Abstract
♦ Background: Peritoneal dialysis (PD)–associated peritonitis clusters within patients. Patient factors contribute to peritonitis risk, but there is also entrapment of organisms within the biofilm that forms on PD catheters. It is hypothesized that this biofilm may prevent complete eradication of organisms, predisposing to multiple infections with the same organism.
♦ Methods: Using data collected in the Canadian multicenter Baxter POET (Peritonitis, Organism, Exit sites, Tunnel infections) database from 1996 to 2005, we studied incident PD patients with 2 or more peritonitis episodes. We determined the proportion of patients with 2 or more episodes caused by the same organism. In addition, using a multivariate logistic regression model, we tested whether prior peritonitis with a given organism predicted the occurrence of a subsequent episode with the same organism.
♦ Results: During their time on PD, 558 patients experienced 2 or more peritonitis episodes. Of those 558 patients, 181 (32%) had at least 2 episodes with the same organism. The organism most commonly causing repeat infection was coagulase-negative Staphylococcus (CNS), accounting for 65.7% of cases. Compared with peritonitis caused by other organisms, a first CNS peritonitis episode was associated with an increased risk of subsequent CNS peritonitis within 1 year (odds ratio: 2.1; 95% confidence interval: 1.5 to 2.8; p < 0.001). Among patients with repeat CNS peritonitis, 48% of repeat episodes occurred within 6 months of the earlier episode.
♦ Conclusions: In contrast to previous data, we did not find a high proportion of patients with multiple peritonitis episodes caused by the same organism. Coagulase-negative Staphylococcus was the organism most likely to cause peritonitis more than once in a given patient, and a prior CNS peritonitis was associated with an increased risk of CNS peritonitis within the subsequent year.
Keywords: Peritonitis, biofilm, microbiology, coagulase-negative Staphylococcus
Despite the decreasing frequency of peritonitis among peritoneal dialysis (PD) patients over time, peritonitis remains a concern because its occurrence has been associated with adverse outcomes (1–4). However, why some patients never develop peritonitis and why others go on to have multiple episodes remains unclear.
Available data suggest that experiencing a first peritonitis episode is associated with an increased risk for developing a subsequent episode. In one study, a first peritonitis episode occurring within the first 6 months after PD initiation was associated with a shorter time to subsequent peritonitis, with a hazard ratio of 2.15 (5). A second study showed a doubling of the peritonitis rate in patients who had experienced a prior episode (6). This increased risk of subsequent peritonitis likely relates in part to patient factors that predispose to the development of peritonitis (5–14). However, an additional consideration may be formation of a biofilm on the PD catheter (15–17). This biofilm is thought to consist of bacteria that attach to the PD catheter and become surrounded by an impenetrable glycocalyx matrix coat. It is hypothesized that the presence of a biofilm may put patients at increased risk of subsequent infection with the same organism because of difficulty in eradicating the organism. That hypothesis was tested in a cohort of 198 patients with multiple peritonitis episodes (18), among whom 80% had at least 1 repeat peritonitis episode, suggesting that bacterial biofilm formation on PD catheters may be playing a role in the frequency of peritonitis in some patients.
The primary objective of the present study was to describe the microbiology of peritonitis among Canadian PD patients with multiple peritonitis episodes, and to determine whether prior peritonitis caused by a given organism predicts subsequent peritonitis with the same organism.
METHODS
PATIENTS
The study included PD patients from 25 centers across Canada for whom data were available through the POET (Peritonitis, Organism, Exit sites, Tunnel infections) database (Baxter Healthcare Corporation, Deerfield, IL, USA). The data from all Canadian PD centers using the POET clinical monitoring system software were collected as previously described (4). For the present study, we included incident PD patients for whom data were prospectively collected from 1 January 1996 until 12 September 2005. Information in the POET database includes patient demographics, cause of infection, catheter complications, and therapy transfers. All patients on automated PD used Baxter cyclers, for which Luer lock connectology first became available in Canada in 2003. Data on exit-site prophylaxis and antibiotic therapy were not available. Before study initiation, approval was obtained from the Research Ethics Board at the University Health Network.
PERITONITIS
Relapsing or recurrent peritonitis episodes were excluded. International Society for Peritoneal Dialysis definitions were used, with “relapse” defined as an episode occurring within 4 weeks of completion of therapy for an earlier infection with a negative culture or the same organism, and “recurrence” defined as an episode occurring within 4 weeks of completion of therapy for an earlier infection with a different organism (19). Consequently, peritonitis episodes occurring within 60 days of a previous episode were excluded based on the assumption that patients were treated with a maximum of 4 weeks of antibiotic therapy.
Peritonitis organisms were classified as follows: coagulase-negative Staphylococcus (CNS), S. aureus, Streptococcus viridans, Corynebacterium, Enterococcus, Neisseria, Escherichia coli, Pseudomonas aeruginosa, Klebsiella, Acinetobacter, anaerobes, culture-negative, and “other organisms.” The determination of the proportion of patients with multiple episodes caused by the same organism excluded all culture-negative and “other organism” peritonitis episodes.
STATISTICAL ANALYSIS
Continuous variables are reported as mean ± standard deviation. For patients with at least 2 episodes of peritonitis, we determined the proportion with 2 or more infections caused by the same organism. To determine whether an earlier peritonitis with a given organism predicted subsequent peritonitis within 1 year, we used a logistic regression model (20,21). Because we were interested in how a previous episode might predict a current episode, we used a transitional model, in which current status was conditional to previous state. If the outcome is binomial (1/0), and only 1 previous state is considered, the transitional model represents a first-order Markov chain. It can be shown that, given the predictors, the model treats repeated transitions by a subject as independent. Therefore a logistic regression model can be fit by treating each transition as a separate observation (22). The model adjusted for covariates including age, sex, race, cause of end-stage renal disease, diabetes status, modality before PD start (new to dialysis, transfer from HD, failed transplant), and PD submodality (continuous ambulatory PD vs automated PD). The association between era of PD initiation (1996 – 2000 vs 2001 – 2005) and repeat peritonitis was also tested. The primary analysis was performed after exclusion of peritonitis episodes occurring within 60 days of a previous episode, but sensitivity analyses were performed to evaluate whether the results could be influenced by differences in the definition of relapsed or recurrent peritonitis. Specifically, analyses were performed
after re-definition of “recurrent” or “relapsed” peritonitis as episodes occurring within 45 days of diagnosis rather than 60 days, and
with inclusion of all peritonitis episodes.
Statistical significance was defined as p <0.05. All statistical analyses were performed using the SAS software application (version 9.1: SAS Institute, Cary, NC, USA).
RESULTS
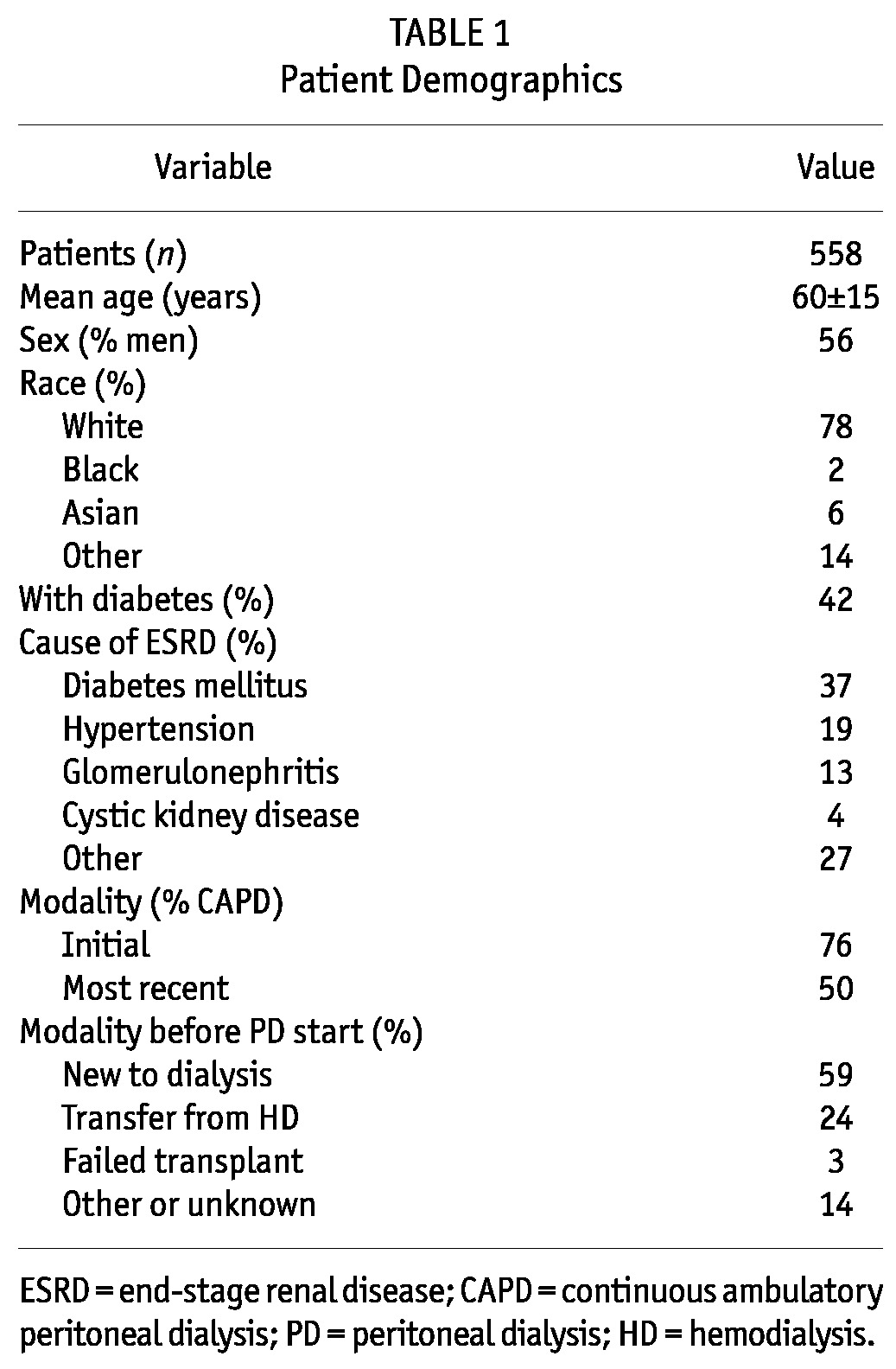
Among the 4247 incident PD patients in the POET database, 1605 patients experienced a total of 3058 episodes of peritonitis. The remaining 2642 patients had no peritonitis. Of the 3058 peritonitis episodes, 503 were excluded because they occurred within 60 days of an earlier episode and were assumed to be recurrent or relapsing events. After restricting the database to patients with at least 2 peritonitis episodes, the study population consisted of 558 patients who had experienced a total of 1508 peritonitis episodes. The median time on PD was 2.6 years. Table 1 shows the demographic characteristics of those patients.
TABLE 1.
Patient Demographics
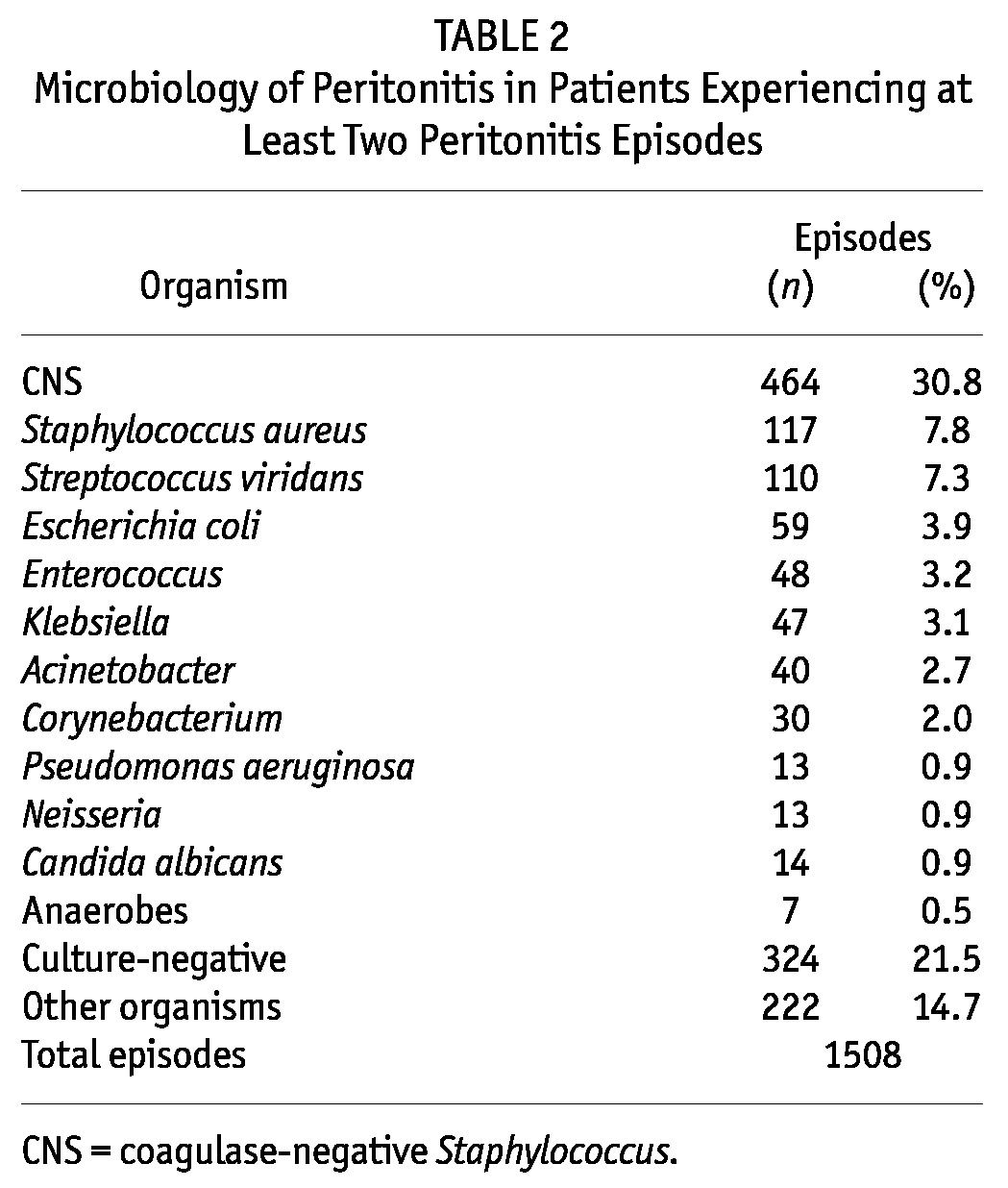
The median number of peritonitis episodes in the study cohort was 2 (range: 2 – 10). Of the cohort patients, 58% had 2 peritonitis episodes, 25% had 3 episodes, 9% had 4 episodes, and 8% had 5 or more episodes. The median time between episodes was 185 days (interquartile range: 106 – 363 days). Table 2 lists the organisms causing the 1508 peritonitis episodes. The most frequent organism was CNS, which accounted for 31% of episodes. Culture-negative peritonitis accounted for 21% of episodes.
TABLE 2.
Microbiology of Peritonitis in Patients Experiencing at Least Two Peritonitis Episodes
Among the 558 patients, 181 (32%) had at least 1 repeat infection with the same organism, including 155 patients (28%) with a repeat peritonitis episode involving a single organism, and 26 patients (5%) with a repeat peritonitis episode involving at least 2 different sets of organisms. Of the latter 26 patients, 18 had 2 episodes of Strep. viridans peritonitis and 2 episodes of a “Streptococcus species” peritonitis (subtype not specified) that likely represented the same organism; however, that assumption could not be confirmed based on the data entered at the time of the episode.
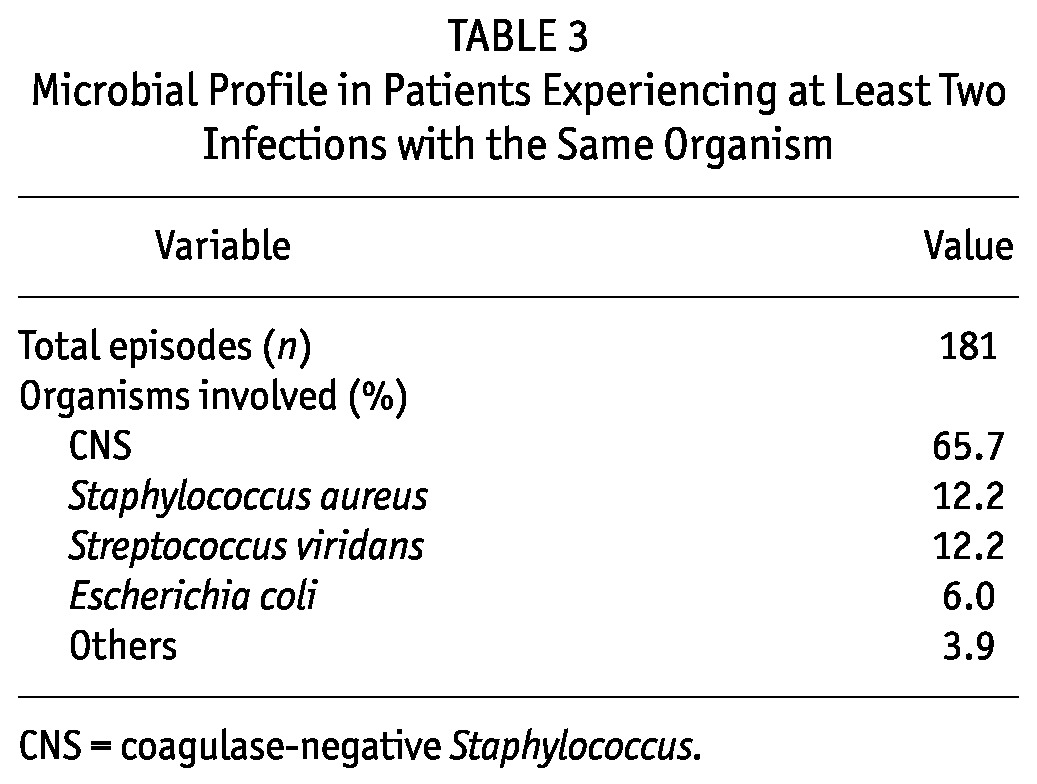
Among all repeat peritonitis episodes, 15% occurred within 3 months of diagnosis of the earlier episode; 33%, between 3 and 6 months; 10%, between 6 and 9 months; 12%, between 9 and 12 months; and 30%, at least 1 year later. The most common infecting organism was CNS, seen in 65.7% of patients. The next most common organisms causing multiple peritonitis episodes were S. aureus (12.2%) and Strep. viridans (12.2%). Table 3 shows the microbial profile for patients with at least 2 infections with the same organism.
TABLE 3.
Microbial Profile in Patients Experiencing at Least Two Infections with the Same Organism
In the multivariate logistic regression model, after adjustments for age, sex, race, cause of end-stage renal disease, diabetes status, modality before PD start, and PD submodality, having a first CNS peritonitis episode was independently associated with an increased risk of subsequent CNS peritonitis within 1 year of the earlier episode (odds ratio: 2.1; 95% confidence interval: 1.5 to 2.8; p < 0.001). The timing of the repeat CNS peritonitis episodes was variable, with 17% occurring within 3 months of diagnosis of the earlier infection; 32%, 3 – 6 months later; 8%, 6 – 9 months later; 13%, 9 – 12 months later; and 30%, at least 1 year later. Era of PD initiation (1996 – 2000 vs 2001 – 2005) did not modify the risk of repeat CNS peritonitis (p = 0.69).
Two sensitivity analyses were performed to ensure that the peritonitis episodes excluded as recurrences or relapses did not have an important effect on the proportion of patients experiencing at least 1 repeat infection with the same organism. Compared with the original analysis, in which 32% of patients had at least 2 peritonitis episodes with the same organism, exclusion of episodes occurring within 45 days of an earlier episode resulted in 37% of patients experiencing at least 2 infections with the same organism. When all relapses and recurrences were included, the proportion of patients experiencing 2 or more episodes with the same organism was 43%.
DISCUSSION
Among Canadian patients initiating PD between 1996 and 2005, we did not find a high proportion of patients with multiple peritonitis episodes caused by the same organism, suggesting that bacterial biofilm did not play a role in most of these infections. The organism most likely to occur more than once in a given patient was CNS, and having an earlier CNS peritonitis episode was associated with an increased risk of subsequent CNS peritonitis within 1 year.
The earliest clinical study to report on the frequency of repeat PD peritonitis with the same organism was conducted by Golper et al. (23). In that study, which examined 96 peritonitis episodes, two thirds of all infections were caused by the same pathogen (genus and species) that had caused the most recent preceding infection. Subsequently, using a larger cohort of American patients, Finkelstein et al. described the microbiology of multiple peritonitis episodes (18). In contrast to our findings, their study reported that, among 198 PD patients with at least 2 peritonitis episodes, 80% had at least 1 repeat infection with the same organism. In addition, among patients whose catheters were changed, only 15% experienced peritonitis episodes with the same organism before and after new catheter placement.
There are several possible reasons for the discrepant findings between the Finkelstein study and the present work. First, differences in the patient populations and the eras in which the studies were conducted likely contributed to overall peritonitis rates that were considerably higher in the Finkelstein study (1 episode in 14.5 patient–months, with a mean of 5 peritonitis episodes per patient in their study cohort vs a median of 2 episodes per patient in the present study). It is plausible that a greater number of peritonitis episodes per patient might partly account for the increased likelihood of patients having more than 1 episode with the same organism. Another potential contributor could be variability in practice with regard to the duration of antibiotic therapy and the threshold for catheter removal, with shorter antibiotic durations and a higher threshold for catheter removal increasing the potential for biofilm-related peritonitis. Importantly, the definition of “relapse” was also different, with the Finkelstein study excluding re-infections with the same organism within 2 weeks of antibiotic completion (rather than within 4 weeks of antibiotic completion in the present study). That difference in definition might have led to the inclusion, in their analyses, of peritonitis episodes as repeat infections that we would have excluded as relapses. However, the results of our sensitivity analysis, performed using definitions that would capture patients similar to those in the Finkelstein study, suggest that this discrepancy is not the explanation for the observed differences in the results.
It is not surprising that CNS was the organism most likely to cause multiple infections and that having an earlier episode of CNS peritonitis was associated with an increased risk of subsequent CNS peritonitis. Although a recent study by Szeto et al. showed a relatively low proportion of repeat CNS peritonitis relative to repeat gram-negative peritonitis (24), our findings are consistent with other studies (18,25). Coagulase-negative Staphylococcus is the most common causative organism in PD peritonitis (25,26), but it is still less common than all the other possible organisms combined, such that having a higher risk of subsequent CNS peritonitis after a first CNS episode cannot be explained on the basis of chance alone.
There are several possible explanations for the propensity for CNS to recur. Because CNS is typically associated with intraluminal introduction of organisms into the peritoneal cavity, multiple CNS peritonitis episodes in a given patient might suggest that a patient with a break in sterile technique would be more likely to have subsequent breaks in technique. Alternatively, other factors that may explain the high proportion of multiple peritonitis episodes with CNS relative to those with other organisms include the shorter duration of antibiotic therapy for CNS peritonitis in some centers (2 weeks for CNS vs 3 weeks for other organisms) and the lower catheter removal rate for CNS infections (25,26). For example, a patient with a S. aureus peritonitis episode is more likely to require catheter removal and is therefore less likely to have multiple peritonitis episodes related to catheter biofilm with that organism. The final possibility is that CNS has a propensity to colonize catheter biofilms, with some limited data to support the contention that CNS is different from other organisms in this regard (27). The variability in the time interval between CNS episodes suggests that the basis for the increased risk of repeat CNS peritonitis is likely multifactorial, with biofilm potentially contributing to the early repeat episodes, and breaks in technique contributing both to early and to late repeat CNS infections.
The current study has several limitations, including a lack of data on the exact speciation of some of the organisms and an absence of information on antimicrobial susceptibilities to support an organism being of the same strain. However, if the missing information were to have affected our results, it would have led to a slight overestimation of the proportion of patients with at least 2 peritonitis episodes with the same organism, and our conclusion with regard to the relatively low frequency of repeat peritonitis in our cohort would therefore remain unchanged. An additional limitation is the lack of precise data on antibiotic choice and duration, and on outcomes such as PD catheter removal after peritonitis. As a result, we cannot determine with certainty whether the lower frequency of repeat peritonitis seen in this study might be a result of increased antibiotic duration and more frequent catheter removal for severe peritonitis episodes. Based on peritonitis outcomes data that were available for 55% of the peritonitis episodes, catheter loss occurred in only 12%, suggesting that the proportion with catheter removal after peritonitis was not higher than expected and would be unlikely to explain the low frequency of repeat peritonitis. Despite these limitations, our study is among the largest to date to report on the patterns of peritonitis microbiology in a contemporary cohort of patients with multiple peritonitis episodes.
CONCLUSIONS
In contrast to previous data, we did not find a high proportion of patients with multiple peritonitis episodes caused by the same organism. Coagulase-negative Staphylococcus was the organism most likely to occur more than once in a given patient, and having an earlier CNS peritonitis predicted repeat CNS peritonitis within the subsequent year.
DISCLOSURES
SJN and JMB have received speaker honoraria from Baxter Healthcare. SVJ has held an investigator-driven grant from OrthoBiotec and has received speaker or consulting fees from Amgen Canada, Baxter Healthcare, and OrthoBiotec within the last 5 years.
Acknowledgments
The authors thank Dr. Ken Story and Dr. Alex Kriukov for statistical support, and the members of the nursing and administrative staffs involved in data entry and maintenance of the POET database. RN gratefully acknowledges the support of the Ontario Ministry of Health and Long-Term Care. This manuscript was presented in abstract form as a poster presentation at the 2009 American Society of Nephrology meeting.
REFERENCES
- 1. Fried L, Abidi S, Bernardini J, Johnston JR, Piraino B. Hospitalization in peritoneal dialysis patients. Am J Kidney Dis 1999; 33:927–33 [DOI] [PubMed] [Google Scholar]
- 2. Fried LF, Bernardini J, Johnston JR, Piraino B. Peritonitis influences mortality in peritoneal dialysis patients. J Am Soc Nephrol 1996; 7:2176–82 [DOI] [PubMed] [Google Scholar]
- 3. Johnson DW, Dent H, Hawley CM, McDonald SP, Rosman JB, Brown FG, et al. Associations of dialysis modality and infectious mortality in incident dialysis patients in Australia and New Zealand. Am J Kidney Dis 2009; 53:290–7 [DOI] [PubMed] [Google Scholar]
- 4. Mujais S. Microbiology and outcomes of peritonitis in North America. Kidney Int Suppl 2006; (103):S55–62 [DOI] [PubMed] [Google Scholar]
- 5. Oo TN, Roberts TL, Collins AJ. A comparison of peritonitis rates from the United States Renal Data System database: CAPD versus continuous cycling peritoneal dialysis patients. Am J Kidney Dis 2005; 45:372–80 [DOI] [PubMed] [Google Scholar]
- 6. Golper TA, Brier ME, Bunke M, Schreiber MJ, Bartlett DK, Hamilton RW, et al. Risk factors for peritonitis in long-term peritoneal dialysis: the Network 9 peritonitis and catheter survival studies. Academic Subcommittee of the Steering Committee of the Network 9 peritonitis and catheter survival studies. Am J Kidney Dis 1996; 28:428–36 [DOI] [PubMed] [Google Scholar]
- 7. McDonald SP, Collins JF, Rumpsfeld M, Johnson DW. Obesity is a risk factor for peritonitis in the Australian and New Zealand peritoneal dialysis patient populations. Perit Dial Int 2004; 24:340–6 [PubMed] [Google Scholar]
- 8. Nessim SJ, Bargman JM, Austin PC, Nisenbaum R, Jassal SV. Predictors of peritonitis in patients on peritoneal dialysis: results of a large, prospective Canadian database. Clin J Am Soc Nephrol 2009; 4:1195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nessim SJ, Bargman JM, Austin PC, Story K, Jassal SV. Impact of age on peritonitis risk in peritoneal dialysis patients: an era effect. Clin J Am Soc Nephrol 2009; 4:135–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lim WH, Johnson DW, McDonald SP. Higher rate and earlier peritonitis in Aboriginal patients compared to non-Aboriginal patients with end-stage renal failure maintained on peritoneal dialysis in Australia: analysis of ANZDATA. Nephrology (Carlton) 2005; 10:192–7 [DOI] [PubMed] [Google Scholar]
- 11. Han SH, Lee SC, Ahn SV, Lee JE, Kim DK, Lee TH, et al. Reduced residual renal function is a risk of peritonitis in continuous ambulatory peritoneal dialysis patients. Nephrol Dial Transplant 2007; 22:2653–8 [DOI] [PubMed] [Google Scholar]
- 12. Chow KM, Szeto CC, Leung CB, Kwan BC, Law MC, Li PK. A risk analysis of continuous ambulatory peritoneal dialysis-related peritonitis. Perit Dial Int 2005; 25:374–9 [PubMed] [Google Scholar]
- 13. Andrews PA, Warr KJ, Hicks JA, Cameron JS. Impaired outcome of continuous ambulatory peritoneal dialysis in immunosuppressed patients. Nephrol Dial Transplant 1996; 11:1104–8 [PubMed] [Google Scholar]
- 14. Wang Q, Bernardini J, Piraino B, Fried L. Albumin at the start of peritoneal dialysis predicts the development of peritonitis. Am J Kidney Dis 2003; 41:664–9 [DOI] [PubMed] [Google Scholar]
- 15. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999; 284:1318–22 [DOI] [PubMed] [Google Scholar]
- 16. Costerton W, Veeh R, Shirtliff M, Pasmore M, Post C, Ehrlich G. The application of biofilm science to the study and control of chronic bacterial infections. J Clin Invest 2003; 112:1466–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dasgupta MK. Biofilms and infection in dialysis patients. Semin Dial 2002; 15:338–46 [DOI] [PubMed] [Google Scholar]
- 18. Finkelstein ES, Jekel J, Troidle L, Gorban–Brennan N, Finkelstein FO, Bia FJ. Patterns of infection in patients maintained on long-term peritoneal dialysis therapy with multiple episodes of peritonitis. Am J Kidney Dis 2002; 39:1278–86 [DOI] [PubMed] [Google Scholar]
- 19. Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, et al. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int 2005; 25:107–31 [PubMed] [Google Scholar]
- 20. Bonney GE. Logistic regression for dependent binary observations. Biometrics 1987; 43:951–73 [PubMed] [Google Scholar]
- 21. Agresti A. An Introduction to Categorical Data Analysis. New York, NY: John Wiley and Sons; 2007. [Google Scholar]
- 22. Bonney GE. Regressive logistic models for familial disease and other binary traits. Biometrics 1986; 42:611–25 [PubMed] [Google Scholar]
- 23. Golper TA, Hartstein AI. Analysis of the causative pathogens in uncomplicated CAPD-associated peritonitis: duration of therapy, relapses, and prognosis. Am J Kidney Dis 1986; 7:141–5 [DOI] [PubMed] [Google Scholar]
- 24. Szeto CC, Kwan BC, Chow KM, Law MC, Pang WF, Leung CB, et al. Repeat peritonitis in peritoneal dialysis: retrospective review of 181 consecutive cases. Clin J Am Soc Nephrol 2011; 6:827–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fahim M, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Coagulase-negative staphylococcal peritonitis in Australian peritoneal dialysis patients: predictors, treatment and outcomes in 936 cases. Nephrol Dial Transplant 2010; 25:3386–92 [DOI] [PubMed] [Google Scholar]
- 26. Szeto CC, Kwan BC, Chow KM, Lau MF, Law MC, Chung KY, et al. Coagulase negative staphylococcal peritonitis in peritoneal dialysis patients: review of 232 consecutive cases. Clin J Am Soc Nephrol 2008; 3:91–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dasgupta MK, Ward K, Noble PA, Larabie M, Costerton JW. Development of bacterial biofilms on Silastic catheter materials in peritoneal dialysis fluid. Am J Kidney Dis 1994; 23:709–16 [DOI] [PubMed] [Google Scholar]


