Abstract
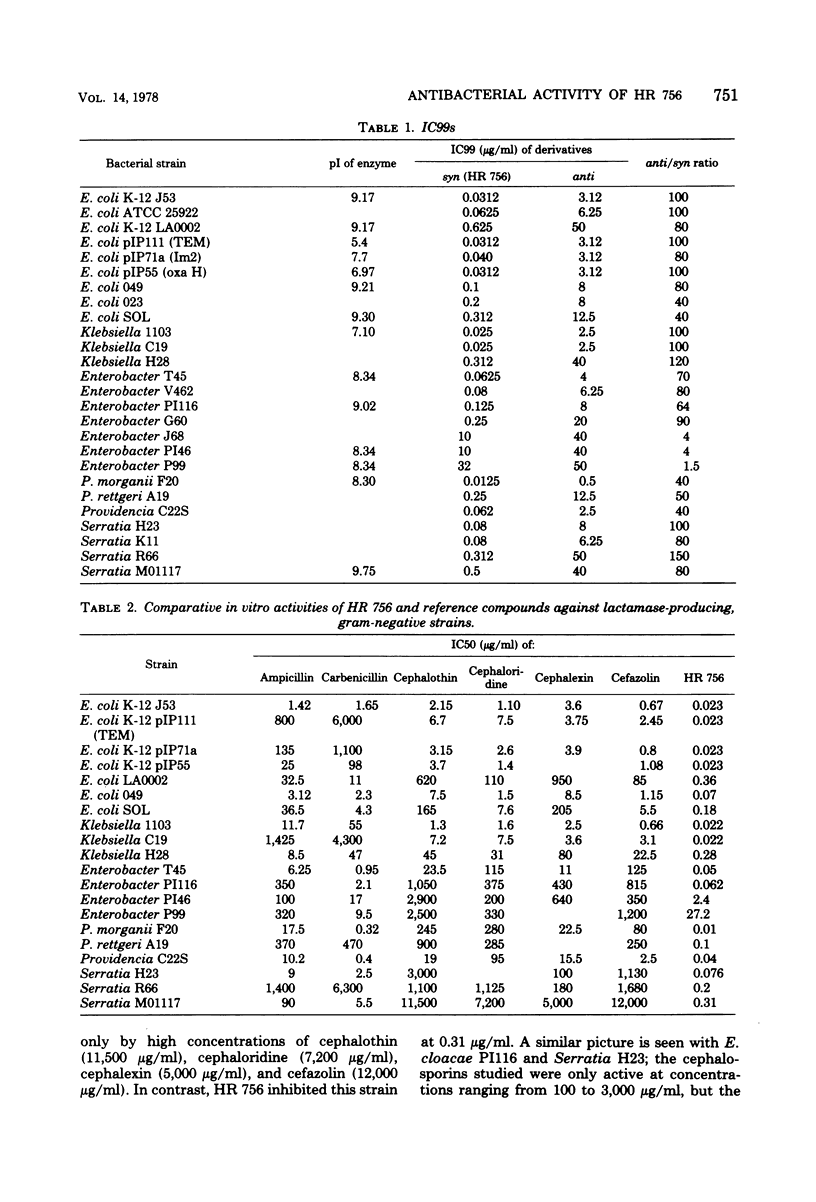
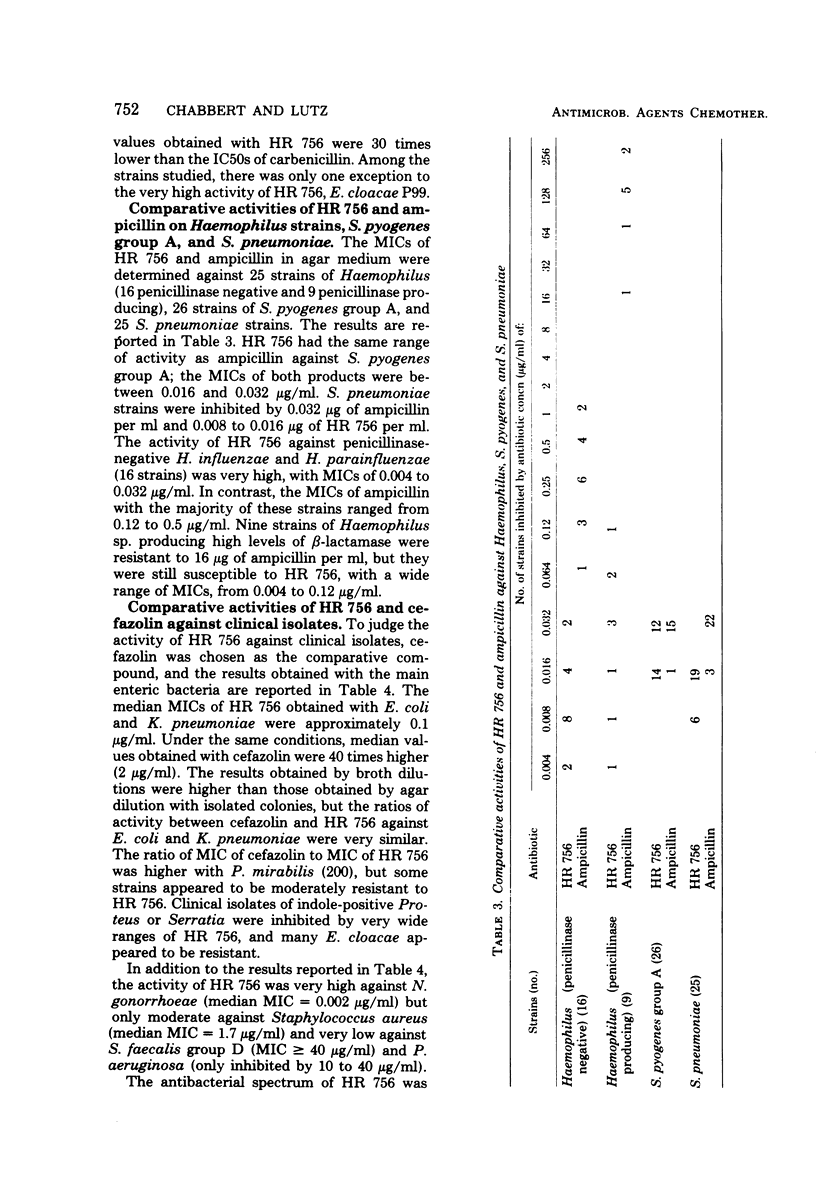
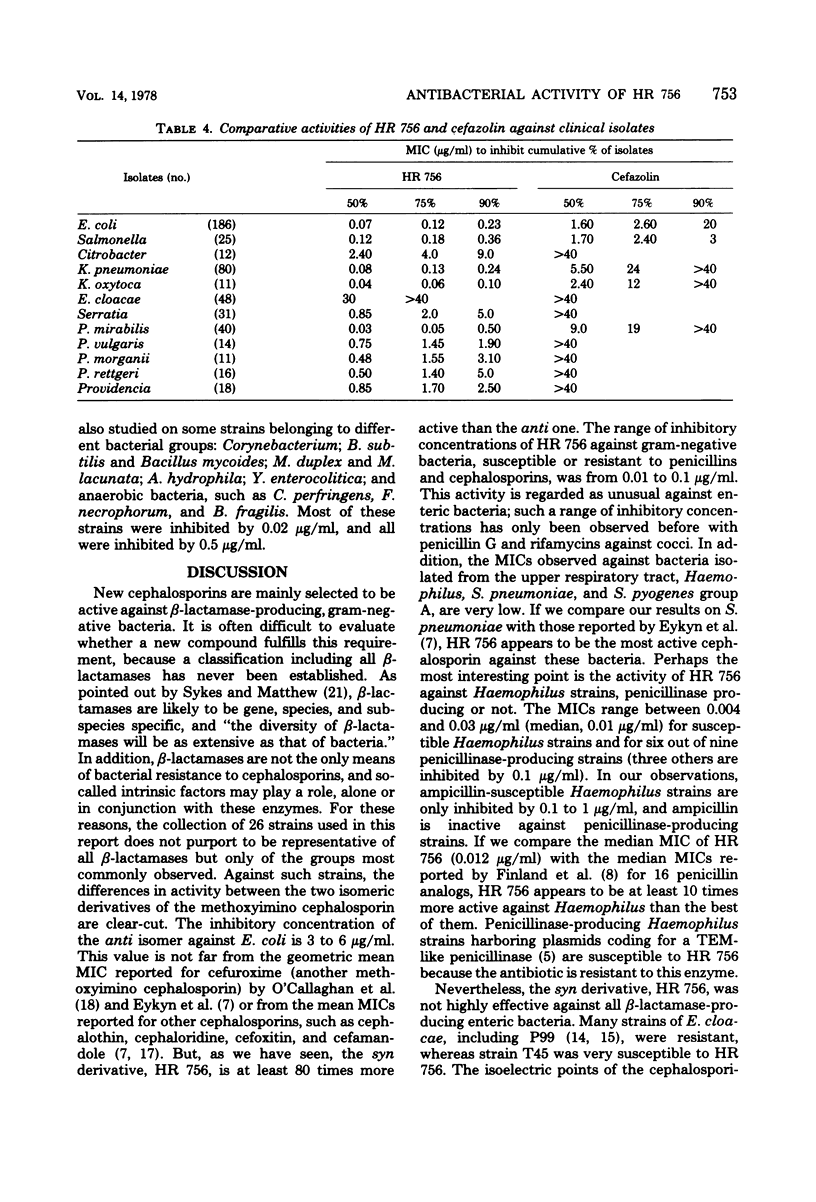
HR 756, the syn derivative of 7-[(2-(2-amino-4-thiazolyl)-2-methoxyimino)acetamido]cephalosporanic acid, is a new semisynthetic cephalosporin. It was 80 times more active than the anti derivative against β-lactamase-producing strains of gram-negative bacteria. The range of inhibitory concentrations of HR 756 against gram-negative bacteria, including Haemophilus influenzae, susceptible or resistant to penicillins and cephalosporins was from 0.01 to 0.1 μg/ml. This activity was consistently higher than those observed with cephalothin, cephaloridine, cephalexin, and cefazolin. Nevertheless, some strains of Enterobacter cloacae were resistant. HR 756 showed very similar activity to that of ampicillin against group A streptococci and Streptococcus pneumoniae.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bucourt R., Heymès R., Lutz A., Pénasse L., Perronnet J. Propriétés antibiotiques inattendues dans le domaine des céphalosphorines. C R Acad Sci Hebd Seances Acad Sci D. 1977 May 9;284(18):1847–1849. [PubMed] [Google Scholar]
- Burman L. G., Nordström K., Boman H. G. Resistance of Escherichia coli to penicillins. V. Physiological comparison of two isogenic strains, one with chromosomally and one with episomally mediated ampicillin resistance. J Bacteriol. 1968 Aug;96(2):438–446. doi: 10.1128/jb.96.2.438-446.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabbert Y. A., Scavizzi M. R., Witchitz J. L., Gerbaud G. R., Bouanchaud D. H. Incompatibility groups and the classification of fi - resistance factors. J Bacteriol. 1972 Nov;112(2):666–675. doi: 10.1128/jb.112.2.666-675.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. R-factor-mediated beta-lactamases that hydrolyze oxacillin: evidence for two distinct groups. J Bacteriol. 1974 Aug;119(2):351–356. doi: 10.1128/jb.119.2.351-356.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., De Graaff J., Seibert D., Falkow S. Plasmid-linked ampicillin resistance in haempohilus influenza type b. Infect Immun. 1975 Aug;12(2):404–410. doi: 10.1128/iai.12.2.404-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eykyn S., Jenkins C., King A., Phillips I. Antibacterial activity of cefuroxime, a new cephalosporin antibiotic, compared with that of cephaloridine, cephalothin, and cefamandole. Antimicrob Agents Chemother. 1976 Apr;9(4):690–695. doi: 10.1128/aac.9.4.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finland M., Garner C., Wilcox C., Sabath L. D. Susceptibility of pneumococci and Haemophilus influenzae to antibacterial agents. Antimicrob Agents Chemother. 1976 Feb;9(2):274–287. doi: 10.1128/aac.9.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labia R., Andrillon J., Le Goffic F. Computerized microacidimetric determination of beta lactamase Michaelis-Menten constants. FEBS Lett. 1973 Jun 15;33(1):42–44. doi: 10.1016/0014-5793(73)80154-1. [DOI] [PubMed] [Google Scholar]
- Labia R., Barthélémy M. Problèmes de la détermination des points isoélectriques des beta lactamases. C R Acad Sci Hebd Seances Acad Sci D. 1977 May 2;284(17):1729–1732. [PubMed] [Google Scholar]
- Labia R., Brunet G., Guionie M., Philippon A., Heitz M., Pitton J. S. Céphalosporinases constitutives de Escherichia coli. Ann Microbiol (Paris) 1976 Nov-Dec;127B(4):453–461. [PubMed] [Google Scholar]
- Labia R., Guionie M., Fabre C. Céphalosporinases inductibles de Proteus morganii. Biochimie. 1976 Nov 13;58(9):1083–1087. doi: 10.1016/s0300-9084(76)80086-7. [DOI] [PubMed] [Google Scholar]
- Labia R., Le Goffic F., Andrillon J. Etude cinétique de deux beta lactamases responsables d'un møeme phénotype. Biochimie. 1974;56(8):1025–1030. doi: 10.1016/s0300-9084(74)80092-1. [DOI] [PubMed] [Google Scholar]
- Marshall M. J., Ross G. W., Chanter K. V., Harris A. M. Comparison of the substrate specificities of the -lactamases from Klebsiella aerogenes 1082E and Enterobacter cloacae P99. Appl Microbiol. 1972 Apr;23(4):765–769. doi: 10.1128/am.23.4.765-769.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Winshell E. B. Relation of beta-lactamase activity and cellular location to resistance of Enterobacter to penicillins and cephalosporins. Antimicrob Agents Chemother. 1972 Feb;1(2):107–111. doi: 10.1128/aac.1.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Edlund T., Grundström T., Bergström S., Wolf-Watz H. Escherichia coli K-12 mutants hyperproducing chromosomal beta-lactamase by gene repetitions. J Bacteriol. 1977 Dec;132(3):912–922. doi: 10.1128/jb.132.3.912-922.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby R., Brorsson J. E., Seeberg S. Comparative study of the in vitro antibacterial activity of cefoxitin, cefuroxine, and cephaloridine. Antimicrob Agents Chemother. 1976 Mar;9(3):506–510. doi: 10.1128/aac.9.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Sykes R. B., Griffiths A., Thornton J. E. Cefuroxime, a new cephalosporin antibiotic: activity in vitro. Antimicrob Agents Chemother. 1976 Mar;9(3):511–519. doi: 10.1128/aac.9.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roupas A., Pitton J. S. R factor-mediated and chromosomal resistance to ampicillin in Escherichia coli. Antimicrob Agents Chemother. 1974 Feb;5(2):186–191. doi: 10.1128/aac.5.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]



