Abstract
Recombinant scfv antibodies specific for CYP1A1 and CYP1B1 P450 enzymes were combined with targeted imaging mass spectrometry to simultaneously detect the P450 enzymes present in archived, paraffin-embedded, human breast cancer tissue sections. By using CYP1A1 and CYP1B1 specific scfv, each coupled to a unique reporter molecule (i.e., a mass tag) it was possible to simultaneously detect multiple antigens within a single tissue sample with high sensitivity and specificity using mass spectrometry. The capability of imaging multiple antigens at the same time is a significant advance that overcomes technical barriers encountered when using present day approaches to develop assays that can simultaneously detect more than a single antigen in the same tissue sample.
Keywords: Targeted multiplex imaging mass spectrometry, Laser desorption ionization mass spectrometry, Imaging mass spectrometry, Single chain fragment variable recombinant antibodies, Immunohistochemistry
Introduction
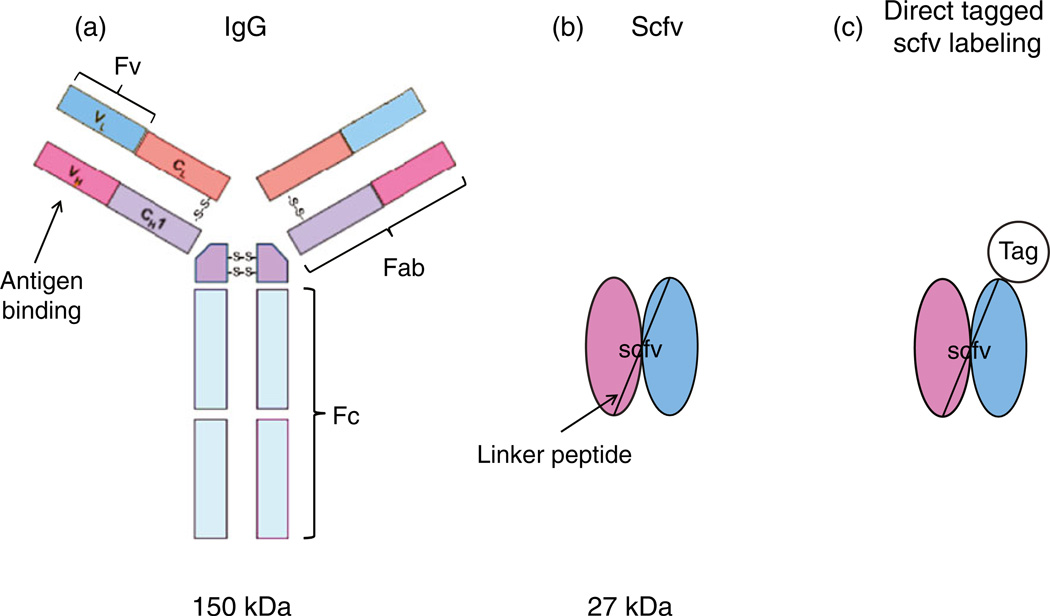
Antibodies are commonly used in clinical studies for both diagnostic and therapeutic applications [1–4]. Single chain fragment variable (scfv) antibodies are small heterodimers composed of the antibody variable heavy-chain (VH) and variable light-chain (VL) domains joined together by a linker peptide (Figure 1) [5, 6]. They represent the smallest functional VH-VL domains of the antibody necessary for high affinity antigen binding [7]. Antigen-specific scfv can be genetically engineered as fusion proteins displayed on the end of a phage or bacterial virus. The phage can infect microbes such as E. coli to produce large quantities of scfv for use in a variety of applications (e.g., enzyme-linked immunosorbent assay (ELISA), Western blot, immunohistochemistry, etc.). Large libraries of phage-displayed scfv antibodies have been created and used to select for antigen-specific recombinant antibodies. The main advantages in using phage displayed scfv libraries are (1) antibody genes have been cloned into microbes so animals are not needed to obtain antigen-specific antibodies, (2) antigen-specific scfv can be readily selected to poorly immunogenic molecules, (3) scfv can be easily conjugated with biotin, dyes or mass tags, and (4) a large number of different antigen-specific scfv can be conjugated with different reporter molecules (e.g., biotin, fluorescent dyes, etc.) and used in a multiplex assay format to simultaneously detect different antigens in a single sample. These advantages have already been explored in other studies [8–11].
Figure 1.
Schematic of (a) a typical 150 kDa IgG, (b) an ~27 kDa single chain (scfv) antibody, and (c) an scfv directly labeled with a mass tag
Estrogens have been identified as key risk factors for breast cancer. The cytochrome P450 enzymes designated CYP1A1 and CYP1B1 are enzymes that metabolize the estrogen known as estradiol. Metabolized estradiol may play a role in breast cancer development and therapy in that its metabolites can lead to the formation of DNA adducts and subsequent mutational events [12]. Additionally, CYP1B1 is also up-regulated in other forms of cancer such as colon, esophageal, lung, ovarian cancers and soft-tissue carcinomas [13] and may serve as a biomarker for tumor development. Although both CYP1A1 and CYP1B1 are involved in similar activities, their location and potential sites for biological activity with respect to one another in breast cancer cells is not known.
Targeted multiplex mass spectrometry imaging (TAMSIM) has been used to specifically detect antigens in situ [14, 15]. The combination of immunohistochemical procedures with Matrix-Assisted Laser Desorption Ionization (MALDI)-imaging mass spectrometry (IMS) [16] provides a method that is exogenous matrix free to simultaneously image multiple antigens using different antigen-specific antibodies, each coupled to a unique mass tag. The mass tags on antibodies bound to different antigens on a tissue section are released from the section by laser desorption ionization (LDI). After tissue scanning, mass spectrometry images are created using the masses of each tag as a surrogate marker for the specific antigens. In this study, we describe the use of antigen-specific monoclonal and recombinant scfv antibodies and IMS to detect CYP1A1 and CYP1B1 in breast cancer tissue section. Results suggest that both antibody formats can be used for IMS to detect antigens in tissue sections.
Materials and Methods
Purified recombinant CYP1A1 and CYP1B1 were used to obtain antigen-specific hybridoma monoclonal and scfv antibodies. Hybridoma monoclonal antibodies were made using CYP1B1 immunized female Balb/c mice as a source of B-cells. Cell fusions and assays to detect CYP1B1 specific monoclonal antibodies were carried out according to modifications of previously published protocols [17]. Anti-CYP1B1 monoclonal antibodies were purified from serum-free tissue culture medium using protein A/G conjugated to sepharose beads.
For phage display, spleens from outbred, newborn and 3–4 wk-old mice and rats were used to create the large (~2.9 billion member) interspecies (mouse × rat), naive phage antibody library to obtain antigen-specific scfv. Recombinant scfv expressed using the phage antibody library bear a tag known as the E-tag. Recombinant E-tagged scfv were detected in assays using an anti-E tag monoclonal antibody conjugated to peroxidase [(Anti-E/HRP) cat. no. 27-9413 G.E. Healthcare, Piscataway, NJ, USA] and affinity purified using an anti-E tag monoclonal antibody column (cat. no. 17-1362-01 G.E. Healthcare) according to product protocols, with some modification. Phage selection, scfv detection, characterization, purification, conjugation [i.e., biotinylation] and use in assays have been described for the anti-CYP1B1 scfv (designated D23/I20) [18]. A similar approach was also used to obtain the anti-CYP1A1 scfv (designated P1).
The production, detection, characterization and use of the anti-rabbit IgG scfv (designated A10B) and anti-CYP1B1 scfv (designated D23) have been described [10, 18]. Since the A10B scfv has been well characterized, is easily expressed and purified, and binds to an inexpensive and readily available antigen (i.e., rabbit IgG), it was initially used as a model system to develop and optimize mass tag labeling and assay conditions.
Enzyme-linked immunosorbent assays (ELISAs) were used to determine biotinylated and mass tag conjugated scfv or avidin binding activity prior to use for IMS. All scfv bear an 11 amino acid peptide sequence recognized by the anti-E tag monoclonal antibody (McAb). The anti-E tag monoclonal antibody conjugated to peroxidase, a peroxidase substrate (H2O2) and color developer [e.g., (ABTS) = 2,2'-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)] were used to detect E-tagged scfv bound to antigens in assays.
Reporter molecules (e.g., biotin, dye, or mass tag) when conjugated directly to antibodies (e.g., scfv) can adversely affect antibody binding activity and specificity. This scenario becomes more probable as the number of reporter molecules used to label an antibody is increased to enhance assay sensitivity. To ascertain the influence of mass tag labeling on assay specificity, scfv were directly conjugated to mass tags and assayed by an ELISA to determine binding activity to positive and negative control antigens. To enhance assay sensitivity, scfv can be labeled with just 1–2 biotins to retain antigen-binding specificity. Biotinylated scfv bound to an antigen in assays can then be indirectly detected using avidin that is conjugated with a large number of reporter molecules or mass tags. Two approaches were followed to develop scfv for IMS. In the first or direct approach, scfv were directly labeled with mass tags at low to high mass tag:scfv molar ratios, then used in assays to detect antigen. In the second or indirect approach, scfv were labeled with biotin at low biotin:scfv ratios, then indirectly detected using avidin labeled with mass tags at low to high mass tag:avidin ratios.
Conjugation of scfv and Monoclonal Antibodies to Biotin and Avidin to Mass Tags
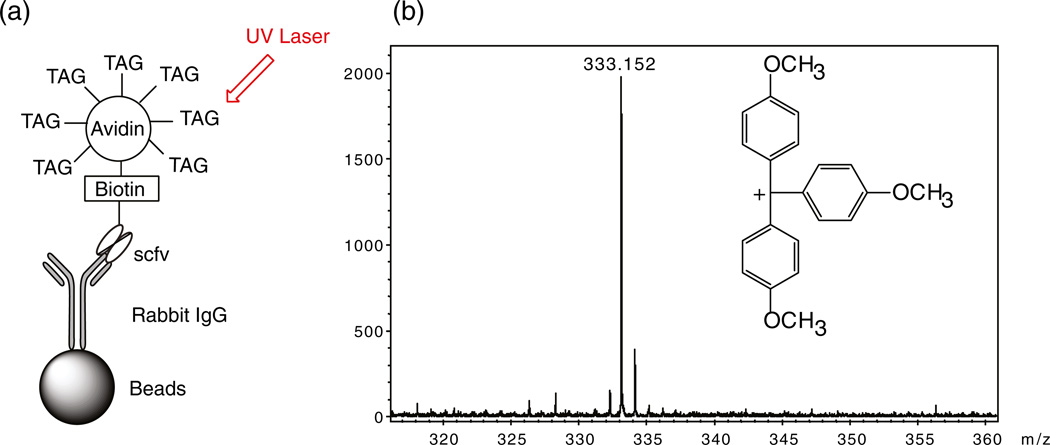
Purified scfv and avidin (cat. no. A9275 Sigma St. Louis, Mo, USA) in phosphate buffered saline (PBS) were conjugated to mass tags (Figures 1 and 2) via their free amines at different (1:1; 2:1; 4:1; 8:1; 16:1; 32:1; 64:1) mass tag:scfv or avidin molar ratios. Unincorporated mass tags were removed from avidin by size exclusion chromatography. Aliquots of the same scfv and an anti-CYP1B1 monoclonal antibody (McAb) were labeled with biotin (biotinamidocaproate-NHS; cat. no. B2643 Sigma) at a 2:1 biotin:antibody molar ratio. An ELISA was used to determine mass-tagged scfv antigen-binding specificity. Purified recombinant CYP1A1, CYP1B1, or rabbit IgG were used, respectively as positive control antigens for anti-CYP1A1, CYP1B1, or A10B antibodies. Commercially available BSA was used as a negative control antigen. For multiplex assays, each biotinylated scfv specific for a different antigen was coupled to avidin (conjugated to a unique mass tag) to form an immune complex (IC). A cocktail of ICs specific for different antigens was applied to tissue in a multiplex assay format to detect different antigens in the same sample.
Figure 2.
(a) Schematic of an assay used to detect mass-tagged avidin bound to biotinylated A10B scfv on rabbit IgG coated beads. (b) The mass spectrum for the mass tag
ELISAs to Detect Antigen-Bound scfv Directly or Indirectly Labeled with Mass Tags
Costar 2595 microtiter plates were coated with either rabbit IgG (positive control antigen) or BSA (negative control antigen) diluted to 10 µg/mL PBS, 25 µL/well for 1 h at room temperature. Wells were emptied and filled with PBS containing 0.1 % Tween 20 (PBS-T) for 15 or more min at room temperature to prevent nonspecific binding of scfv or avidin to microtiter wells. A10B scfv labeled directly with mass tags, biotinylated A10B scfv (only), and biotinylated A10B scfv premixed with mass-tagged conjugated avidin were added to rabbit IgG and BSA-coated wells, incubated for 1 h at room temperature, then microtiter wells washed with PBS-T. For wells in which biotinylated A10B scfv (only) was added, mass tags conjugated to avidin were added, incubated for 1 h at room temperature, then washed with PBS-T to remove unbound mass-tagged conjugated avidin. Scfv bound to rabbit IgG and BSA in microtiter plates were detected using Anti-E/HRP, H2O2 and ABTS. Absorbance (405 nm) readings for microtiter wells were determined using a BioTek ElxNB800 plate reader.
ELISAs to Indirectly Detect Antigen-Bound, Biotinylated scfv Using Mass-Tagged Avidin
Biotinylated scfv in combination with avidin, streptavidin, or neutravidin conjugated to reporter molecules (e.g., dyes and enzymes) can be used to specifically detect antigens in assays [18]. Avidin was conjugated directly to mass tags at 1:1, 2:1, 4:1, 8:1, 16:1, 32:1, and 64:1 mass tags:avidin ratio. Two types of biotin scfv and avidin-mass tag immunoassays were carried out, one-step assay and two-step assay.
Immune Complex (IC) Assay
One step assay: Biotinylated A10B scfv was pre-mixed with mass-tagged avidin at a ratio of 1 biotinylated scfv: 1 mass-tagged avidin for 20 minutes at room temperature to form an IC. The IC was then applied to rabbit IgG and BSA coated wells as described previously, incubated for 1 h at room temperature, then wells washed using PBS-T. Two-step assay: In a more traditional approach, biotinylated A10B scfv was added to antigen-coated wells, incubated for 1 h at room temperature, then wells washed with PBS-T. Mass-tagged avidin was then added to antigen-coated wells, incubated for 1 h and wells washed again. Anti-E/HRP diluted 1:8,000 in PBS-T was then added to detect antigen-bound E-tagged A10B scfv plus mass-tagged avidin in both ICs, one-step and two-step assays. Wells were washed with PBS-T to remove unbound Anti-E/HRP. Twenty-five µL of H2O2/ABTS was added to each well for color development. Plates were then read as described previously.
IMS Using Mass Tag Conjugated scfv
Six µm thick, paraffin-embedded breast cancer tissue sections were cut and mounted on glass slides. Sections were deparaffinized with xylene and rehydrated with ethanol (100 %–70 %–50 %) then water. Sections were incubated with 10 mM citrate buffer to unmask antigen epitopes, then blocked with PBS-containing 0.1 % Tween 20 (PBS-T) to prevent nonspecific antibody binding. Sections were incubated with mass tag directly conjugated to scfv antibody at different concentration values 2, 5, 10, 20 µg/mL for 1 h at room temperature, washed with PBS-T and water, dried then visualized using LDI MS.
Enzyme and Mass Tag-Antibody Conjugate Methods
Deparaffinized and rehydrated sections were incubated with 10 mM citrate buffer to unmask antigen epitopes as described above. Endogenous peroxidase activity in tissue sections was quenched with PBS containing 3 % H2O2 and 30 % methanol. Tissue sections were blocked with PBS-T, then incubated with 5 µg/mL of biotinylated D-23 scfv or anti-CYP1B1 IgG monoclonal antibody for 1 h at room temperature, washed with PBS-T, then probed with streptavidin conjugated to either alkaline phosphatase or peroxidase. D23 scfv and anti-CYP1B1 IgG monoclonal antibody-binding activities were detected with either the alkaline phosphatase chromogen Fast red, the peroxidase chromogen DAB or 5 µg/mL biotinylated scfv coupled to mass-tagged avidin.
Mass Tag Compound
Trityl mass tag was synthesized from Tris(4-methoxyphenyl)-methanol (Catalog No. B24516, Alfa Aesar, Ward Hill, MA, USA) and N-hydroxysuccinimide (Sigma). Trityl mass tag was synthesized as N-Hydroxysuccinimide ester (Catalog No. 130672, Sigma-Aldrich) that binds to proteins (e.g., scfv, avidin) via free amines under neutral pH buffer conditions [19].
Mass Spectrometry Analysis
A MALDI TOF/TOF mass spectrometer (UltrafleXtreme; Bruker Daltonik GmbH, Bremen, Germany) equipped with a 1 kHz, 355 nm Nd:YAG laser was used for IMS experiments. Mass spectra (500 shots/spectra) were acquired in positive ion reflectron time-of-flight mode. Spatial resolution was 45 µm. FlexImaging (Bruker Daltonic GmbH, Bremen, Germany) was used to create MS images.
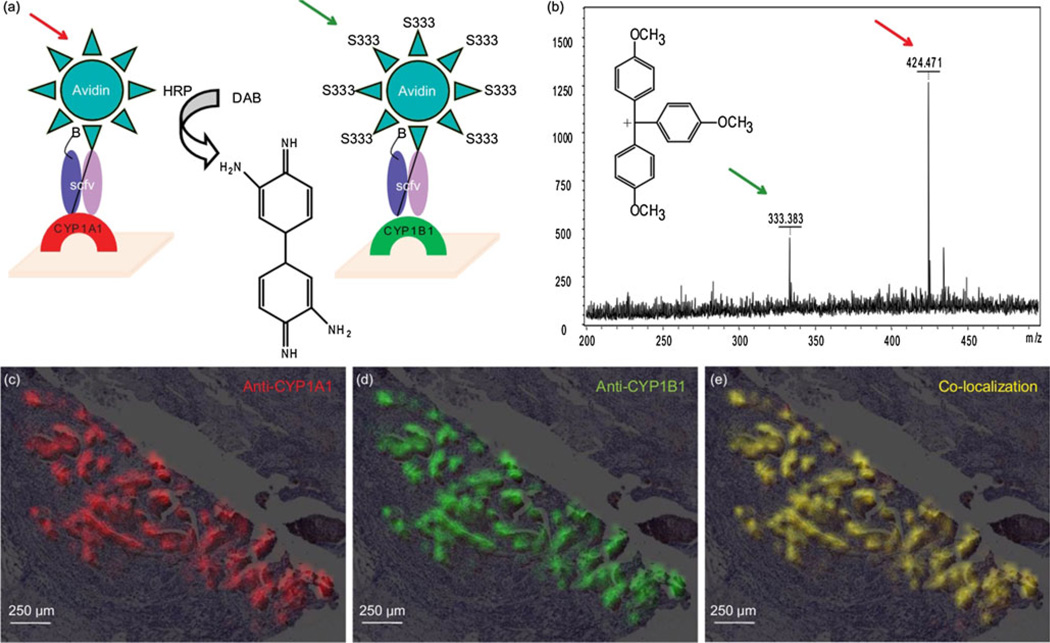
Identification of the precipitable peroxidase substrate m/z 424 ions were made by mass accuracy and MS/MS data from a MALDI FT-ICR mass spectrometer (9.4 Tesla Apex-Qe; Bruker Daltonics, Billerica, MA, USA) equipped with an Apollo II dual ions source and a 355 nm solid-state laser. External calibration was done using a peptide mixture of bradykinin 1–7, angiotensin II, and [Glu1]-fibrinopeptide with the MALDI matrix α-cyano-4-hydroxycinnamic acid (CHCA). Mass accuracies of better than 2 ppm were achieved using a series of CHCA clusters and the peptide standards for calibration. Fragmentation was performed in the source region of the instrument that has a mass-selecting quadrupole and an accumulation hexapole that is held at elevated Ar pressure (~1 × 10−5 mbar) for collision-induced dissociation (CID) experiments.
Results
Antibody scfv structure and mass tag labeling approaches are illustrated in Figures 1 and 2. The A10B scfv and rabbit IgG (the antigen for A10B) were used in ELISAs and mass spectrometry as a model scfv:antigen system to optimize scfv mass tag-labeling.
ELISA and IMS Results for scfv Directly Conjugated with Mass Tags
When A10B scfv was conjugated directly to mass tags and used in ELISA to detect the rabbit IgG antigen, the scfv retained antigen-binding specificity when conjugated with four or fewer mass tags. When conjugated with more than four mass tags, the scfv lost binding specificity and bound both the positive (rabbit IgG) and negative (BSA) control antigens. Additionally, A10B scfv conjugated directly with fewer than eight mass tags could not be resolved by LDI MS when bound to rabbit IgG on beads (data not shown). Based on these results, it was concluded that the scfv could not be directly labeled with a sufficient number of mass tags for use in IMS without loss of scfv antigen-binding specificity and/or sensitivity.
ELISA Results for Biotinylated scfv Coupled to Mass Tag Conjugated Avidin
When the A10B scfv was biotinylated and coupled to avidin conjugated to mass tags (at 1:1 on up to 64:1 mass tags: avidin ratios), scfv antigen-binding specificity, and sensitivity were retained.
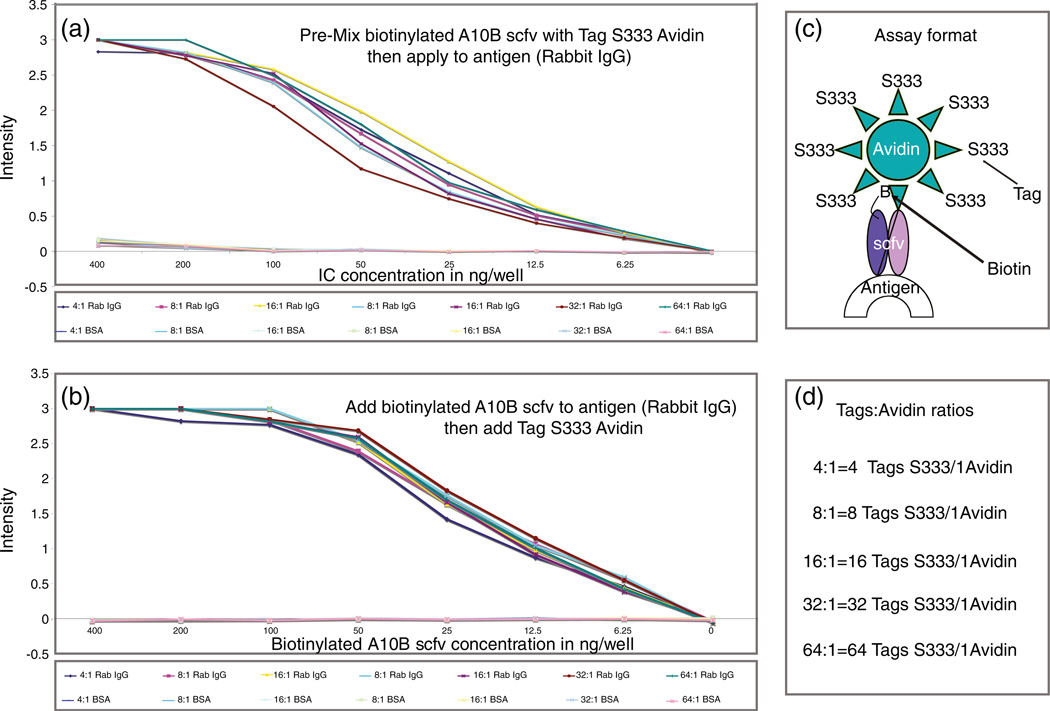
Two different ELISA formats were used to determine if biotinylated scfv, coupled to mass tag conjugated avidin, could be used to specifically detect antigens in assays. In the one-step format, biotinylated A10B scfv was pre-mixed with mass tag conjugated avidin to form an immune complex (IC), then applied to microtiter wells coated with either positive (rabbit IgG) or negative (BSA) control antigens. In the two-step format, biotinylated A10B scfv was applied to microtiter wells coated with the same antigens, washed from the wells after incubation, and probed with mass tag conjugated avidin.
In both the one-step and two-step ELISA formats, biotinylated A10B scfv and mass tag avidin interacted specifically with the positive (but not negative) control antigens, regardless of the number of mass tags conjugated to avidin (Figure 3).
Figure 3.
ELISA results for biotinylated A10B scfv linked to mass tag (m/z 333) conjugated avidin on positive (rabbit IgG) and negative (BSA) control antigens. Results are presented for the premixed or one-step (a) and two-step (b) formats
Immunoactivity of scfv Versus McAb Using IMS
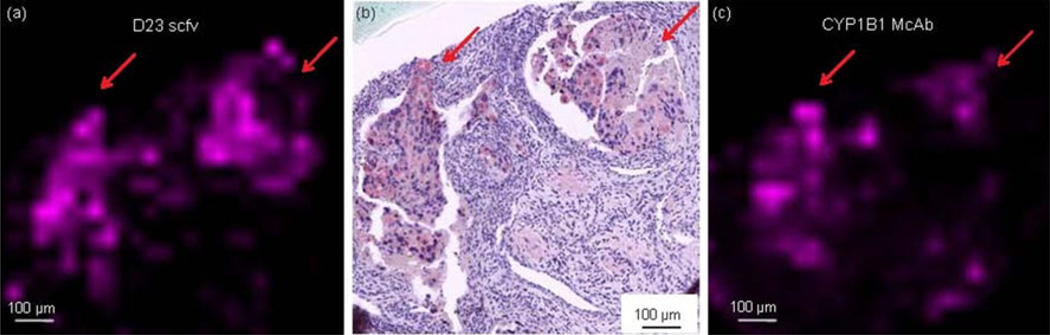
The anti-CYP1B1 D23 scfv and an anti-CYP1B1 IgG McAb were labeled with biotin and used to detect CYP1B1 in breast cancer tumor tissue sections. Biotinylated antibody bound to CYP1B1 in sections was detected using tagged-avidin. Both biotinylated anti-CYP1B1 D23 scfv and McAb produced similar results when used for LDI MS to detect CYP1B1 in serial sections of breast cancer tissue samples (Figure 4).
Figure 4.
IMS activity of anti-CYP1B1 scfv (D23) and monoclonal antibody on breast cancer tissue. (a) MS image of anti-CYP1B1 scfv (D23). (b) Light microscopy image of anti-CYP1B1 scfv (D23). (c) MS image anti-CYP1B1 monoclonal antibody. This figure demonstrates that scfv and monoclonal antibodies (McAb) to CYP1B1 exhibit comparable IHC/IMS antigen-binding activity
Multiplexing Antigen Detection Using Biotinylated scfv, Enzyme, or Mass Tag Conjugated Avidin and IMS
A biotinylated anti-CYP1A1 scfv (designated P1) and anti-CYP1B1 scfv were used to simultaneously detect, respectively, CYP1A1 and CYP1B1 on the same tissue section. The biotinylated P1 scfv was incubated and then, avidin conjugated to peroxidase was added for CYP1A1 detection (Figure 5a). The biotinylated D23 scfv was premixed with mass tag-conjugated avidin to form an IC. The IC was applied to tissue sections to detect CYP1B1, and binding was detected at m/z 333 (Figure 5b). A peroxidase precipitable color developer [DAB (m/z 424)] was used to visualize anti-CYP1A1 binding activity for IMS (Figure 5a). Both the anti-CYP1A1 and anti-CYP1B1 scfv simultaneously bound respective antigens in tissue sections (Figure 5c and d, respectively) and co-localized to the same location within the tissue (Figure 5e).
Figure 5.
LDI MS multiplexing assay results for anti-CYP1B1scfv and anti-CYP1A1scfv on breast cancer tissue. (a) Assays formats used to simultaneously image CYP1A1 and CYP1B1 on a breast tumor tissue section. (b) Mass spectra of the two reporter groups (m/z 333, mass tag; m/z 424, peroxidase precipitable substrate) used to simultaneously image CYP1A1 and CYP1B1. (c) MS image of CYP1A1 on tissue section. (d) MS image of CYP1B1 on tissue section. (e) MS image of CYP1A1 and CYP1B1 co-localized on the same tissue section
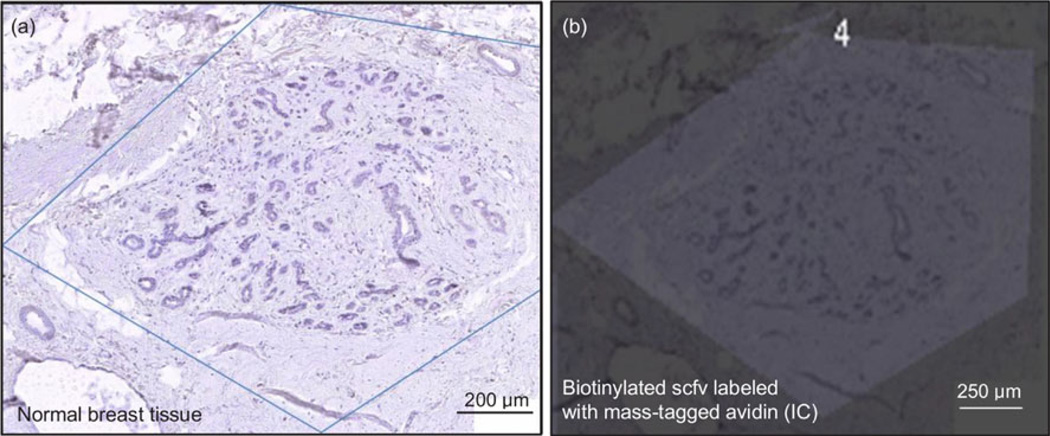
Figure 6 illustrates the immunolabeling control. Biotinylated scfv labeled with mass-tagged avidin (IC) was applied on a normal breast tissue and at no time did the IC bind to normal breast.
Figure 6.
Negative control of biotinylated anti-CYP1B1 scfv labeled with mass-tagged avidin (IC) on normal breast tissue. (a) Optical image of normal breast tissue stains with hematoxylin and eosin. (b) MS image of normal breast tissue treated by biotinylated anti-CYP1B1 scfv labeled with mass-tagged avidin antibody. This image demonstrates that the labeling scfv (IC) does not bind on normal cells
The LDI FT-ICR mass spectrum of DAB (the precipitate peroxidase reaction product), is presented in Figure S1 (Supplemental Information). The ion m/z 424.21191 has been identified as the covalently bound dimer of the precipitable peroxidase substrate. Mass accuracy (C24H24N8•+ theoretical m/z: 424.21184) shows the loss of 2 H2 upon dimerization suggesting that, during desorption and ionization, the highly energetic molecular species undergo hydrogen extraction followed by Diels-Alder cycloaddition to form the proposed covalently bound dimer. Fragmentation data (not shown) is consistent with this structure highlighted by neutral losses such as C3H5N2 (69.04524 Da, mass accuracy: 0.43 ppm), C6H7N2 (107.06109 Da, mass accuracy: 1.59 ppm) and C9H10N3 (160.08750 Da, mass accuracy: 0.19 ppm).
Discussion
In this study, we report the feasibility of using reporter molecule-scfv conjugate methods in LDI MS to image scfv binding to antigens in breast tumor tissue sections. Recombinant scfv and monoclonal IgG antibodies were used to specifically detect CYP1A1 or CYP1B1 in breast tumor tissue sections. In preliminary studies, it was determined that scfv needed to be conjugated with eight or more mass tags to be detected when bound to antigen. However, scfv lost antigen-binding specificity when conjugated directly to four or more mass tags. To circumvent this problem, scfv were conjugated, via a spacer, to biotin. The biotinylated scfv were then mixed and coupled to avidin conjugated to mass tags to form an immune complex (IC). Essentially, the IC approach allowed scfv to be linked to mass tags through biotin and avidin. The scfv, linked to mass tags using an IC approach, could be used to specifically detect antigens in assays (Figure 3). Biotinylated antibodies and tagged-avidin were used to detect CYP1A1 or CYP1B1 in breast tumor tissue sections. Antibody binding to CYP1A1 or CYP1B1 was detected by imaging reporter molecules as indicators of antibody binding. Anti-CYP1B1 scfv and IgG McAb demonstrated similar antigen-binding sensitivities and specificities when used as imaging agents (Figure 4), suggesting that either antibody format (scfv versus McAb) is suitable for targeted mass spectrometry imaging. Immune complexes containing either (biotin-anti-CYP1A1scfv + avidin-peroxidase) or (biotin-anti-CYP1B1scfv + avidin-mass tag) could be combined and used to detect, respectively, CYP1A1 and CYP1B1 in the same breast tumor tissue section (Figures 5c and d). It was also determined that CYP1A1 and CYP1B1 co-localized within the tumor tissue sample (Figure 5e). At no time did biotinylated scfv labeled with mass-tagged avidin (IC) bind on normal breast tissues (Figure 6) and no m/z 333 signal was obtained when mass tag conjugated avidin itself was applied to tumor tissue. These results demonstrate that scfv specifically bound their antigens and can be used in LDI MS in a multiplex assay format to simultaneously detect more than one antigen in a single tissue section.
Conclusion
Recombinant scfv and monoclonal IgG antibodies can be successfully used in targeted IMS assays to visualize antigen/antibody interactions in tumor tissue sections. Recombinant scfv antibodies can be successfully conjugated to both biotin and mass tags. However, antigen-binding specificity can be lost when scfv antibodies are directly conjugated to mass tags. Biotin scfv antibody and mass tag avidin conjugation methodologies were used to overcome this loss of scfv antibody-binding specificity. Biotinylated scfv antibody and mass tag conjugated avidin can be combined, prior to use in assays, to form an IC. The IC links the scfv antigens on tissue sections without compromising assay sensitivity and specificity. This provides a unique opportunity to use enzyme or fluorescent-labeled ICs to select a specific group of cells on tissue by light or fluorescence microscopy and then study the immunoreactivity of these cells, on the same section, using mass tag ICs to detect antigens of interest. A sub-cellular spatial resolution could be easily reached using laser desorption ionization in transmission geometry and thus, this study could be ultimately be carried out on individual cells [20].
Lastly, it is likely that avidin can be conjugated to a large number of unique mass tags. If so, then unique avidin mass tag conjugates could be used interchangeably to link different biotinylated antigen-specific scfv antibodies to unique mass tags. Such an approach would provide the flexibility needed to optimize multiplex assays for targeted IMS.
Supplementary Material
Acknowledgments
The authors acknowledge support for this work in part by funds from the Vanderbilt Institute for Chemical Biology to R.L.M. RMC wishes to acknowledge NIH/NCRR grant 1P41RR03146-01 and NIH/NIGMS grant 8P41GM103391-02
Abbreviations
- LDI
Laser Desorption Ionization
- TOF
Time of Flight
- MS
Mass Spectrometry
- MALDI
Matrix-Assisted Laser Desorption Ionization
- IMS
Imaging Mass Spectrometry
- scfv
single chain fragment variable
- IC
Immune complex
- IHC
Immunohistochemistry
- McAb
Monoclonal Antibody
- ELISA
Enzyme-linked immunosorbent assay
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s13361-012-0423-x) contains supplementary material, which is available to authorized users.
References
- 1.Cerio R, Dupuy PF, Allen MH, MacDonald DM. Monoclonal antibody labeling of mononuclear cell surface antigens in formaldehyde-fixed paraffin-embedded cutaneous tissue. J. Invest. Dermatol. 1986;87(4):499–503. doi: 10.1111/1523-1747.ep12455571. [DOI] [PubMed] [Google Scholar]
- 2.Cerio R, MacDonald DM. Effect of routine paraffin wax processing on cell membrane immunoreactivity in cutaneous tissue. J. Clin. Lab. Immunol. 1986;20(2):97–100. [PubMed] [Google Scholar]
- 3.Cerio R, MacDonald DM. Routine diagnostic immunohistochemical labeling of extracellular antigens in formol saline solution-fixed, paraffin-embedded cutaneous tissue. J. Am. Acad. Dermatol. 1988;19(4):747–753. doi: 10.1016/s0190-9622(88)70232-7. [DOI] [PubMed] [Google Scholar]
- 4.Cerio R, Wilson-Jones E. New cell markers for routine diagnostic dermatopathology. Clin. Exp. Dermatol. 1989;14(3):177–180. doi: 10.1111/j.1365-2230.1989.tb00926.x. [DOI] [PubMed] [Google Scholar]
- 5.Bird RE, Hardman KD, Jacobson JW, Johnson S, Kaufman BM, Lee SM, Lee T, Pope SH, Riordan GS, Whitlow M. Single-chain antigen-binding proteins. Science. 1988;242(4877):423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- 6.Huston JS, Levinson D, Mudgett-Hunter M, Tai MS, Novotny J, Margolies MN, Ridge RJ, Bruccoleri RE, Haber E, Crea R, Oppermann H. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 1988;85(16):5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padlan EA. Anatomy of the antibody molecule. Mol. Immunol. 1994;31(3):169–217. doi: 10.1016/0161-5890(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 8.Bradley C, van der Meer R, Roodi N, Yan H, Chandrasekharan MB, Sun ZW, Mernaugh RL, Parl FF. Carcinogen-induced histone alteration in normal human mammary epithelial cells. Carcinogenesis. 2007;28(10):2184–2192. doi: 10.1093/carcin/bgm100. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Liu Y, Mernaugh RL, Zeng X. Single chain fragment variable recombinant antibody functionalized gold nanoparticles for a highly sensitive colorimetric immunoassay. Biosens. Bioelectron. 2009;24(9):2853–2857. doi: 10.1016/j.bios.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen Z, Mernaugh RL, Yan H, Yu L, Zhang Y, Zeng X. Engineered recombinant single-chain fragment variable antibody for immunosensors. Anal. Chem. 2005;77(21):6834–6842. doi: 10.1021/ac0507690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen Z, Stryker GA, Mernaugh RL, Yu L, Yan H, Zeng X. Single-chain fragment variable antibody piezoimmunosensors. Anal. Chem. 2005;77(3):797–805. doi: 10.1021/ac048655w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke P, Justenhoven C, Brauch H, Dawling S, Roodi N, Higginbotham K, Plummer W, Schuyler P, Sanders M, Page D, Smith J, Dupont W, Parl F. Estrogen metabolism and exposure in a genotypic-phenotypic model for breast cancer risk prediction. Canc. Epidemiol. Biomarkers Prev. 2011;20:1502–1515. doi: 10.1158/1055-9965.EPI-11-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McFadyen MC, Melvin WT, Murray GI. Cytochrome P450 enzymes: novel options for cancer therapeutics. Mol. Cancer Ther. 2004;3(3):363–371. [PubMed] [Google Scholar]
- 14.Thiery G, Shchepinov MS, Southern EM, Audebourg A, Audard V, Terris B, Gut IG. Multiplex target protein imaging in tissue sections by mass spectrometry—TAMSIM. Rapid Comm. Mass Spectrom. 2007;21(6):823–829. doi: 10.1002/rcm.2895. [DOI] [PubMed] [Google Scholar]
- 15.Thiery G, Anselmi E, Audebourg A, Darii E, Abarbri M, Terris B, Tabet JC, Gut IG. Improvements of Targeted multiplex mass spectrometry imaging. Proteomics. 2008;8(18):3725–3734. doi: 10.1002/pmic.200701150. [DOI] [PubMed] [Google Scholar]
- 16.Caprioli RM, Farmer TB, Gile J. Molecular imaging of biological samples: localization of peptides and proteins using MALDI-TOF MS. Anal. Chem. 1997;69(23):4751–4760. doi: 10.1021/ac970888i. [DOI] [PubMed] [Google Scholar]
- 17.Mernaugh R, Mernaugh G. Methods for the production of mouse monoclonal antibodies. In: Singh RP, Singh US, editors. Molecular Methods in Plant Pathology. Boca Raton FL: CRC Press; 1995. pp. 343–358. [Google Scholar]
- 18.Shen Z, Yan H, Parl FF, Mernaugh RL, Zeng X. Recombinant antibody piezoimmunosensors for the detection of cytochrome P450 1B1. Anal. Chem. 2007;79(4):1283–1289. doi: 10.1021/ac061211a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ustinov AV, Shmanai VV, Patel K, Stepanova IA, Prokhorenko IA, Astakhova IV, Malakhov AD, Skorobogatyi M, Bernad PL, Jr, Khan S, Shahgholi M, Southern EM, Korshun VA, Shchepinov MS. Reactive trityl derivatives: stabilized carbocation mass-tags for life sciences applications. Org. Biomol. Chem. 2008;6(24):4593–4608. doi: 10.1039/b810600b. [DOI] [PubMed] [Google Scholar]
- 20.Thiery G, Zavalin AI, Caprioli RM. Targeted Multiplex Mass Spectrometry Imaging in Transmission Geometry LDI for a Sub-Cellular Resolution; Proceedings of the 58th Annual Conference on Mass Spectrometry; May, 2010; Salt Lake City, UT. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.