clin Toxicol (Phila) 2009; 47:840] [DOI] [PubMed] [Google Scholar]
- 10. Basu RK, Wheeler DS, Goldstein S, Doughty L. Acute renal replacement therapy in pediatrics. Int J Nephrol 2011; 2011:785392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kandoth PW, Agarwal GJ, Dharnidharka VR. Acute renal failure in children requiring dialysis therapy. Indian Pediatr 1994; 31:305–9 [PubMed] [Google Scholar]
- 12. Kohli HS, Arora P, Kher V, Gupta A, Sharma RK, Bhaumik SK. Daily peritoneal dialysis using a surgically placed Tenckhoff catheter for acute renal failure in children. Ren Fail 1995; 17:51–6 [DOI] [PubMed] [Google Scholar]
- 13. Phadke KD, Dinakar C. The challenges of treating children with renal failure in a developing country. Perit Dial Int 2001; 21(Suppl 3):S326–9 [PubMed] [Google Scholar]
- 14. Hui–Stickle S, Brewer ED, Goldstein SL. Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kidney Dis 2005; 45:96–101 [DOI] [PubMed] [Google Scholar]
- 15. Bailey D, Phan V, Litalien C, Ducruet T, Mérouani A, Lacroix J, et al. Risk factors of acute renal failure in critically ill children: a prospective descriptive epidemiological study. Pediatr Crit Care Med 2007; 8:29–35 [DOI] [PubMed] [Google Scholar]
- 16. Williams DM, Sreedhar SS, Mickell JJ, Chan JC. Acute kidney failure: a pediatric experience over 20 years. arch Pediatr adolesc Med 2002; 156:893–900 [DOI] [PubMed] [Google Scholar]
- 17. Goldstein SL. Overview of pediatric renal replacement therapy in acute kidney injury. Semin Dial 2009; 22:180–4 [DOI] [PubMed] [Google Scholar]
- 18. Bunchman TE, McBryde KD, Mottes TE, Gardner JJ, Maxvold NJ, Brophy PD. Pediatric acute renal failure: outcome by modality and disease. Pediatr Nephrol 2001; 16:1067–71 [DOI] [PubMed] [Google Scholar]
- 19. Menendez C, Fleming AF, Alonso PL. Malaria-related anaemia. Parasitol Today 2000; 16:469–76 [DOI] [PubMed] [Google Scholar]
- 20. Flynn JT, Kershaw DB, Smoyer WE, Brophy PD, McBryde KD, Bunchman TE. Peritoneal dialysis for management of pediatric acute renal failure. Perit Dial Int 2001; 21:390–4 [PubMed] [Google Scholar]
- 21. Wong SN, Geary DF. Comparison of temporary and permanent catheters for acute peritoneal dialysis. arch Dis Child 1988; 63:827–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chadha V, Warady BA, Blowey DL, Simckes AM, Alon US. Tenckhoff catheters prove superior to cook catheters in pediatric acute peritoneal dialysis. Am J Kidney Dis 2000; 35:1111–16 [DOI] [PubMed] [Google Scholar]
- 23. Bonilla–Félix M. Peritoneal dialysis in the pediatric intensive care unit setting. Perit Dial Int 2009; 29(Suppl 2):S183–5 [PubMed] [Google Scholar]
- 24. Gabriel DP, Caramori JT, Martim LC, Barretti P, Balbi AL. High volume peritoneal dialysis vs daily hemodialysis: a randomized, controlled trial in patients with acute kidney injury. Kidney Int Suppl 2008; (108):S87–93 [DOI] [PubMed] [Google Scholar]
- 25. Gong WK, Tan TH, Foong PP, Murugasu B, Yap HK. Eighteen years experience in pediatric acute dialysis: analysis of predictors of outcome. Pediatr Nephrol 2001; 16:212–15 [DOI] [PubMed] [Google Scholar]
- 26. Arora P, Kher V, Rai PK, Singhal MK, Gulati S, Gupta A. Prognosis of acute renal failure in children: a multivariate analysis. Pediatr Nephrol 1997; 11:153–5 [DOI] [PubMed] [Google Scholar]
- 27. Alarabi AA, Petersson T, Danielson BG, Wikström B. Continuous peritoneal dialysis in children with acute renal failure. adv Perit Dial 1994; 10:289–93 [PubMed] [Google Scholar]
- 28. Gallego N, Gallego A, Pascual J, Liaño F, Estepa R, Ortuño J. Prognosis of children with acute renal failure: a study of 138 cases. Nephron 1993; 64:399–404 [DOI] [PubMed] [Google Scholar]
- 29. Moghal NE, Brocklebank JT, Meadow SR. A review of acute renal failure in children: incidence, etiology and outcome. Clin Nephrol 1998; 49:91–5 [PubMed] [Google Scholar]


