Abstract
♦ Objective: Although peritoneal dialysis (PD) is a widely accepted form of renal replacement therapy, concerns remain regarding the bioincompatible nature of standard PD fluid (PDF). Short-term studies of new biocompatible PDFs low in glucose degradation products (GDPs) reveal divergent results with respect to peritoneal integrity.
♦ Methods: We studied 125 patients on maintenance PD who were assigned, by simple randomization, to receive either conventional or low-GDP PDF at PD initiation. Parameters of dialysis adequacy and peritoneal transport of small solutes were determined at initiation and after a period of maintenance PD at the time when serum and overnight effluent dialysate were simultaneously collected and assayed for various cytokines, chemokines, adipokines, and cardiac biomarkers. All patients were further followed prospectively for an average of 15 months from the day of serum and effluent collection to determine patient survival and cardiovascular events.
♦ Results: Patients treated with conventional or low-GDP PDF were matched for sex, age, duration of dialysis, dialysis adequacy, and incidence of cardiovascular disease or diabetes. After an average of 2.3 years of PD treatment, the weekly total and peritoneal creatinine clearance, and the total and peritoneal Kt/V were comparable in the groups. However, urine output was higher in patients using low-GDP PDF despite there having been no difference between the groups at PD initiation. Patients using low-GDP PDF also experienced a slower rate of decline of residual glomerular filtration and urine output than did patients on conventional PDF. Compared with serum concentrations, effluent concentrations of tumor necrosis factor α, hepatocyte growth factor, macrophage migration inhibitory factor, interleukins 8 and 6, C-reactive protein, and leptin were found to be higher in both groups of patients after long-term PD, suggesting that the peritoneal cavity was the major source of those mediators. Compared with patients on low-GDP PDF, patients on conventional fluid showed elevated leptin and reduced adiponectin levels in serum and effluent. The effluent concentration of interleukin 8 was significantly lower in patients using low-GDP PDF. The survival rate and incidence of cardiovascular complications did not differ between these groups after maintenance PD for an average of 3.6 years.
♦ Conclusions: It appears that low-GDP PDF results in an improvement of local peritoneal homeostasis through a reduction of chronic inflammatory status in the peritoneum.
Keywords: Glucose degradation products, low-GDP dialysate, chemokines, adipokines, cardiovascular event
During chronic peritoneal dialysis (PD), the peritoneal membrane, lined with a monolayer of mesothelial cells, is repeatedly exposed to a non-physiologic hypertonic environment that may lead to peritoneal fibrosis and ultrafiltration (UF) failure. Conventional PD fluid (PDF) contains dextrose as the osmotic agent. Long-term exposure to glucose has been well recognized to cause metabolic and cardiovascular abnormalities.
Two pathways of glucose degradation play an important role in peritoneal mesothelial biology:
degradation into glucose degradation products (GDPs) during heat sterilization and storage; and
formation of advanced glycation end-products (AGEs) after nonenzymatic reactions with free amino groups in proteins (1).
Accumulation of AGEs in the peritoneal tissues of continuous ambulatory PD (CAPD) patients promotes peritoneal expression of various growth factors and subsequently leads to deterioration of UF capacity during CAPD (2). Newer PDFs, which are thought to be more biocompatible by being less acidic, or containing the physiologic buffer bicarbonate, or having lower concentrations of GDPs, have been developed in the last few years (3). Two observational studies suggested that the newer low-GDP PDFs might lead to less frequent therapy failure and improved patient and technique survival (4,5). Low-GDP PDF appears to better preserve peritoneal membrane integrity, as indirectly suggested by a higher concentration of cancer antigen 125 and a lower concentration of hyaluronan in overnight effluent (6–8). Although newer solutions have been implicated as causing higher levels of inflammatory markers in effluent, it is unclear whether those levels result from an inflammatory response or from a lessening of impaired peritoneal defense mechanisms, leading to better healing of the peritoneal membrane (as suggested by elevated cancer antigen 125). Furthermore, in vivo data on inflammatory markers in effluent remain conflicting (9,10).
In the present study, patients were randomly assigned to one of two groups: those receiving treatment with conventional or with low-GDP PDF. After an average PD treatment duration of 2.3 years, laboratory profiles of cytokines, growth factors, adipokines, and cardiac biomarkers were determined in serum and effluent. The patients were then followed prospectively for dialysis adequacy, patient survival, and cardiovascular events (CVEs) for an average of 15 months from the day of serum and effluent collection.
METHODS
STUDY DESIGN AND PARTICIPANTS
Commencing in July 2003, our study recruited 125 clinically stable patients on maintenance CAPD for a period of about 30 months from 4 regional renal centers in Hong Kong (Figure 1). Patients were excluded if they had an underlying malignancy, systemic lupus erythematosus, or chronic valvular or congenital heart disease. At initiation of their maintenance PD program, these subjects had been assigned, using simple randomization, to receive either conventional PDF (n = 67) or low-GDP PDF (n = 58) by the individual dialysis centers.
Figure 1.
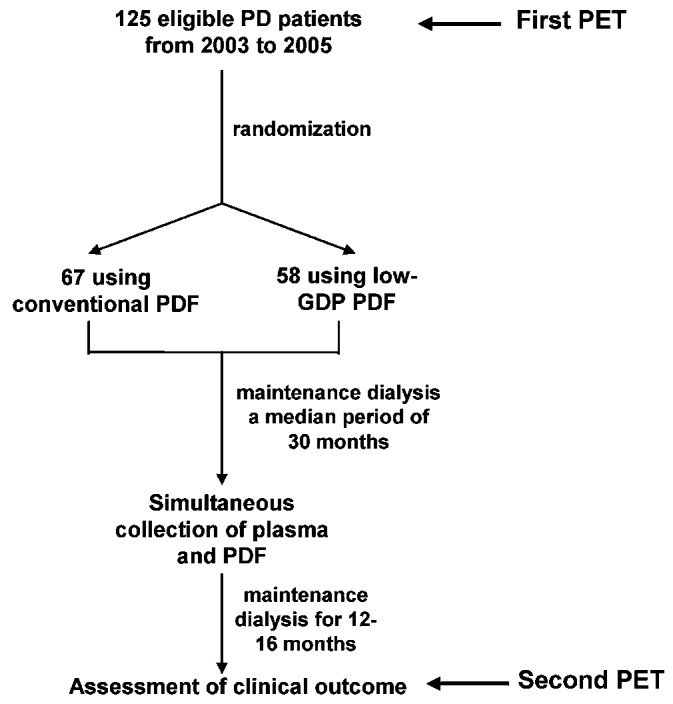
— Study design and flow diagram. PD = peritoneal dialysis; PET = peritoneal equilibration test; PDF = peritoneal dialysis fluid; GDP = glucose degradation products.
The random PDF assignments were made by the patient’s training nursing officer at the individual renal center. Neither patients nor nurses were informed of the study at the time the PD solution was being selected. They were informed only afterwards at the time of serum and effluent collection (with informed consent) after an average stable PD duration of 2.3 years. Most patients adopted a 4×2-L regime using 1.5% dextrose bags.
The conventional PDFs were lactate-buffered glucose-based Dianeal PD-2 [Baxter, Shanghai, China (43 patients)] or ANDY-Disc [Fresenius Medical Care, Bad Homburg, Germany (24 patients)]. The low-GDP PDFs were Gambrosol Trio (Gambro Lundia AB, Lund, Sweden), Physioneal 40 (Baxter), and Balance (Fresenius Medical Care), which were used by 41, 12, and 5 patients respectively. The study was approved by an Institutional Review Board and Ethics Committee, and all participating patients gave written informed consent. The trial was registered at HKClinicalTrials.com (http://www.hkclinicaltrials.com) with the number HKCTR-576.
The study patients were maintained on CAPD for an average duration of 2.3 years before their biochemical profiles were studied. All patients were clinically stable and non-edematous, with a targeted blood pressure of less than 140/90 mmHg achieved by careful fluid balance and antihypertensive treatment. Serum and overnight effluent were collected from each patient at the time of recruitment. The morning exchange was performed with the patients fasting and with a 1.5% glucose concentration having been used for the overnight dwell. All patients were free of peritonitis or systemic infection for the 6 months before the sample collection.
BIOCHEMICAL MEASUREMENTS
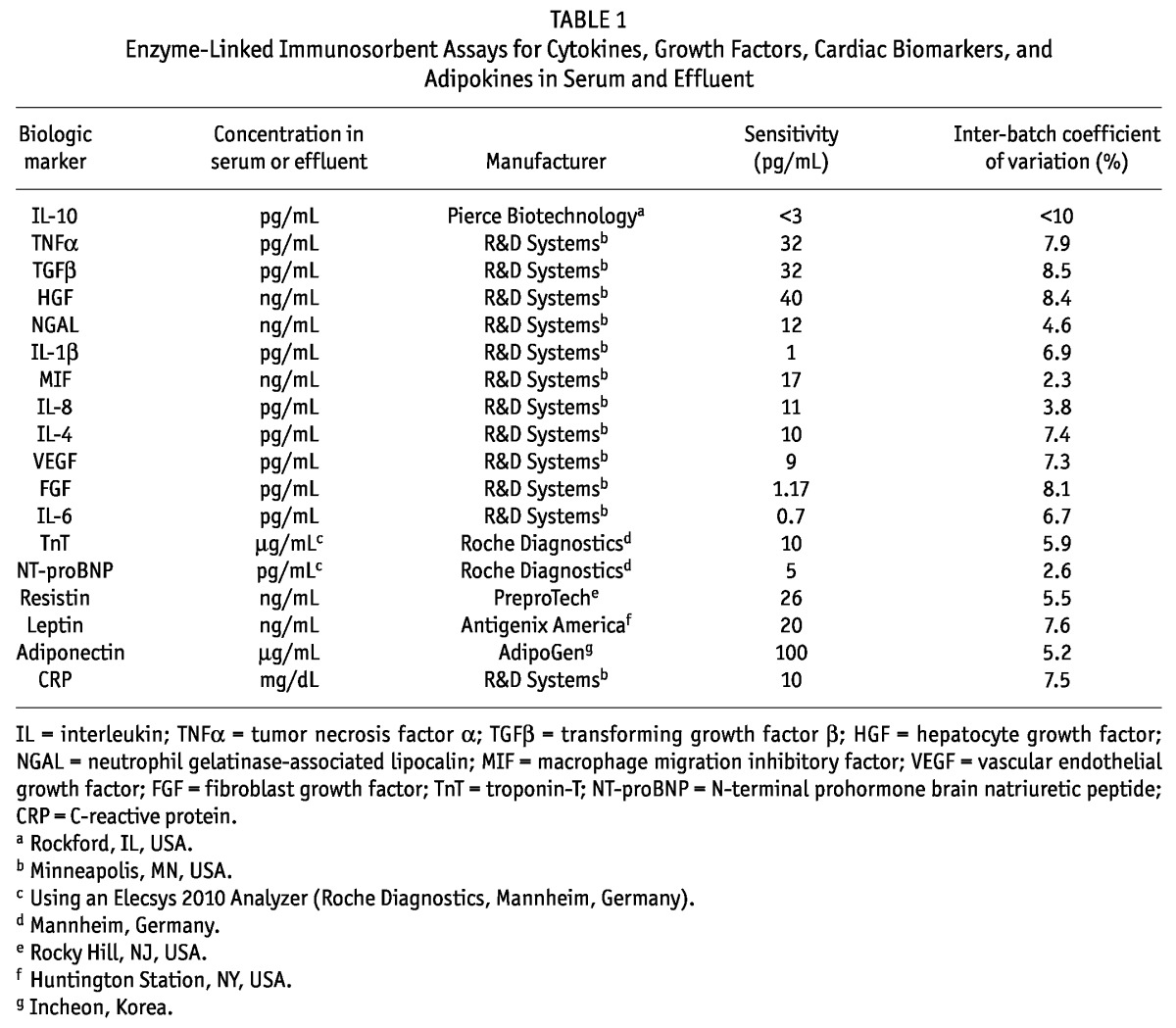
Venous blood was collected from the fasting patients on the morning that overnight effluent was collected. All cytokines, growth factors, chemokines, and adipokines were determined using enzyme-linked immunosorbent assays. Troponin T (TnT) and N-terminal prohormone brain natriuretic peptide (NT-proBNP) were measured using an Elecsys 2010 Analyzer (Roche Diagnostics, Mannheim, Germany). Table 1 summarizes the sensitivities, inter-batch coefficients of variation, and manufacturers of the assays.
TABLE 1.
Enzyme-Linked Immunosorbent Assays for Cytokines, Growth Factors, Cardiac Biomarkers, and Adipokines in Serum and Effluent
DIALYSIS ADEQUACY
Two standard peritoneal equilibration tests (PETs) with determination of UF capacity were performed for each patient—one at PD initiation and the other at the end of the post-sampling follow-up (“census”). For the PETs, 2 L of 2.27% glucose PDF was used. The dialysate-to-plasma ratio (D/P) of creatinine at 4 hours and the protein catabolic rate were determined as previously described (11). Residual glomerular filtration rate (GFR) was measured as the average of the 24-hour urine urea and creatinine clearances (12). Adequacy of dialysis was estimated using the standard method to measure total weekly urea and creatinine clearances (13). The contributions of the PD and renal components to total urea clearance was separately estimated.
STUDY OUTCOMES
It was not feasible to recruit a sample large enough to provide adequate statistical power for an assessment of the individual endpoints of death, cause-specific death, hospitalization, or other events. Hence, we selected two composite co-primary outcomes:
biochemical profile of cytokines, growth factors, adipokines, and cardiac biomarkers determined after stable PD treatment for an average duration of 2.3 years, and
dialysis adequacy determined by GFR and daily urine output at initiation and at the time of census after stable PD for an average duration of 3.6 years.
Despite the limitations of a relatively short follow-up period and a study underpowered for determining the patient survival rate, we attempted to analyze clinical outcomes, including death from all causes, cardiovascular death, and first fatal or nonfatal CVE. Fatal and nonfatal CVEs documented from commencement of maintenance dialysis included electrocardiographically documented myocardial ischemia or infarction, congestive heart failure, sustained atrial or ventricular arrhythmia, transient ischemic attack, ischemic cerebrovascular event, peripheral vascular disease, and sudden death. Peripheral vascular disease was defined as the presence of intermittent claudication (with angiographic or sonographic detection of 50% or more stenosis of the major arteries of the lower limb, with or without revascularization procedures), ischemic leg ulceration, gangrene with or without amputation, and aortic aneurysm. Sudden death was defined as unexpected natural death within 1 hour from onset of symptoms and without any earlier condition that would appear fatal. The exact cause of death and the nature of the first CVE were provided by the attending physician. In the case of death out of hospital, family members were interviewed by telephone to ascertain the circumstances of the death. For patients who experienced multiple CVEs, the CVE survival analysis was limited to the first event.
STATISTICAL ANALYSIS
We used a two-sided two-sample t-test with Bonferroni correction to provide a significance level (α) of 0.01 when considering the two co-primary composite outcomes. Assuming a 20% difference in those covariates between patients receiving conventional and low-GDP PDF, we estimated that the enrolment of 58 patients into each arm would achieve 83% power in the present study.
Continuous data are expressed as mean ± standard error of the mean or as median and interquartile range, depending on the distribution. Between-group comparisons used the t-test for continuous data or the Wilcoxon signed rank test for non-continuous data, as appropriate, and the chi-square test for categorical data. Cumulative patient survival was calculated by the Kaplan–Meier method, and comparisons between groups were made using the log-rank test. Correlations between serum and effluent concentrations of adipokines and growth factors were determined using the Spearman rho (ρ). Statistical analyses were performed using the SPSS software application (version 13.0: SPSS, Chicago, IL, USA).
RESULTS
BASELINE CHARACTERISTICS AND BIOCHEMISTRY AFTER LONG-TERM MAINTENANCE DIALYSIS
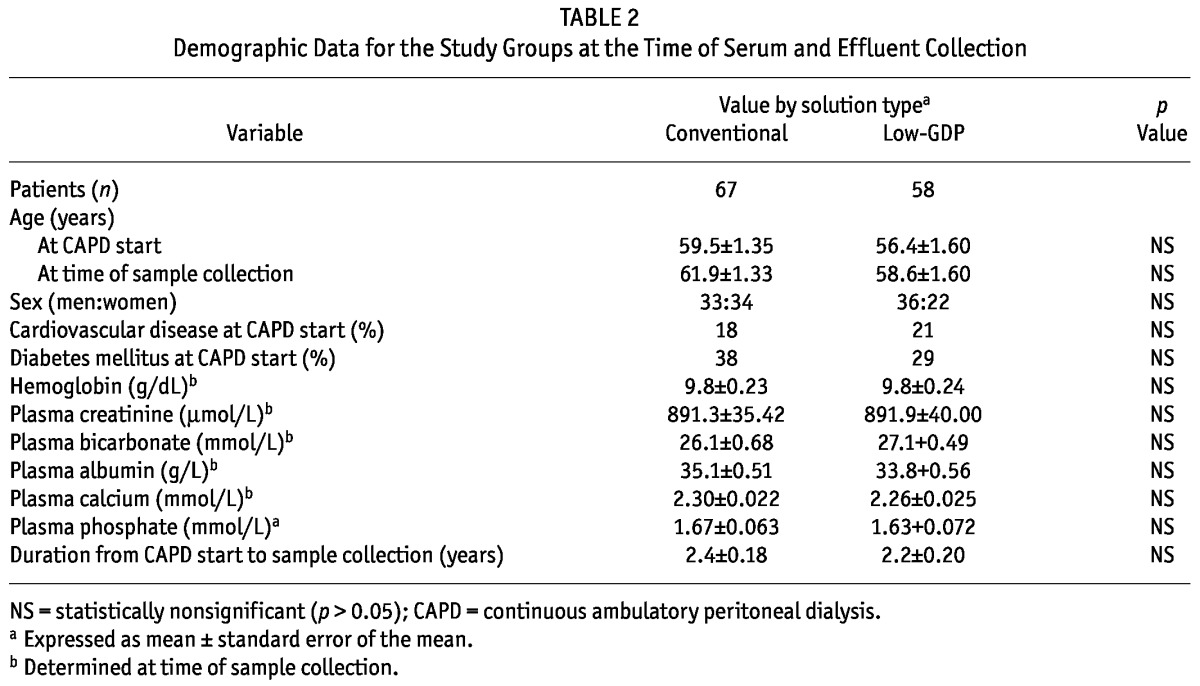
Age at dialysis start, the incidences of cardiovascular disease and diabetes mellitus, and duration of dialysis at the time of sample collection were similar between the groups (Table 2). The underlying causes of renal failure were also comparable (data not shown). No difference in dialysis adequacy or residual GFR was observed at the time of PD initiation, but the low-GDP PDF group had a higher D/P creatinine at 4 hours (p < 0.01). After an average of 2.3 years of stable maintenance dialysis (median: 2.2 years), hemoglobin and blood biochemistries were again comparable between the groups (Table 2).
TABLE 2.
Demographic Data for the Study Groups at the Time of Serum and Effluent Collection
DIALYSIS ADEQUACY AT THE TIME OF CENSUS
Compared with baseline values at the initiation of dialysis, total Kt/V, total weekly creatinine clearance, residual GFR, and daily urine output were all lower in patients on maintenance dialysis with conventional PDF for an average of 3.6 years. Reduced urine output was partly compensated by increased peritoneal Kt/V despite an unchanged 4-hour D/P creatinine (Table 3). The patients using conventional PDF also experienced a decrease in protein catabolic rate. By contrast, after an average 3.6 years on maintenance dialysis, patients on low-GDP PDF demonstrated only reduced total weekly creatinine clearance and residual GFR, with elevated UF. No significant fall in total Kt/V or urine output was observed. Their 4-hour D/P creatinine and their protein catabolic rate remained unchanged.
TABLE 3.
Dialysis Adequacy in the Study Groups
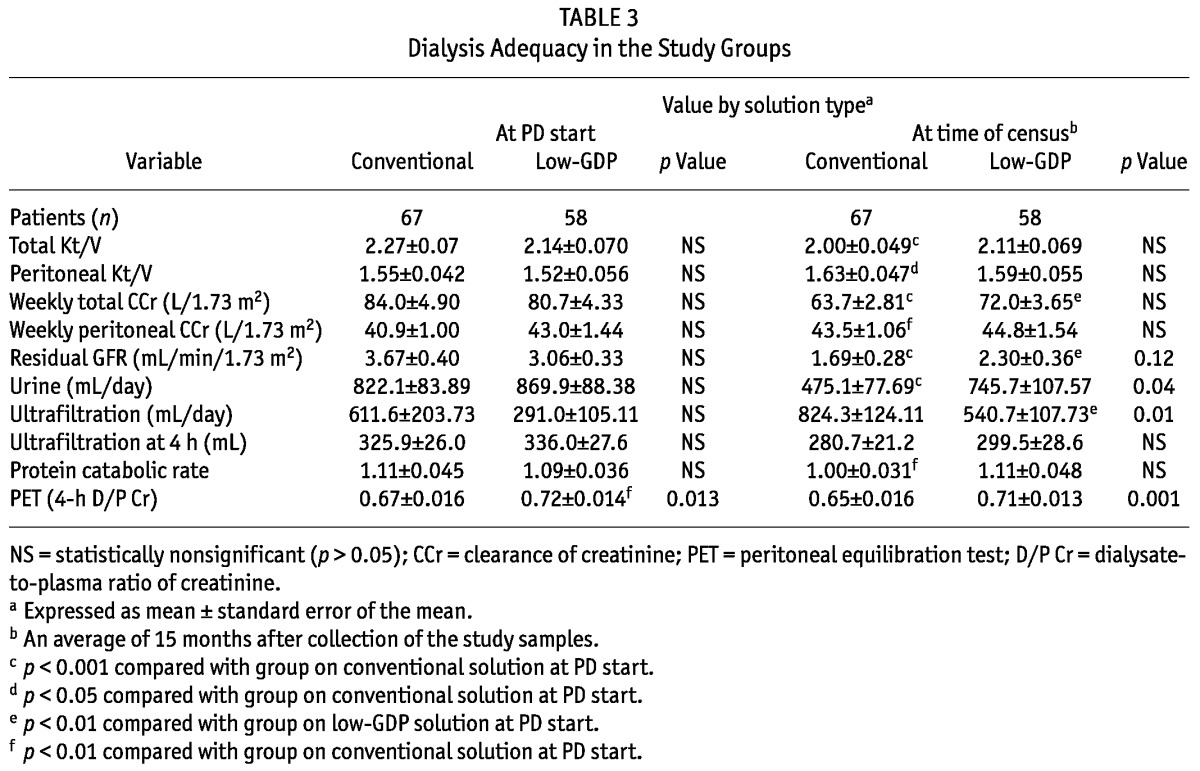
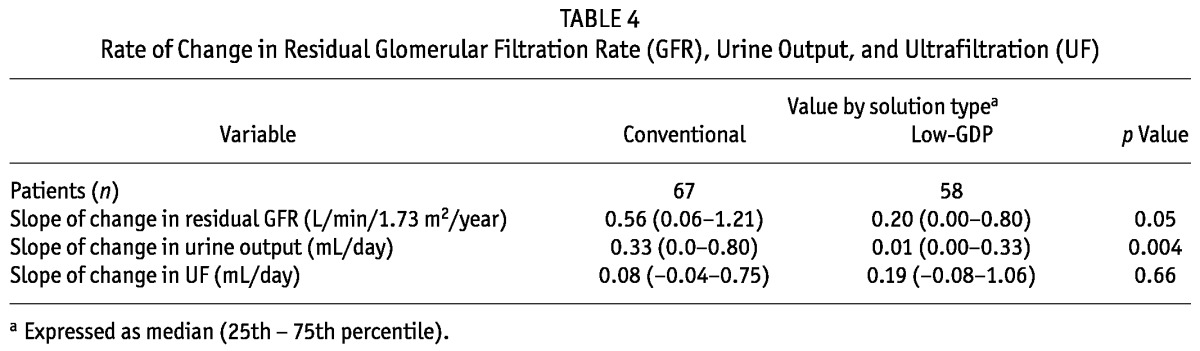
Compared with values at the time of serum and effluent collection, we observed no significant differences between the groups in total or peritoneal Kt/V, weekly total creatinine clearance, and peritoneal creatinine clearance (Table 3). As observed at the time of PD initiation, the low-GDP group still had a higher 4-hour D/P creatinine. Compared with patients using conventional PDF, the patients on low-GDP PDF had higher urine output at census despite baseline values being similar in both groups at PD initiation. We also compared the rates for loss of residual GFR, reduction of urine output, and increase in UF between the groups (Table 4). Most interestingly, compared with patients on conventional PDFs, those on low-GDP PDFs experienced a slower rate of decline in residual GFR and urine output.
TABLE 4.
Rate of Change in Residual Glomerular Filtration Rate (GFR), Urine Output, and Ultrafiltration (UF)
CARDIAC BIOMARKERS AND ADIPOKINES
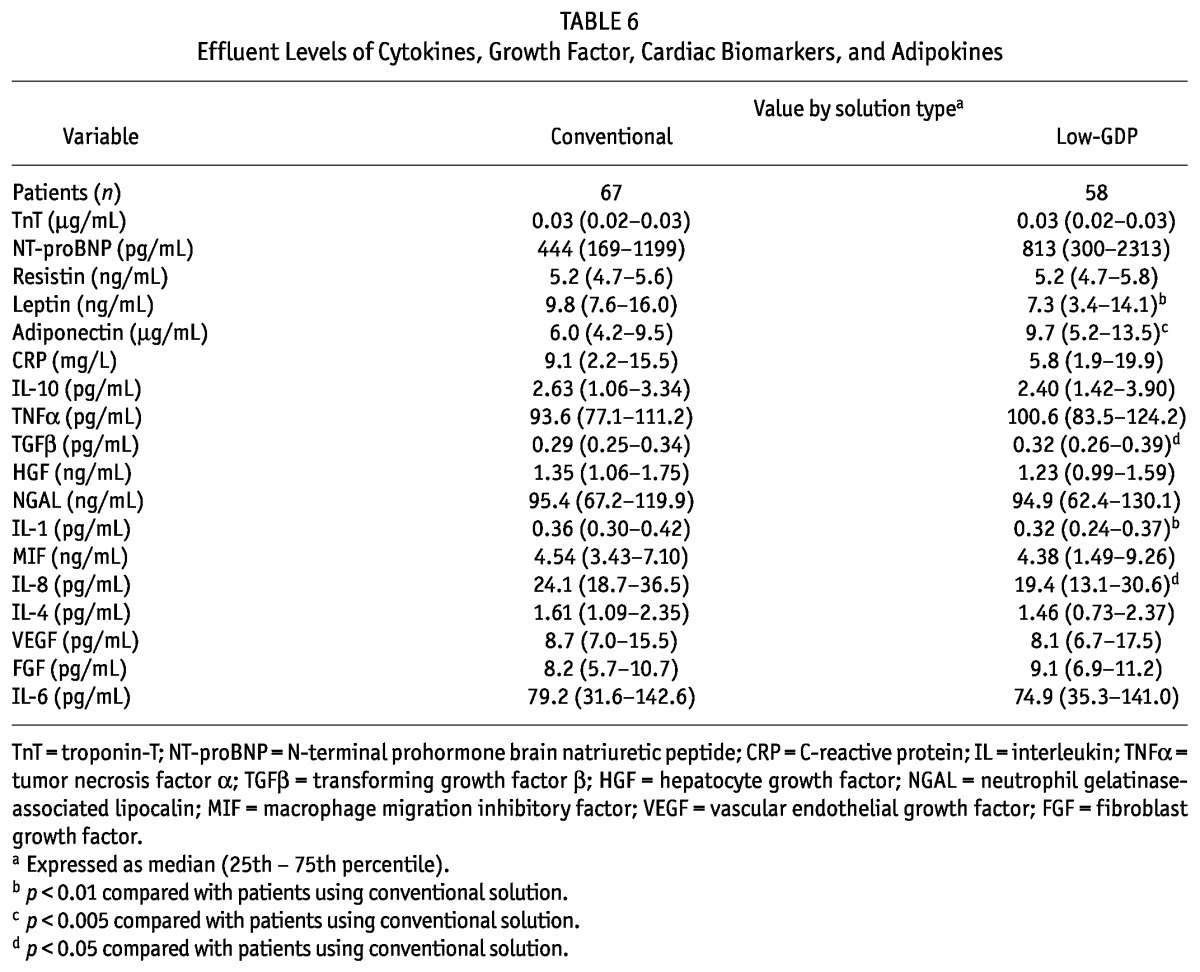
Compared with patients on low-GDP PDFs, those on conventional PDFs had a higher serum concentration of leptin and a lower serum concentration of adiponectin (Tables 5 and 6). Similar results were observed in effluent. We observed no differences in serum or effluent levels of TnT, NT-proBNP, resistin, or C-reactive protein (CRP) between patients using conventional or low-GDP PDF for long-term dialysis.
TABLE 5.
Serum Levels of Cytokines, Growth Factor, Cardiac Biomarkers, and Adipokines
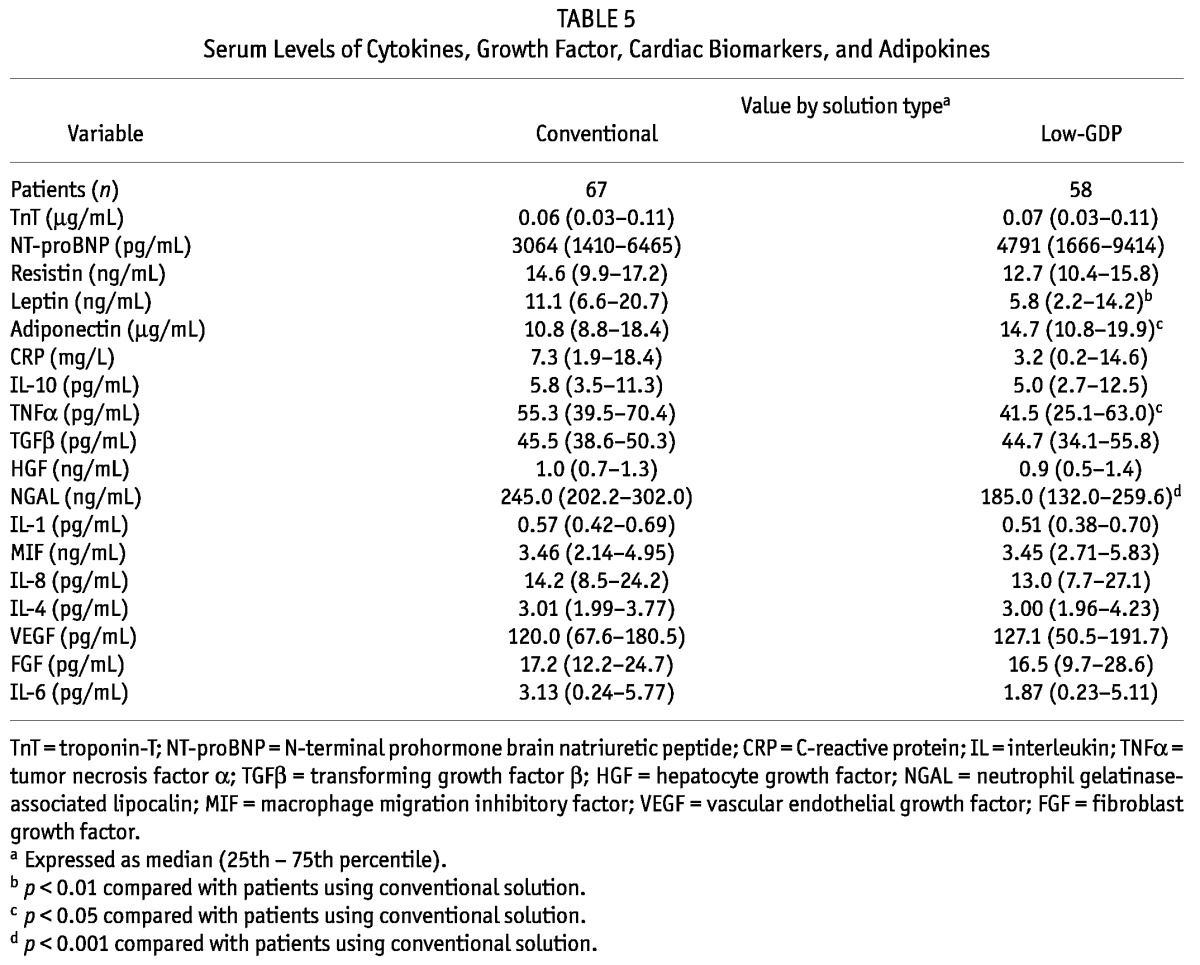
TABLE 6.
Effluent Levels of Cytokines, Growth Factor, Cardiac Biomarkers, and Adipokines
CYTOKINES AND GROWTH FACTORS
Compared with patients on low-GDP PDFs, those on conventional PDFs had higher serum concentrations of TNFα and neutrophil gelatinase-associated lipocalin (NGAL) (Tables 5 and 6). In effluent, concentrations of interleukins 1 (IL-1) and 8 (IL-8) were significantly higher in patients on conventional PDFs than in those on low-GDP PDFs. We observed no differences between the groups in serum or effluent levels of IL-10, transforming growth factor β (TGFβ), hepatocyte growth factor (HGF), macrophage migration inhibitory factor (MIF), IL-4, vascular endothelial growth factor, fibroblast growth factor, or IL-6.
PERITONEUM AS A MAJOR SOURCE OF CYTOKINE, GROWTH FACTOR, AND ADIPOKINE PRODUCTION
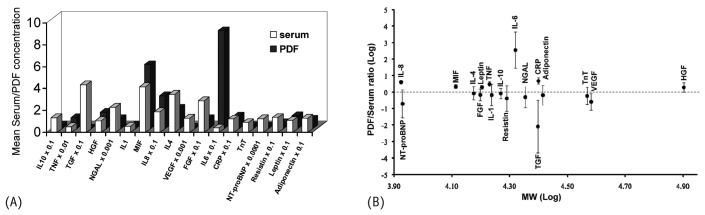
Given the relatively smaller tissue mass of the peritoneal cavity compared with the entire body, serum concentrations of cytokines, growth factors, cardiac markers, and adipokines are usually higher than those in effluent by factors of 10 – 100. Figure 2(A) shows mean serum and effluent concentrations. If high effluent concentrations are a result of size-selective peritoneal transport rather than of local synthesis, the effluent-to-serum ratio should exhibit an inverse correlation with the molecular weight of these mediators (14). Such correlations were not demonstrated when the dialysate-to-serum ratios of these mediators were plotted against their molecular weights [Figure 2(B)]. A dialysate-to-serum ratio exceeding or near unity suggests that the peritoneal cavity is a major source of the particular mediator. Based on our observations, TNFα, MIF, IL-8, IL-6, HGF, leptin, and CRP are likely to be significantly produced in the peritoneal cavity as a result of chronic inflammation secondary to exposure to less-biocompatible dialysate. With the exception of TNFα, we observed good correlation between effluent and serum concentrations of those mediators, specifically: MIF (ρ = 0.30, p < 0.01), IL-8 (ρ = 0.32, p < 0.001), IL-6 (ρ = 0.40, p < 0.001), HGF (ρ = 0.35, p < 0.001), leptin (ρ = 0.58, p < 0.001), and CRP (ρ = 0.67, p < 0.001).
Figure 2.
— (A) Mean serum or dialysate concentration, and (B) dialysate-to-serum ratio (expressed as mean ± standard error of the mean) of measured cytokines, growth factors, adipokines, and cardiac biomarkers. The dotted line at the zero mark represents a dialysate-to-serum ratio of unity in the logarithmic scale. IL = interleukin; TNF = tumor necrosis factor α; TGF = transforming growth factor β; HGF = hepatocyte growth factor; NGAL = neutrophil gelatinase-associated lipocalin; MIF = macrophage migration inhibitory factor; VEGF = vascular endothelial growth factor; FGF = fibroblast growth factor; CRP = C-reactive protein; TnT = troponin-T; NT-proBNP = N-terminal prohormone brain natriuretic peptide.
SURVIVAL AND CARDIOVASCULAR EVENTS
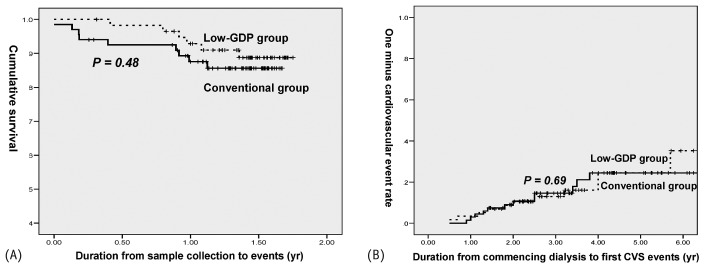
Patients also underwent prospective, scheduled assessments for an average of 15 months from the day of serum and effluent collection. The duration from PD initiation to final clinical assessment (“census”) was comparable between the two groups (3.6 ± 0.18 years vs 3.6 ± 0.20 years). No patient was lost to follow-up. At the time of censoring, 82% and 77.6% of patients using conventional and low-GDP PDF respectively remained on maintenance CAPD (Table 7). Cumulative survival in the conventional PDF group (86.6%) was similar to that in the low-GDP PDF group [89.6%, Figure 3(A)]. Most mortality (73.3%) was related to sepsis. Figure 3(B) shows the 1 – CVE rate of the 125 patients since PD initiation. During the average treatment period of 3.6 years, 18% of patients in the conventional PDF group and 20.7% in the low-GDP PDF developed a first fatal or nonfatal CVE.
TABLE 7.
Clinical Outcomes in the Study Groups
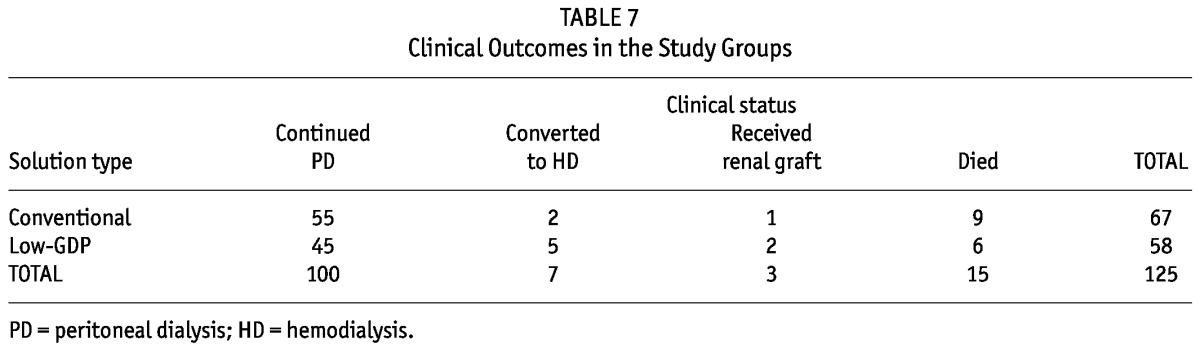
Figure 3.
— (A) Kaplan–Meier analysis of cumulative survival in two groups of patients over a mean follow-up period of 15-month after the collection of serum and effluent samples. Causes of death: peritonitis (n = 3), pneumonia (n = 5), acute myocardial infarction (n = 2), biliary sepsis (n = 1), ischemic colitis (n = 1), gastrointestinal infection (n = 1), undetermined (n = 2). (B) Kaplan–Meier analysis of the likelihood a first fatal or nonfatal cardiovascular event (CVS) over a mean 36-month follow-up period after dialysis start in two groups of patients. Patients permanently transferred to alternative renal replacement therapy (including hemodialysis or kidney transplantation) were censored from these survival and cardiovascular outcomes analyses. GDP = glucose degradation products.
DISCUSSION
The GDPs in conventional PDF cause mesothelial injury and reduce mesothelial regeneration, hence predisposing the patient to peritoneal fibrosis (15). In addition, accumulation of AGEs in the peritoneal tissue correlates with the development of severe interstitial fibrosis and microvascular sclerosis in PD (2,16). In vitro experiments have demonstrated that GDPs exert harmful effects in human peritoneal mesothelial cells through increased production of vascular endothelial growth factor (17), overexpression of the receptor for AGE (17), downregulation of tight junction–associated protein (18), and enhanced epithelial–mesenchymal transition (19).
Since the early 2000s, new biocompatible double-chambered bicarbonate/lactate– or bicarbonate-based PDFs have been introduced, with the rationale that their low GDP content will reduce UF failure, technique failure, and patient mortality. No large, long-term prospective randomized “hard endpoint” study on technique and patient survival is currently available, and often it is not feasible to recruit a sample large enough to provide adequate statistical power to assess the individual endpoints of death, cause-specific death, hospitalization, and other events (20). Results in the literature regarding the short-term impact of the new low-GDP PDFs on peritoneal UF capacity and other peritoneal membrane indices are divergent. Better peritoneal integrity with the low-GDP PDFs is hypothesized as being indirectly suggested by the higher cancer antigen 125 and lower hyaluronan levels observed in effluent (6–10,21–23). However, results concerning the beneficial effect on peritoneal net UF of the new biocompatible PDFs compared with conventional PDFs have been conflicting (6–8,10,24–27). Low-GDP or neutral dialysates are supposed to induce lower levels of inflammatory markers in effluent, and yet the effluent level of IL-6 has been reported to be high (28), low (9,21,29), or unchanged (10,23). The reason that use of the new biocompatible PDFs has failed to translate into better clinical outcomes is unclear. It may be a result of the short treatment period [only one study has considered treatment for 24 months (6)] and of differences in the selection of biomarkers for the examination of systemic and peritoneal inflammation.
In the present study, we recruited 125 patients on maintenance CAPD who were initially assigned at random by individual dialysis centers to receive either conventional or low-GDP PDF. Cytokine and biomarker profiles and dialysis adequacy were used as composite co-primary outcomes. At PD initiation, these groups of patients were comparable except for a higher 4-hour D/P creatinine in the group using low-GDP PDF. No patient was lost to follow-up, and 80% remained on maintenance CAPD at study completion. After an average CAPD treatment duration of 3.6 years (median: 3.4 years), the weekly total creatinine clearance, peritoneal creatinine clearance, total Kt/V, and peritoneal Kt/V were comparable in the groups. As they had at baseline, patients on low-GDP PDF had a higher 4-hour D/P creatinine at census, but this index of peritoneal transport was unchanged within the dialysis groups after 3.6 years of CAPD. However, patients receiving low-GDP PDF were observed to have higher urine output, a finding similar to that in two other short-term prospective studies of small sample size (7,27). Furthermore, compared with patients on conventional PDF, those on low-GDP PDF showed a slower rate of decline of residual GFR and urine output despite the lack of a change in UF at 4 hours in a standard PET. Those findings suggest that the use of low-GDP PDF might be associated with better preservation of residual renal function.
We also studied cytokines, growth factors, cardiac biomarkers, and adipokines in overnight effluent, and we simultaneously collected serum samples from these patients undergoing stable long-term maintenance CAPD. Compared with the group using low-GDP PDF, the group using conventional PDF showed higher serum concentrations of TNFα, NGAL, and leptin, and a lower serum concentration of adiponectin. Likewise, effluent concentrations of IL-1, IL-8, and leptin were higher in patients on conventional PDF than in those on low-GDP PDF. Patients on conventional PDF also had a lower concentration of adiponectin in effluent. From among those results, we are interested in mediators with high effluent concentrations as defined by their dialysate-to-serum ratio, because serum concentrations should normally be 10 – 100 times higher than effluent concentrations, given the relatively smaller mass of the peritoneal tissue compared with the whole body. That the dialysate-to-serum ratios of TNFα, HGF, MIF, IL-8, IL-6, CRP, and leptin exceed unity suggests that the peritoneal cavity is a major site of synthesis of those mediators because of chronic inflammation after exposure to less-biocompatible dialysate. The lack of an inverse correlation between the dialysate-to-serum ratios and the molecular weights of these mediators further supports the hypothesis that their higher effluent concentrations are the result of significant local intraperitoneal synthesis rather than of size-selective peritoneal transport (14).
Human peritoneal mesothelial cells synthesize TNFα, IL-6, IL-8, and HGF (30–32). Peritoneal macrophages and adipocytes also synthesize IL-6 (33,34), and peritoneal fibroblasts and macrophages respectively synthesize IL-8 and MIF (33,35). Tumor necrosis factor α and IL-6 are proinflammatory; MIF and IL-8 are chemotactic. By contrast, HGF ameliorates epithelial–mesenchymal transition induced by high glucose in the peritoneal mesothelium. Leptin and adiponectin are adipokines with pro-atherogenic and anti-atherogenic properties respectively. Teta et al. (36) reported in vitro findings that pH-neutral dialysate specifically induces leptin secretion from 3T3-L1 mouse adipocytes. Axelsson et al. (37) demonstrated that truncal (visceral) but not non-truncal (subcutaneous) fat mass is a contributor to inflammation in end-stage renal disease. Earlier, we also showed that glucose increases synthesis of leptin in human peritoneal adipocytes (34). The Janus kinase signal transducer and activator of transcription pathway in mesothelial cells was activated by leptin derived from adipocytes, and it in turn induced the release of TGFα by mesothelial cells. The TGFα synthesis induced by leptin was amplified by glucose through increased leptin receptor expression. In nonrenal patients, hyperleptinemia and hypoadiponectinemia are associated with cardiovascular risks (38). In patients on PD, the leptin/adiponectin ratio is markedly elevated, which is speculated to be associated with increased cardiovascular complications (39). In vitro studies revealed that glucose-sparing PD regimens improve the leptin/adiponectin ratio (39). The present study revealed that PD with conventional or low-GDP PDF (independent of complications from peritonitis) induces synthesis of selected proinflammatory cytokines, chemokines, and adipokines in the peritoneum, where maintenance of a subclinical low-grade inflammation favors the development of the malnutrition–inflammation–atherosclerosis syndrome (40).
More interestingly, compared with patients using conventional PDF, those using low-GDF PDF showed lower serum and effluent levels of leptin and higher serum and effluent levels of adiponectin. The effluent concentration of the chemoattractant IL-8 was also significantly lower in patients using low-GDP PDF.
Previous studies showed that serum TnT and NT-proBNP had predictive value for survival in CAPD patients and that measurement of those cardiac biomarkers may be of value in guiding risk stratification and, potentially, targeted therapeutic interventions (41,42). Our study failed to demonstrate differences between our groups of patients in serum levels of TnT and NT-proBNP. As occurred in previous studies, we also failed to observe a difference in either patient survival or first CVE between patients on low-GDP PDF and those on conventional PDFs. That finding possibly reflects several factors. First, it is not feasible in dialysis studies to recruit a sample large enough to provide statistical power adequate to assess the individual endpoints of death, cause-specific death, hospitalization, and other events (20). Second, compared with other dialysis populations, our patients are healthier, with a higher protein catabolic rate, a lower incidence of diabetes, and fewer cardiovascular complications (42,43). Lastly, adjustments were not made in our study for other comorbid factors such as obesity, smoking, and exercise.
A weakness of our study is that our assessment of hydration status was based on clinical assessment of blood pressure and edema combined with UF by standard PET. We did not use body composition analysis techniques (such as bioelectric impedance) that are used to study physiologic processes such as growth, development, aging, and exercise physiology (44). However, the accuracy and interpretation of such techniques in pathologic states such as PD have not been confirmed (45).
CONCLUSIONS
Peritoneal dialysis with conventional or low-GDP PDF induces synthesis of selected proinflammatory cytokines, chemokines, and adipokines in the peritoneum. Compared with patients using conventional PDFs, patients using low-GDP PDFs have an improved serum and effluent profile for adipokines. Patients on low-GDP PDFs also show a slower rate of decline in residual GFR and urine output. It would appear that low-GDP PDF results in an improvement in local peritoneal homeostasis by ameliorating the chronic inflammatory state in the peritoneum.
DISCLOSURES
The authors have no financial conflicts of interest to declare. No sponsorship for PD fluid was received for the present work.
Acknowledgments
The work reported here was supported in part by a Renal Discoveries–International Society of Nephrology grant and a Baxter extramural grant. JCKL was supported by the L&T Charitable Foundation and the House of INDOCAFE.
REFERENCES
- 1. Brownlee M, Cerami A, Vlassara H. Advanced glycation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med 1988; 318:1315–21 [DOI] [PubMed] [Google Scholar]
- 2. Nakamura S, Tachikawa T, Tobita K, Miyazaki S, Sakai S, Morita T, et al. Role of advanced glycation end products and growth factors in peritoneal dysfunction in CAPD patients. Am J Kidney Dis 2003; 41(Suppl 1):S61–7 [DOI] [PubMed] [Google Scholar]
- 3. Chaudhary K, Khanna R. Biocompatible peritoneal dialysis solutions: do we have one? Clin J Am Soc Nephrol 2010; 5:723–32 [DOI] [PubMed] [Google Scholar]
- 4. Lee HY, Choi HY, Park HC, Seo BJ, Do JY, Yun SR, et al. Changing prescribing practice in CAPD patients in Korea: increased utilization of low GDP solutions improves patient outcome. Nephrol Dial Transplant 2006; 21:2893–9 [DOI] [PubMed] [Google Scholar]
- 5. Lee HY, Park HC, Seo BJ, Do JY, Yun SR, Song HY, et al. Superior patient survival for continuous ambulatory peritoneal dialysis patients treated with a peritoneal dialysis fluid with neutral pH and low glucose degradation product concentration (Balance). Perit Dial Int 2005; 25:48–55 [PubMed] [Google Scholar]
- 6. Rippe B, Simonsen O, Heimbürger O, Christensson A, Haraldsson B, Stelin G, et al. Long-term clinical effects of a peritoneal dialysis fluid with less glucose degradation products. Kidney Int 2001; 59:348–57 [DOI] [PubMed] [Google Scholar]
- 7. Williams JD, Topley N, Craig KJ, Mackenzie RK, Pischetsrieder M, Lage C, et al. The Euro-Balance Trial: the effect of a new biocompatible peritoneal dialysis fluid (Balance) on the peritoneal membrane. Kidney Int 2004; 66:408–18 [DOI] [PubMed] [Google Scholar]
- 8. Szeto CC, Chow KM, Lam CW, Leung CB, Kwan BC, Chung KY, et al. Clinical biocompatibility of a neutral peritoneal dialysis solution with minimal glucose-degradation products—a 1-year randomized control trial. Nephrol Dial Transplant 2007; 22:552–9 [DOI] [PubMed] [Google Scholar]
- 9. Do JY, Kim YL, Park JW, Chang KA, Lee SH, Ryu DH, et al. The association between the vascular endothelial growth factor–to–cancer antigen 125 ratio in peritoneal dialysis effluent and the epithelial-to-mesenchymal transition in continuous ambulatory peritoneal dialysis. Perit Dial Int 2008; 28(Suppl 3):S101–6 [PubMed] [Google Scholar]
- 10. Kim S, Oh J, Kim S, Chung W, Ahn C, Kim SG, et al. Benefits of biocompatible PD fluid for preservation of residual renal function in incident CAPD patients: a 1-year study. Nephrol Dial Transplant 2009; 24:2899–908 [DOI] [PubMed] [Google Scholar]
- 11. Lai KN, Lo WK. Optimal peritoneal dialysis for patients from Hong Kong. Perit Dial Int 1999; 19(Suppl 3):S26–31 [PubMed] [Google Scholar]
- 12. van Olden RW, Krediet RT, Struijk DG, Arisz L. Measurement of residual renal function in patients treated with continuous ambulatory peritoneal dialysis. J Am Soc Nephrol 1996; 7:745–50 [DOI] [PubMed] [Google Scholar]
- 13. Nolph KD, Moore HL, Twardowski ZJ, Khanna R, Prowant B, Meyer M, et al. Cross-sectional assessment of weekly urea and creatinine clearances in patients on continuous ambulatory peritoneal dialysis. ASAIO J 1992; 38:M139–42 [DOI] [PubMed] [Google Scholar]
- 14. Zemel D, Imholz AL, de Waart DR, Dinkla C, Struijk DG, Krediet RT. Appearance of tumor necrosis factor-alpha and soluble TNF-receptors I and II in peritoneal effluent of CAPD. Kidney Int 1994; 46:1422–30 [DOI] [PubMed] [Google Scholar]
- 15. Jörres A. Glucose degradation products in peritoneal dialysis: from bench to bedside. Kidney Blood Press Res 2003; 26:113–17 [DOI] [PubMed] [Google Scholar]
- 16. Honda K, Nitta K, Horita S, Yumura W, Nihei H, Nagai R, et al. Accumulation of advanced glycation end products in the peritoneal vasculature of continuous ambulatory peritoneal dialysis patients with low ultra-filtration. Nephrol Dial Transplant 1999; 14:1541–9 [DOI] [PubMed] [Google Scholar]
- 17. Lai KN, Leung JC, Chan LY, Li FF, Tang SC, Lam MF, et al. Differential expression of receptors for advanced glycation end-products in peritoneal mesothelial cells exposed to glucose degradation products. Clin Exp Immunol 2004; 138:466–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leung JC, Chan LY, Li FF, Tang SC, Chan KW, Chan TM, et al. Glucose degradation products downregulate ZO-1 expression in human peritoneal mesothelial cells: the role of VEGF. Nephrol Dial Transplant 2005; 20:1336–49 [DOI] [PubMed] [Google Scholar]
- 19. Oh EJ, Ryu HM, Choi SY, Yook JM, Kim CD, Park SH, et al. Impact of low glucose degradation product bicarbonate/lactate–buffered dialysis solution on the epithelial–mesenchymal transition of peritoneum. Am J Nephrol 2010; 31:58–67 [DOI] [PubMed] [Google Scholar]
- 20. Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, et al. on behalf of the FHN Trial Group. Incenter hemodialysis six times per week versus three times per week. N Engl J Med 2010; 363:2287–300 [Erratum in: N Engl J Med 2011; 364:93] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fusshöller A, Plail M, Grabensee B, Plum J. Biocompatibility pattern of a bicarbonate/lactate-buffered peritoneal dialysis fluid in APD: a prospective, randomized study. Nephrol Dial Transplant 2004; 19:2101–6 [DOI] [PubMed] [Google Scholar]
- 22. Choi HY, Kim DK, Lee TH, Moon SJ, Han SH, Lee JE, et al. The clinical usefulness of peritoneal dialysis fluids with neutral pH and low glucose degradation product concentration: an open randomized prospective trial. Perit Dial Int 2008; 28:174–82 [PubMed] [Google Scholar]
- 23. Weiss L, Stegmayr B, Malmsten G, Tejde M, Hadimeri H, Siegert CE, et al. Biocompatibility and tolerability of a purely bicarbonate-buffered peritoneal dialysis solution. Perit Dial Int 2009; 29:647–55 [PubMed] [Google Scholar]
- 24. Fang W, Mullan R, Shah H, Mujais S, Bargman JM, Oreopoulos DG. Comparison between bicarbonate/lactate and standard lactate dialysis solution in peritoneal transport and ultrafiltration: a prospective, crossover single-dwell study. Perit Dial Int 2008; 28:35–43 [PubMed] [Google Scholar]
- 25. Fan SL, Pile T, Punzalan S, Raftery MJ, Yaqoob MM. Randomized controlled study of biocompatible peritoneal dialysis solutions: effect on residual renal function. Kidney Int 2008; 73:200–6 [DOI] [PubMed] [Google Scholar]
- 26. Pajek J, Kveder R, Bren A, Gucek A, Bucar M, Skoberne A, et al. Short-term effects of bicarbonate/lactate–buffered and conventional lactate-buffered dialysis solutions on peritoneal ultrafiltration: a comparative crossover study. Nephrol Dial Transplant 2009; 24:1617–25 [DOI] [PubMed] [Google Scholar]
- 27. Haag–Weber M, Krämer R, Haake R, Islam MS, Prischl F, Haug U, et al. Low-GDP fluid (Gambrosol Trio) attenuates decline of residual renal function in PD patients: a prospective randomized study. Nephrol Dial Transplant 2010; 25:2288–96 [DOI] [PubMed] [Google Scholar]
- 28. Martikainen TA, Teppo AM, Grönhagen–Riska C, Ekstrand AV. Glucose-free dialysis solutions: inductors of inflammation or preservers of peritoneal membrane? Perit Dial Int 2005; 25:453–60 [PubMed] [Google Scholar]
- 29. Pajek J, Kveder R, Bren A, Gucek A, Ihan A, Osredkar J, et al. Short-term effects of a new bicarbonate/lactate–buffered and conventional peritoneal dialysis fluid on peritoneal and systemic inflammation in CAPD patients: a randomized controlled study. Perit Dial Int 2008; 28:44–52 [PubMed] [Google Scholar]
- 30. Topley N, Brown Z, Jörres A, Westwick J, Davies M, Coles GA, et al. Human peritoneal mesothelial cells synthesize interleukin-8. Synergistic induction by interleukin-1beta and tumor necrosis factor-alpha. Am J Pathol 1993; 142:1876–86 [PMC free article] [PubMed] [Google Scholar]
- 31. Leung JC, Chan LY, Tam KY, Tang SC, Lam MF, Cheng AS, et al. Regulation of CCN2/CTGF and related cytokines in cultured peritoneal cells under conditions simulating peritoneal dialysis. Nephrol Dial Transplant 2009; 24:458–69 [DOI] [PubMed] [Google Scholar]
- 32. Yu MA, Shin KS, Kim JH, Kim YI, Chung SS, Park SH, et al. HGF and BMP-7 ameliorate high glucose-induced epithelial-to-mesenchymal transition of peritoneal mesothelium. J Am Soc Nephrol 2009; 20:567–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ikeda Y, Murakami A, Fujimura Y, Tachibana H, Yamada K, Masuda D, et al. Aggregated ursolic acid, a natural triterpenoid, induces IL-1beta release from murine peritoneal macrophages: role of CD36. J Immunol 2007; 178:4854–64 [DOI] [PubMed] [Google Scholar]
- 34. Leung JC, Chan LY, Tang SC, Chu KM, Lai KN. Leptin induces TGF-beta synthesis through functional leptin receptor expressed by human peritoneal mesothelial cell. Kidney Int 2006; 69:2078–86 [DOI] [PubMed] [Google Scholar]
- 35. Witowski J, Tayama H, Ksiazek K, Wanic–Kossowska M, Bender TO, Jörres A. Human peritoneal fibroblasts are a potent source of neutrophil-targeting cytokines: a key role of IL-1beta stimulation. Lab Invest 2009; 89:414–24 [DOI] [PubMed] [Google Scholar]
- 36. Teta D, Maillard M, Tedjani A, Passlick–Deetjen J, Burnier M. The effect of pH-neutral peritoneal dialysis fluids on adipokine secretion from cultured adipocytes. Nephrol Dial Transplant 2007; 22:862–9 [DOI] [PubMed] [Google Scholar]
- 37. Axelsson J, Rashid Qureshi A, Suliman ME, Honda H, Pecoits–Filho R, Heimbürger O, et al. Truncal fat mass as a contributor to inflammation in end-stage renal disease. Am J Clin Nutr 2004; 80:1222–9 [DOI] [PubMed] [Google Scholar]
- 38. Xu L, Jiang CQ, Lam TH, Lin JM, Yue XJ, Cheng KK, et al. The metabolic syndrome is associated with subclinical atherosclerosis independent of insulin resistance: the Guangzhou Biobank Cohort Study-CVD. Clin Endocrinol (oxf) 2010; 73:181–8 [DOI] [PubMed] [Google Scholar]
- 39. Teta D, Maillard M, Halabi G, Burnier M. The leptin/adiponectin ratio: potential implications for peritoneal dialysis. Kidney Int Suppl 2008; (108):S112–18 [DOI] [PubMed] [Google Scholar]
- 40. Lai KN, Leung JC. Inflammation in peritoneal dialysis. Nephron Clin Pract 2010; 116:c11–18 [DOI] [PubMed] [Google Scholar]
- 41. Han SH, Choi HY, Kim DK, Moon SJ, Lee JE, Yoo TH, et al. Elevated cardiac troponin T predicts cardiovascular events in asymptomatic continuous ambulatory peritoneal dialysis patients without a history of cardiovascular disease. Am J Nephrol 2009; 29:129–35 [DOI] [PubMed] [Google Scholar]
- 42. Paniagua R, Amato D, Mujais S, Vonesh E, Ramos A, Correa–Rotter R, et al. Predictive value of brain natriuretic peptides in patients on peritoneal dialysis: results from the ADEMEX trial. Clin J Am Soc Nephrol 2008; 3:407–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lam MF, Tang C, Wong AK, Tong KL, Yu AW, Li CS, et al. ASPD: a prospective study of adequacy in Asian patients on long term, small volume, continuous ambulatory peritoneal dialysis. Perit Dial Int 2006; 26:466–74 [PubMed] [Google Scholar]
- 44. Nichols J, Going S, Loftin M, Stewart D, Nowicki E, Pickrel J. Comparison of two bioelectrical impedance analysis instruments for determining body composition in adolescent girls. Int J Body Compos Res 2006; 4:153–60 [PMC free article] [PubMed] [Google Scholar]
- 45. Woodrow G. Body composition analysis techniques in adult and pediatric patients: how reliable are they? how useful are they clinically? Perit Dial Int 2007; 27(Suppl 2):S245–9 [PubMed] [Google Scholar]