Abstract
The cytotoxic effect of pomolic acid (PA), a pentacyclic triterpene isolated from flowers of Osmanthus fragrans var. aurantiacus Makino, was investigated in SK-OV-3 human ovarian adenocarcinoma cells. PA dose-dependently inhibited the viability of SK-OV-3 cells. PA-induced apoptosis was further characterized by detection of cell surface annexin V and sub-G1 apoptotic cell populations. The number of cells immunostained with annexin V-fluorescein isothiocyanate (FITC) increased following treatment with PA. The sub-G1 cell populations also increased in PA-treated SK-OV-3 cells. PA induced the activation of caspase-8, -9 and -3, critical mediators of apoptosis signaling. PA decreased the mitochondrial transmembrane potential (ΔΨm), resulting in the activation of caspase-9. In addition, PA increased the expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis signaling-related death receptor 5 (DR5), mediating caspase-8-involved extrinsic pathway. Taken together, our results indicate that PA induces apoptosis in SK-OV-3 cells, which is mediated by the mitochondrial-mediated intrinsic and death receptor-induced extrinsic pathways.
Keywords: apoptosis, mitochondrial transmembrane potential (ΔΨm), death receptor, pomolic acid, SK-OV-3 cells
Introduction
Terpenes are widespread natural compounds involved in various biological activities, playing a significant role in human medicine. They include triterpenes, saponines and other triterpenoids, representing one of numerous classes of natural compounds. The main groups of triterpenes and saponines are represented by tetracyclic derivatives of protostane, cycloartane, dammarane, euphane and pentacyclic derivatives of ursane, gammacerane, lupane and hopane (1). Pentacyclic triterpenes are secondary metabolites that are based on a 30-carbon skeleton and synthesized in many plants by the cyclization of squalene (2). These are widely distributed in fruit peel, leaves and stem bark (3). It has been demonstrated that several triterpenes have the ability to induce cancer cell apoptosis and prevent oxidative stress, inflammation and hypertension (4,5).
Pomolic acid (PA) is a pentacyclic triterpene isolated from the flowers of Osmanthus fragrans var. aurantiacus Makino that are found in China, Japan and the southern portion of Korea. The flowers are used by the Chinese to give an aroma to tea or wine and in cosmetics for hair and skin (6,7). Also, dried flowers have neuroprotective, free radical scavenging and anti-oxidative effects (8). PA inhibited the growth of leukemia cell line HL-60 and induced mitochondria-dependent apoptotic cell death (9). PA has been shown to induce apoptosis by activating the caspase cascade through loss of the mitochondrial transmembrane potential (ΔΨm), independently of anti-apoptotic Bcl-2 expression in leukemia cells (9,10). PA is suggested to overcome multidrug resistance mediated by overexpression of anti-apoptotic Bcl-2 proteins (10,11). PA also showed anti-proliferative activities against human gastric adenocarcinoma (MK-1), human uterine carcinoma (HeLa) and murine melanoma (B16F10) cells (12). However, the apoptotic mechanisms of PA in human carcinoma cells were not investigated in detail. In our preliminary experiment on cytotoxic effects, PA reduced the viabilities of human ovarian adenocarcinoma SK-OV-3, human colon carcinoma HCT-116, human breast adenocarcinoma MCF-7, SK-BR-3 and human melanoma SK-MEL-5 cells. The greatest inhibition of PA was observed in SK-OV-3 cells (data not shown). To the best of our knowledge, the apoptotic effect of PA on SK-OV-3 cells has not yet been examined. Thus, in this study, we report the PA-induced apoptosis and its molecular mechanism in human ovarian adenocarcinoma SK-OV-3 cells.
Materials and methods
Ethical considerations
The study was approved by the Kyung Hee University Institutional Animal Care and Use Committee (Yongin, Korea).
Extraction and isolation of pomolic acid
The dried and powdered flowers (800 g) of Osmanthus fragrans var. aurantiacus Makino, were extracted with 80% aqueous methanol (MeOH) (3 liters x3). Extracts were partitioned with ethyl acetate (EtOAc; 3 liters x3) and H2O (3 liters). The soluble fraction of EtOAc extract (30 g) was subjected to SiO2 column (8x15 cm) chromatography (c.c.), eluted with n-hexane-EtOAc (5:1 → 1:1, 5 liters of each) and monitored using thin-layer chromatography (TLC) to produce 30 fractions (OSE-1 to OSE-30). Non-soluble precipitate (OSE-P, 2.69 g) of EtOAc extracts was applied to the octadecylsilica gel (ODS) c.c. and eluted with MeOH-H2O (4:1, 18 liters) to produce 8 fractions (OSE-P-1 to OSE-P-8). OSE-P-4 [Ve/Vt (elution volume/total volume) 0.27–0.38, 224 mg] was subjected to SiO2 c.c. and eluted with CHCl3-MeOH (25:1, 2.1 liters) to give 14 fractions (OSE-P-4-1 to OSE-P-4-14). The subfraction OSE-P-4-4 yielded 20 mg PA (Ve/Vt 0.21–0.28, ODS TLC Rf 0.21, MeOH-H2O=13:1).
Cell culture
Human ovarian adenocarcinoma SK-OV-3 cells were obtained from the Korea Cell Line Bank (KCLB, Seoul, Korea). Cells were grown at 37°C with 5% CO2 in RPMI-1640 medium with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin-streptomycin. All cell culture media and reagents were purchased from Thermo Scientific Hyclone (Waltham, MA, USA).
Cytotoxic assay
The cytotoxicity of PA was measured using an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma, St. Louis, MO, USA] colorimetric assay. Cells were seeded onto 96-well plates at a density of 1x104 cells/well in 100 μl RPMI-1640 supplemented with 10% (v/v) FBS. Following 24 h incubation at 37°, the cells were treated with serum-free RPMI-1640, containing various concentrations of PA. Following a further 24h incubation, 50 μl MTT [5 mg/ml in phosphate-buffered saline, (PBS)] was added to each well. The cells were incubated at 37°C for 2 h. Following removal of the medium, the cells were treated with 100 μl dimethyl sulfoxide (DMSO) for 5 min and then the optical density was measured using a microplate reader (Bio-Tek, Winooski, VT, USA) at 550 nm. Cell viability was calculated as a percentage of viable cells in the PA-treated group (5, 15, 25 and 50 μM) vs. a control group using the following equation: Cell viability (%) = [(ODCompound - ODBlank)/(ODContol - ODBlank)] × 100.
Annexin V assay
Modulation of phosphatidylserine externalization during apoptosis was identified using annexin V conjugated with the fluorescent dye FITC. SK-OV-3 cells were seeded onto 6-well plates at a density of 3x105 cells/well. Following treatment with 25 μM PA for 6 h, the cells were stained with annexin V-FITC conjugate and then imaged under x40 objective magnification using a confocal laser scanning microscope (LSM 510 Meta, Carl Zeiss, Oberkochen, Germany).
Cell cycle analysis
SK-OV-3 cells were seeded onto 6-well plates at a density of 3x105 cells/well. Following dose-dependent treatment with PA for various times up to 12 h, cells were collected and washed twice with ice-cold PBS. Cell pellets were fixed in 70% (v/v) cold ethanol overnight at −20°C. Fixed cells were centrifuged, washed and resuspended in 100 μl PBS, then mixed with 100 μl RNase A (1 mg/ml, Sigma) and incubated for 30 min at 37°. The cells were stained by adding 400 μl propidium iodide (PI; 50 μg/ml, Sigma). After filtering through a nylon mesh (40 μm), the DNA content of stained cells was analyzed using the FACSVantage SE and CellQuest program (BD Biosciences, San Jose, CA, USA).
Mitochondrial membrane potential (ΔΨm) assay
SK-OV-3 cells treated with 25 μM PA for 3 or 6 h were labeled for 30 min with 0.1 μM tetramethylrhodamine ethyl ester (TMRE; Sigma), a cationic, lipophilic dye that accumulates in the negatively charged mitochondrial matrix and collected by trypsinization. The fluorescence intensity was monitored at 582 nm (FL2 channel) by FACSVantage SE (BD Biosciences).
Real-time polymerase chain reaction (PCR) analysis
Total RNA was isolated using an RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. DNase I (Takara Bio Inc., Shiga, Japan)-treated total RNA was transcribed into cDNA using an ImProm-II Reverse Transcription System (Promega, Madison, WI, USA). Quantitative real-time PCR was performed using the ABI prism 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA). The reaction was carried out in a total volume of 20 μl containing 10 μl 2X SYBR Premix EX Taq™ (Takara Bio Inc.), 1 μl each oligonucleotide primer (10 μM) and 1 μl DNA. The sequences of the sense and antisense primers for death receptor (DR) 5, DR4 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were as follows: DR5 sense: 5′-TGC ACC ACG ACC AGA AAC AC-3′; DR5 antisense: 5′-ATC ACC GAC CTT GAC CAT CC-3′; DR4 sense: 5′-AGG GTC TCA GAG GAG GAG GC-3′; DR4 antisense: 5′-GGA GTC AAA GGG CAC GAT GT-3′; GAPDH sense: 5′-AGG AGG CAT TGC TGA TGA TC-3′; GAPDH antisense: 5′-AGT GAG GGT CTC TCT CTT CC-3′. Following completion of PCR, the data were analyzed using ABI prism 7000 sequence detection system software. The expression of mRNA was normalized with GAPDH.
Western blot analysis
After seeding onto 6-well plates at a density of 3x105 cells/well, cells were treated with 25 μM PA for various time periods up to 12 h, then lysed with RIPA buffer (Thermo Fisher Scientific Inc., Rockford, IL, USA) supplemented with a protease inhibitor cocktail (Roche, Basel, Switzerland). Protein concentrations were determined using an RC/DC Bio-Rad assay kit (Bio-Rad, Hercules, CA, USA) following the manufacturer’s instructions. Protein samples were separated using electrophoresis on 10–15% polyacrylamide-SDS gel. The electrophoresized proteins on the gel were transferred onto a polyvinylidene fluoride (PVDF) membrane (Pall Life Sciences, Port Washington, NY, USA), blocked with 5% (w/v) skimmed milk (BD Biosciences), incubated with anti-human cleaved caspase-3 and -8, anti-human caspase-9 (Cell Signaling Technology Inc., Danvers, MA, USA), anti-human DR4 and DR5 (Imgenex, San Diego, CA, USA), anti-human PARP and anti-human α-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA, USA), then probed with horseradish peroxidase-conjugated anti-rabbit or mouse IgG antibody (GE Healthcare Life Sciences, Stockholm, Sweden). The membrane was detected using an enhanced chemiluminescent western blotting detection system (GE Healthcare Life Sciences).
Statistical analysis
All data are presented as mean ± standard deviation (SD). Student’s t-test was used to compare different data groups. P<0.05, P<0.01 and P<0.001 were considered to indicate statistically significant differences.
Results
PA demonstrates dose-dependent cytotoxic effects
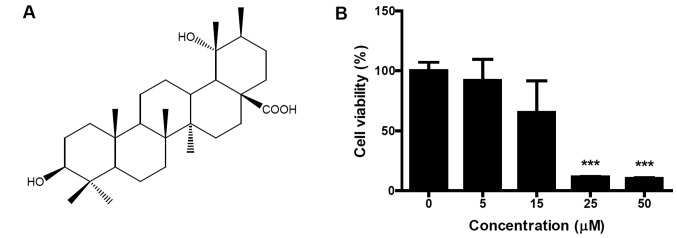
PA was isolated from flowers of Osmanthus fragrans var. aurantiacus Makino (Fig. 1A). Identification of the structure was confirmed on the basis of several spectroscopic analyses, including infrared (IR), 1H- and 13C-nuclear magnetic resonance (NMR) and 2D-NMR (correlation spectroscopy, COSY; heteronuclear single quantum coherence, HSQC and heteronuclear multiple-bond correlation spectroscopy, HMBC) (data not shown). To evaluate the cytotoxic effect of PA on SK-OV-3 cells, cells were treated with different concentrations (5, 15, 25 and 50 μM) of PA for 24 h and cell viabilities were measured using an MTT assay. PA dose-dependently inhibited the viability of SK-OV-3 cells (Fig. 1B). When 50 μM PA was treated for 24 h, the viability of SK-OV-3 cells decreased to 10.3% of that for untreated control cells. IC50 (50% inhibitory concentration) value occurred between 15 and 25 μM.
Figure 1.
Structure of pomolic acid (PA) and effect of PA on the viability of human ovarian adenocarcinoma SK-OV-3 cells. (A) Structure of PA. (B) PA dose-dependently decreased the viability of SK-OV-3 cells. Cells were treated with different concentrations (0, 5, 15, 25, 50 μM) of PA. Following 24 h of incubation, cell viability was assessed using an MTT assay. Data are presented as mean ± standard deviation (SD). ***P<0.001, between treated and control cells.
PA induces apoptosis in SK-OV-3 cells
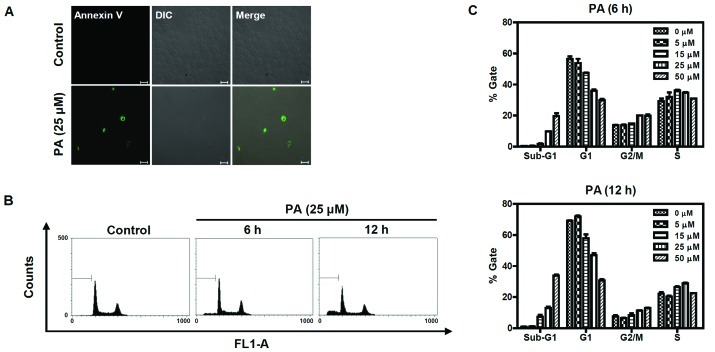
To determine whether the cytotoxic effect of PA was caused by apoptosis, PA-treated SK-OV-3 cells were immunostained with annexin V-FITC conjugate and monitored under a confocal microscope (Fig. 2A). Annexin V-FITC-stained cells were observed in PA-treated cells, but not in an untreated control group. Cell cycle analysis was performed to determine the sub-G1 apoptotic population of PA-treated SK-OV-3 cells. Cells were treated with different concentrations (5, 15, 25 and 50 μM) of PA for 6 or 12 h and their DNA contents were analyzed using flow cytometry following propidium iodide (PI) staining. As shown in Fig. 2B and C, the number of cells in the sub-G1 population increased in a time- and dose-dependent manner. Following 6 h incubation with 5, 15, 25 and 50 μM PA, the sub-G1 populations increased to 0.6, 1.8, 9.8 and 19.8%, respectively. The sub-G1 populations of cells treated with 5, 15, 25 and 50 μM PA for 12 h increased to 1.01, 7.41, 13.01 and 33.93%, respectively. Taken together, these results indicate that PA induces apoptosis in SK-OV-3 cells.
Figure 2.
Annexin V and flow cytometric analysis of PA-treated SK-OV-3 cells. (A) PA increased apoptotic cells immunostained with annexin V-FITC. Cells were treated with 25 μM PA for 6 h and stained with annexin V-FITC, then imaged under x40 objective magnification using a confocal microscope (bar=20 μm). DIC represents the differential interference contrast. (B) PA induced apoptosis in a dose and time-dependent manner. Cells were treated with different concentrations (0, 5, 15, 25 and 50 μM) of PA for 6 h or 12 h, then sub-G1 populations were measured using a flow cytometer after staining with PI. The sub-G1 populations of cells treated 25 μM of PA for 6 h or 12 h are represented as a diagram. FL1-A indicates fluorescence 1 area for the measurement of the PI fluorescence intensity (excitation, 488 nm; emission, 530 nm). (C) Three independent experiments of (B) were performed and are represented as a bar diagram. PA, pomolic acid; SK-OV-3 cells, human ovarian adenocarcinoma cells; PI, propidium iodide.
PA induces the activation of the caspase cascade
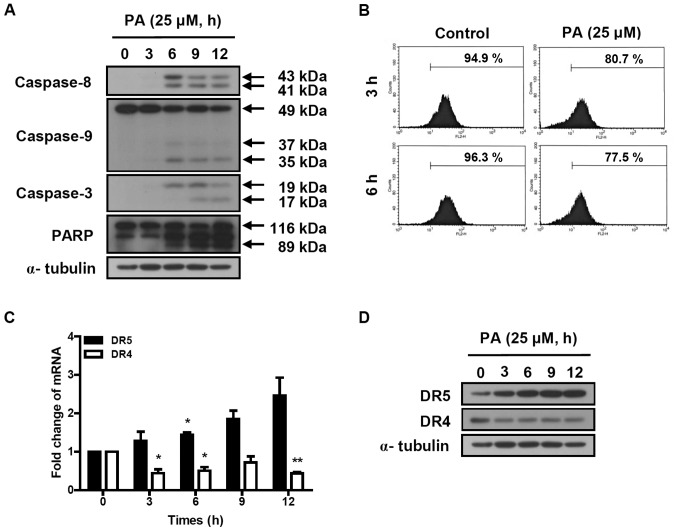
The activation of the caspase cascade was determined in SK-OV-3 cells treated with 25 μM PA for indicated times (3, 6, 9 and 12 h). Cleaved caspase-8 (41 and 43 kDa) and caspase-3 (17 and 19 kDa) were increased by the treatment of PA (Fig. 3A). PA induced the reduction of procaspase-9 (49 kDa) and increased the cleaved caspase-9 (35 and 37 kDa). PA also induced the cleavage of poly ADP-ribose polymerase (PARP). These results indicate that PA induces apoptosis in SK-OV-3 cells via the activation of the caspase cascade.
Figure 3.
Effect of PA on caspase activation cascade and TRAIL signaling-related death receptors DR4 and DR5. (A and D) Protein extracts were prepared from 25 µM PA-treated cells. The cleavage of caspase-8, -3, -9 and PARP (A) and the amounts of DR4 and DR5 proteins (D) were determined using western blot analysis. α-tubulin was used as a control. (B) Cells were treated with 25 μM PA for the indicated times. Flow cytometry was performed to determine the loss of mitochondrial transmembrane potential (ΔΨm). The percentages of cells showing fluorescence intensities up to 10 are represented in the diagram. (C) Real-time PCR was performed to determine the transcript levels of DR5 and DR4. The expression of the mRNA was normalized with GAPDH. Data are presented as mean ± standard deviation (SD). *P<0.05 and **P<0.01 were considered to indicate statistically significant differences between treatment and control groups. PA, pomolic acid; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; PARP, poly ADP ribose polymerase; PCR, polymerase chain reaction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
PA reduces the mitochondrial transmembrane potential (ΔΨm) and increased the expression of DR5 in SK-OV-3 cells
To determine the effect of PA on mitochondrial transmembrane potential (ΔΨm), SK-OV-3 cells were treated with 25 μM PA for 3 or 6 h and labeled with TMRE. The fluorescence intensity was measured using flow cytometry. The number of TMRE-stained cells showing fluorescence intensities up to 10 were 80.7 and 77.5% following 3 and 6 h incubation of 25 μM PA, respectively (Fig. 3B). This means that the treatment of 25 μM PA for 3 and 6 h reduced the ΔΨm by 15 and 19.5%, respectively, compared to PA-nontreated cells. This indicates that PA induces the loss of mitochondrial transmembrane potential, resulting in the activation of caspase-9.
To investigate the effect of PA on death receptor-induced extrinsic apoptosis pathway, the expression levels of DR4 and DR5 were analyzed using real-time PCR and western blot analysis. As shown in Fig. 3C, DR5 transcript was time-dependently increased in PA-treated SK-OV-3 cells, resulting in 1.3, 1.7, 2.3 and 2.5-fold increases following 3, 6, 9 and 12 h incubation with 25 μM PA, respectively. The protein levels of DR5, based on densitometry (Fig. 3D), increased 3.5, 5.1, 6 and 6.1-fold, respectively, following 3, 6, 9 and 12 h incubation with 25 μM PA. However, DR4 transcript and protein levels decreased in PA-treated SK-OV-3 cells. This indicates that PA increases expression of DR5, resulting in the activation of caspase-8, a caspase cascade mediator of the death receptor-induced extrinsic apoptosis pathway.
Discussion
PA inhibited the growth of leukemia HL-60 cells (9) and showed anti-proliferative activities in several human carcinoma cells including human gastric adenocarcinoma (MK-1), human uterine carcinoma (HeLa) and murine melanoma (B16F10) cells (12). Although mitochondria-dependent apoptotic cell death was evaluated in leukemia HL-60 cells, PA-induced apoptotic mechanisms of human carcinoma cells were not investigated in detail. In a preliminary experiment, we determined the cytotoxic effect of PA on the viabilities of human carcinoma cells. The greatest inhibition of PA was observed in SK-OV-3 cells (data not shown). We chose SK-OV-3 cells to investigate PA-induced apoptotic mechanisms of human carcinoma cells. In this study, we examined the effect of PA on the viability of human ovarian adenocarcinoma SK-OV-3 cells and investigated the mechanisms involved in PA-induced apoptosis. PA dose-dependently inhibited the viability of SK-OV-3 cells (Fig. 1B) and induced apoptosis, which was characterized by detection of cell surface annexin V and sub-G1 apoptotic cell populations (Fig. 2).
The caspase-3 activation cascade played a central role in apoptotic mechanisms (13) as determined in PA-treated SK-OV-3 cells. Cleaved caspase-8, -9 and -3 levels increased following treatment with PA (Fig. 3A). Our findings indicate that PA activates two major apoptotic pathways, intrinsic and extrinsic, as is the case for other reported triterpenoids, to induce apoptosis through mitochondria-mediated intrinsic and death receptor-induced extrinsic pathways (14,15). PA has been reported to induce apoptosis by activation of caspase-9 and -3 through the loss of mitochondrial transmembrane potential (ΔΨm) in leukemia cells (9). In SK-OV-3 cells, PA was also found to reduce the mitochondrial transmembrane potential (ΔΨm) (Fig. 3B). This implies that PA induces apoptosis in SK-OV-3 cells via a mitochondria-mediated intrinsic pathway.
In addition, PA induced activation of caspase-8, a caspase cascade mediator of the death receptor-induced extrinsic pathway. This indicates that PA induces apoptosis through the death receptor-induced extrinsic pathway. Recently, triterpenoids such as celastrol and ursolic acid have been suggested to potentiate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through upregulation of DR4 and/or DR5 (16,17). In our previous study, 3-O-acetyloleanolic acid was also shown to induce apoptosis in HCT-116 cells, which is mediated by upregulation of DR5 (18). TRAIL is a TNF family member showing anti-tumor activity in various cancer cell types as well as minimal cytotoxicity to normal cells and tissues (19). The binding between TRAIL and the two death receptors DR4 (TRAILR1/APO-2) and DR5 (TRAILR2/KILLER) leads to oligomerization of the death receptors and activation of the death receptor-induced extrinsic pathway (20). DR5 and DR4 signaling induces apoptosis via caspase-8-mediated activation of the caspase cascade (21,22). PA increased the expression of DR5, increasing induction of the caspase-8-mediated extrinsic pathway. To the best of our knowledge, this is the first report showing that PA induces apoptosis in SK-OV-3 cells as well as upregulation of TRAIL signaling-related DR5 in PA-mediated apoptosis.
In conclusion, our results demonstrated for the first time PA-induced apoptotic mechanisms in human ovarian adenocarcinoma SK-OV-3 cells. Our findings suggest that PA increases expression of TRAIL signaling-related death receptor DR5 and induces apoptosis in SK-OV-3 cells via the mitochondria-mediated intrinsic and the death receptor-induced extrinsic pathway.
Acknowledgments
This study was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012001575) and from Kyung Hee University in 2010 (KHU-20110257).
References
- 1.Patocka J. Biologically active pentacyclic triterpenes and their current medicine signification. J Appl Biomed. 2003;1:7–12. [Google Scholar]
- 2.Phillips DR, Rasbery JM, Bartel B, Matsuda SP. Biosynthetic diversity in plant triterpene cyclization. Curr Opin Plant Biol. 2006;9:305–314. doi: 10.1016/j.pbi.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Jager S, Trojan H, Kopp T, Laszczyk MN, Scheffler A. Pentacyclic triterpene distribution in various plants - rich sources for a new group of multi-potent plant extracts. Molecules. 2009;14:2016–2031. doi: 10.3390/molecules14062016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baek JH, Lee YS, Kang CM, Kim JA, Kwon KS, Son HC, Kim KW. Intracellular Ca2+ release mediates ursolic acid-induced apoptosis in human leukemic HL-60 cells. Int J Cancer. 1997;73:725–728. doi: 10.1002/(sici)1097-0215(19971127)73:5<725::aid-ijc19>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi Y, Yamada K, Yoshikawa N, Nakamura K, Haginaka J, Kunitomo M. Corosolic acid prevents oxidative stress, inflammation and hypertension in SHR/NDmcr-cp rats, a model of metabolic syndrome. Life Sci. 2006;79:2474–2479. doi: 10.1016/j.lfs.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Duke JA, Ayensu ES. Medicinal Plants of China. Vol. 2. Reference Publications Inc; Algonac, MI, USA: 1985. p. 381. [Google Scholar]
- 7.Hedrick UP. Sturtevant’s Edible Plants of the World. Dover Publications; Mineola, NY, USA: 1972. p. 686. [Google Scholar]
- 8.Lee HH, Lin CT, Yang LL. Neuroprotection and free radical scavenging effects of Osmanthus fragrans. J Biomed Sci. 2007;14:819–827. doi: 10.1007/s11373-007-9179-x. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes J, Weinlich R, Castilho RO, Kaplan MAC, Amarante-Mendes GP, Gattass CR. Pomolic acid triggers mitochondria dependent apoptotic cell death in leukemia cell line. Cancer Lett. 2005;219:49–55. doi: 10.1016/j.canlet.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes J, Weinlich R, Castilho RO, Amarante-Mendes P, Gattass CR. Pomolic acid may overcome multidrug resistance mediated by overexpression of anti-apoptotic Bcl-2 proteins. Cancer Lett. 2007;245:315–320. doi: 10.1016/j.canlet.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Vasconcelos FC, Gattass RR, Rumjanek VM, Maia RC. Pomolic acid-induced apoptosis in cells from patients with chronic myeloid leukemia exhibiting different drug resistance profile. Invest New Drugs. 2007;25:525–533. doi: 10.1007/s10637-007-9064-5. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida M, Fuchigami M, Nagao TT, et al. Antiproliferative constituents from umbelliferase plants VII. Active triterpenes and rosmarinic acid from Centella asiatica. Biol Pharm Bull. 2005;28:173–175. doi: 10.1248/bpb.28.173. [DOI] [PubMed] [Google Scholar]
- 13.Zheng TS, Schlosser SF, Dao T, Hingorani R, Crispe IN, Boyer JL, Flavell RA. Caspase-3 controls both cytoplasmic and nuclear events associated with Fas-mediated apoptosis in vivo. Proc Natl Acad Sci USA. 1998;95:13618–13623. doi: 10.1073/pnas.95.23.13618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 15.Lee B, Lee DY, Yoo KH, Baek NI, Park JH, Chung IS. Calenduloside E 6’-methyl ester induces apoptosis in CT-26 mouse colon carcinoma cells and inhibits tumor growth in a CT-26 xenograft animal model. Oncol Lett. 2012;4:22–28. doi: 10.3892/ol.2012.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sung B, Park B, Yadav VR, Aggarwal BB. Celastrol, a triterpene, enhances TRAIL-induced apoptosis through the down-regulation of cell survival proteins and up-regulation of death receptors. J Biol Chem. 2010;285:11498–11507. doi: 10.1074/jbc.M109.090209. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Prasad S, Yadav VR, Kannappan R, Aggarwal BB. Ursolic acid, a pentacyclin triterpene, potentiates TRAIL-induced apoptosis through p53-independnet up-regulation of death receptors. J Biol Chem. 2011;286:5546–5557. doi: 10.1074/jbc.M110.183699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Yoo KH, Park JH, Cui EJ, et al. 3-O-Acetyloleanolic acid induces apoptosis in human colon carcinoma HCT-116 cells. Phytother Res. 2012 doi: 10.1002/ptr.4616. [DOI] [PubMed] [Google Scholar]
- 19.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Therapy. 2005;12:228–237. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 21.Ashkenazi A, Dixit VM. Death receptors: Signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 22.Wajant H, Gerspach J, Pfizenmaier K. Tumor therapeutics by design: targeting and activation of death receptors. Cytokine Growth Factor Rev. 2005;16:55–76. doi: 10.1016/j.cytogfr.2004.12.001. [DOI] [PubMed] [Google Scholar]