Abstract
One target of protective immunity against the Plasmodium liver stage in BALB/c mice is represented by the circumsporozoite protein (CSP), and mainly involves its recognition by IFN-γ producing specific CD8+T-cells. In a previous in vitro study we showed that primary hepatocytes from BALB/c mice process Plasmodium berghei (Pb) CSP (PbCSP) and present CSP-derived peptides to specific H-2kd restricted CD8+T-cells with subsequent killing of the presenting cells. We now extend these observations to an in vivo infection model in which infected hepatocytes and antigen specific T-cell clones are transferred into recipient mice inducing protection from sporozoite (SPZ) challenge. In addition, using a similar protocol, we suggest the capacity of hepatocytes in priming of naïve T-cells to provide protection, as further confirmed by induction of protection after depletion of cross-presenting dendritic cells (DCs) by cytochrome c (cyt c) treatment or using traversal deficient parasites. Our results clearly show that hepatocytes present Plasmodium CSP to specific-primed CD8+T-cells, and could also prime naïve T-cells, leading to protection from infection. These results could contribute to a better understanding of liver stage immune response and design of malaria vaccines.
Introduction
Immunization of rodents and humans with radiation- or genetically-attenuated sporozoites (SPZ) (RAS, GAS) confers pre-erythrocytic stage specific protective immunity to an infectious challenge [1], [2]. This protective immunity is mediated in part by CD8+ T-cells specific for the CSP and other not yet identified proteins [2]–[5]. Recently, it was demonstrated that both infected and sporozoite-traversed mouse primary hepatocytes can process the PbCSP and present CSP-derived peptides to a specific H-2Kd-restricted CD8+ T-cell clone in vitro but recognition of infected hepatocytes was the only relevant step in the elimination of infection [6]. Using bone marrow cell transfer into totally irradiated mice, it was also concluded that activation of protective CD8+ T-cell clones was due to antigen presentation by nonhematopoietic parenchymal cells [7]. Thus, the role of hepatocytes as antigen presenting cells (APCs) in the activation of primed T-cells to provide sterile protection seems to be accepted. Nevertheless, the role of liver cells and, in particular, hepatocytes in the activation of Plasmodium-specific CD8+ T-cells is not clearly elucidated. Chakravarty et al. [7] presented data supporting the role of lymph node dentritic cells (DCs) in presentation to parasite-specific T-cells while Leiriao et al. [8] supported the notion that apoptotic Plasmodium-infected hepatocytes provide antigen to liver DCs. While both pathways of antigen presentation co-exist, their role in providing a protective CD8+ T-cell response has not been established. In both studies, critical experiments such as the elimination of DCs and its consequence on sterile protection are missing. On the other hand, several publications point to the role of liver cells and hepatocytes in the mechanism of protection [2], [5], [9]–[11]. In addition, intravenous (iv) injection in mice of RAS gives rise to a more robust immune response and to sterile immunity compared to intradermal (id) immunization [12]. On the other hand, this difference in protection between iv and id injection is overcome by a higher dose of sporozoites [13]. Furthermore, immunization of mice with GAS is associated with a better immune response, probably due to the development of GAS liver stages and presentation of antigens other than CSP to specific T-cells [14].
It has been shown that CD8+ T-cells are primed by the DCs in the skin, and are then thought to exit the priming site and migrate to the liver where they can eliminate infection after recognizing antigens presented by hepatocytes [2], [7], [9], [15]. Thus, it was concluded that initiation of the CD8+ T-cell mediated immunity requires antigen presentation by only DCs [16]. On the other hand, the infected hepatocyte is the cell where SPZs develop and proliferate into the next liver forms. Furthermore, infection of hepatocytes is crucial for maintenance of anti- SPZ protective CD8+ T-cell response since protection was abrogated if hepatic stages were eliminated [2], [9], [15]. However, the ability of hepatocytes to prime naïve CD8+ T-cells and induce protective immunity remains unclear. It has also been illustrated that hepatocytes present CSP that is secreted directly into the cytosol, unlike DCs that cross-present Plasmodium antigens via endosomes [16]. These findings suggested that both in DCs and hepatocytes, the presentation of antigens requires the transporter associated with antigen processing (TAP) proteins [16]–[18] and that the cross-presenting DCs could be abrogated in mice after treatment with cytochrome c (cyt c) [16]. Determining if hepatocytes in vivo process and present Plasmodium antigens to naïve and primed T-cells may help in the rational identification of pre-erythrocytic vaccine candidates. To this purpose, we have established an in vivo protocol to address two questions: are hepatocytes capable of 1) in vivo stimulation of primed CD8+ T-cells and 2) priming naïve T-cells to protect mice against parasite challenge? We used different methods to address these two questions. First, we used intrasplenic (IS) transfer of PbSPZ-infected hepatocytes from BALB/c mice into naïve, TAP−/− deficient mice (H-2Kb ) and BALB/c mice (H-2Kd) in the presence or absence of H-2Kd-specific CD8+ T-cells (C7 clone) in order to determine the ability of primary hepatocytes to present antigen to primed CD8+ T-cells. Secondly, to show that hepatocytes could present antigen in the absence of dendritic cells, mice treated with cyt c were injected with irradiated SPZ (iSPZ) and challenged with live SPZ. Thirdly, mice were immunized with iSPZs deficient for the sporozoite microneme protein essential for cell traversal (spect (−) iSPZs), known for being incapable of cell traversal, but capable of infection and normal development [6], [19], [20] in order to show the role of infected hepatocytes in inducing a protective immune response. In all cases, mice were partially or totally protected from an infectious SPZ challenge as assessed by their level of blood parasitemia up to 14 days post infection.
Materials and Methods
Peptides
Peptides PbCS245–253 (YIPSAEKI) and PbCS253–260 modified with iodo-azidosalicylic acid (IASA) and azidobenzoic acid (ABA) groups, (IASA)-YIPSAEK(ABA)I representing the epitope for C7 clone (H-2Kd-restricted and PbCSP specific CTL) and S14 (H-2Kd-restricted and an irrelevant CTL), respectively [3], [21], were synthesized by solid-phase F-moc chemistry. Peptide stock solutions (2 mg/ml) were prepared in PBS and stored at −20°C.
Parasites
Plasmodium berghei ANKA wild-type (wt) and spect (−) SPZ were obtained after salivary gland dissection of infected female Anopheles stephensi mosquitos raised in the mosquito facility at the Department of Biochemistry, University of Lausanne, Switzerland as described previously [6], [20]. After dissection, salivary glands were homogenized in a glass grinder and released SPZ were counted and then diluted in sterile Dulbecco’s Modified Eagle Medium, DMEM (Gibco®, Life Technologies™, New York, NY).
Animals
Six-to-12-week-old BALB/c (H-2Kd) or TAP−/− (H-2Kb) mice were obtained from Harlan Laboratories B.V. (Venray, Netherlands) or bred at the animal facility at the Department of Biochemistry (University of Lausanne, Switzerland). All mice were housed under pathogen-free conditions and handled according to the guidelines of the authorization N° 805.7 of the Service de la consommation et des affaires vétérinaires (Lausanne, Switzerland).
Hepatocyte Isolation
SPZ-infected and naïve hepatocytes from BALB/c mice were obtained after collagenase perfusion of the liver as previously described [6], [20], [22]. Briefly, mice were sacrificed by CO2 inhalation, dissected, and the biggest lobule of liver was cut out. The lobule was perfused for 10 min with Ca2+-free HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer, pH 7.6 (Gibco® Invitrogen™, New York, NY) at 37°C at a rate of 5 ml/min. The lobule was then perfused with type IV collagenase (Sigma-Aldrich®, Steinheim, Germany) (HEPES buffer containing 0.04% type IV collagenase and 0.075% CaCl2-2H2O) for 5 min at 37°C. The perfused lobule was incubated for 10 min at 37°C in the collagenase solution. Using sterile pipettes, the tissue was gently teased apart to release cells and washed once with Ca2+-free HEPES buffer at 800 rpm for 30 s at 4°C. The pellet was gently re-suspended in DMEM, layered on 60% Percoll (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and centrifuged at 2000 rpm for 2 min at 4°C. The resulting pellet was re-suspended in complete culture medium (DMEM supplemented with 10% FCS, 1% penicillin streptomycin, 1% HEPES, and 0.05 mM of β-mecarptoethanol (β-ME, Sigma-Aldrich®), and centrifuged again at 800 rpm for 30 s at 4°C. The pellet was finally re-suspended again in 5 ml of DMEM for counting. Viability of isolated hepatocytes was assessed by light microscopy and trypan blue dye exclusion. As reported previously [6] hepatocyte contamination with Kupffer/dendritic cell markers is less than 1% as determined by FACS analysis [6], [10], [23], [24]. Then, mice were injected iv with 7×105 live hepatocytes re-suspended in 200 µl of sterile DMEM. To obtain infected hepatocytes, 1×106 of live or irradiated wt PbSPZ were injected iv through the tail vein of BALB/c mice. Two hours later, the liver was removed from the infected mouse and perfused as described above.
Intrasplenic (IS) Transfer
Hepatocellular transplantation was carried out by direct injection of 7×105 hepatocytes in 200 µl of sterile DMEM with a syringe into the splenic parenchyma of recipient mice that were anaesthetized with Isoflurane (Provet AG, Berne, Switzerland). Briefly, under aseptic and anaesthesia conditions, cautiously, with a chisel and a clip, the abdomen of the mouse was opened on the left flank. Using a clamp fitted, the spleen was gently pulled out and placed on a sterile piece of paper. The injection of cell suspension in the spleen parenchyma was carefully carried out at a rate of 10 µl per 10 respiratory cycles of the mouse. Suturing and clamping of the skin minimized leakage from the site of operation. Four hours later, 20 million PbCSP-restricted CD8+ T-cells (C7-clone) or irrelevant CD8+ T-cells (clone S14) were injected iv through the tail vein in a volume of 500 µl of DMEM. Control mice received only hepatocytes.
T-cell Clone Re-stimulation
The C7 and irrelevant S14 clones [3], [21] were re-stimulated weekly, maintained at 37°C and used as effector cells. Briefly, P815 cells (mastocytoma cells as antigen presenting cells, APC) were re-suspended (1×106 cells/ml) in complete culture medium (DMEM supplemented with 10% FCS +1% of pyrimethamine-streptomycin +0.1% of β-ME and 1% of HEPES) and pulsed for 1 hour with PbCSP-epitope peptides (1 µg/ml) specific for C7-and S14 clones. C7 and S14 were washed and re-suspended (2×106/ml/well) in CTL culture medium (complete culture medium supplemented by 30 U/ml of mouse IL-2) in a 6-well plate. For re-stimulation, the respective clones were added to the 1×106 pulsed and irradiated P815 cells (10000 rads/20 minutes) in the presence of 15×106/well of irradiated-BALB/c spleen cells (5000 rads/10 minutes) in 6-well flat bottom plates. In the intrasplenic experiment, clones were re-stimulated and kept for two weeks in culture to make sure they were resting at the time of injection.
Cytochrome c Treatment and Induction of CD8+ T-cells
BALB/c mice were depleted of cross-presenting DCs by iv treatment for 3 days with 15 mg/ml (5 mg/ml per day) of horse cyt c (Sigma-Aldrich®, St Louis, LA) in 100 µl of PBS (Gibco® Invitrogen™). Control mice received 100 µl of PBS alone. On the last day of treatment, mice were immunized iv with 1×105 wt PbiSPZ). Seven-to-ten days later, the frequency of both PE-conjugated SYIPSAEKI-tetramer and FITC-conjugated anti-mouse CD8 antibody (BD Biosciences, Allschwil, Switzerland) specific CD8+ T-cells was measured in different mouse tissues (blood = PBL, spleen, liver and lymph nodes (LN)) by flow cytometry.
Real Time PCR (RT-PCR)
In vivo assessment of parasite loads was performed by RT-PCR as described previously [25]. Briefly, BALB/c mice were injected iv with 1×105 PbiSPZ (wt or spect (−)). Two hours later, total livers and spleens were isolated, perfused with PBS alone and total RNA was extracted. Then, cDNA was synthesized using specific primers for the P. berghei 18S rRNA (forward: 5′ AAGCATTAAATAAAGCGAATACATCCTTAC-3′ and reverse: 5′ GGAGATTGGTTTTGAC GTTTATGTG-3′) as described previously [25]. The DNA was thus amplified in the LightCycler 2.0 Instrument (Roche Diagnostics, Basel, Switzerland) using the program Roche LightCycler Run 5.32, and the relative parasite DNA load was thus determined in liver and spleen for each type of SPZ.
Parasitemia Assessment
At different time points after the IS transfer or challenge with live PbSPZs (iv), parasitemia was assessed by 10% Giemsa (Fluka®, Sigma-Aldrich®)-stained blood smears. Blood smear slides were air-dried and read by light microscopy (Olympus CH-2, Microscope Company, Hicksville, NY) to determine infected red blood cells (iRBC). Animals were protected against malaria if they remained negative in Giemsa-stained blood smears 2 weeks after receiving infected hepatocytes or live SPZ. Control animals were included to verify infectivity of SPZ or infected hepatocytes. In each control animal, parasitemia was detectable 7 days after IS transfer or challenge.
Statistical Analysis
Different statistic tests were performed using GraphPad Prism software (version 6). Fisher’s exact test compares the proportion of mouse protection among various groups. The Mann-Whitney test was performed to compare parasite DNA load and frequency of PbCSP-specific CD8+ T-cells in PBL in two independent experiments or in different organs in the same experiment in which mice underwent different treatments (wild-type and Spect (−) iSPZ or PBS and cyt c groups). All p-values equal to or lower than 0.05 were considered significant.
Results
Infected Hepatocytes Present the PbCSP-specific Epitope to Cloned CD8+ T-cells with Subsequent Protection against Malaria
To show that infected hepatocytes process and present PbCSP, TAP−/− mice received BALB/c PbSPZ-infected hepatocytes by IS transfer followed by iv injection of PbCSP specific (clone C7) or irrelevant (S14 clone) CD8+ T-cells 4 h later. TAP −/− mice were selected to bypass the possibility that presentation could be performed by professional APCs through processing of apoptotic, infected hepatocytes or live SPZs possibly externally associated with BALB/c hepatocytes. In addition, the host vs graft immune response is minimized. All mice (8/8; 100%) that received infected hepatocytes and the specific C7 clone were protected from an infective sporozoite challenge ( Table 1 , group A). In contrast, all mice receiving the irrelevant S14 clone were infected ( Table 1 , group B). In addition, 75% of naive TAP−/− mice treated with infected BALB/c hepatocytes were infected ( Table 1 , group C).
Table 1. Infected hepatocytes present a PbCSP-specific epitope to primed CD8+ T-cells and protect mice against SPZ challenge.
| Treatment | |||||
| TAP−/− mouse group | BALB/c- SPZ infected hepatocytes | T cell clones | Mice protected/total injected | % of protection | p-value |
| A | yes | C7 | 8/8 | 100 | A versus B = 0.006 |
| B | yes | S14 | 0/3 | 0 | B versus C = 1 |
| C | yes | None | 1/4 | 25 | A versus C = 0.018 |
Each recipient TAP−/− mouse (H-2Kb) received 7×105 PbSPZ-infected BALB/c (H-2kd) hepatocytes by IS transfer as described in Materials and Methods. Before IS injection, infected hepatocytes were isolated from BALB/c mice that were injected (iv) with 106 ANKA wt PbSPZ 2 h earlier. C7 and S14 (20 million cells per mouse, iv) were injected into the corresponding group 4 h after IS transfer. Mice were protected if they remained parasite negative 2 weeks after infected hepatocyte transfer.
In order to show that the protective immune response is specific and not due to a bystander effect or a continuous secretion of cytokine by the C7 clone, TAP−/− mice were first infected with live PbSPZ (1000/mouse in iv) or not. Ten hours later, mice received infected or naïve BALB/c hepatocytes together or without C7 clone as indicated in Table 2. Parasitemia determination of group C showed that only 1/7 (14%) of mice was protected ( Table 2 , group C). Other controls showed that protection occurred only in the group that received infected BALB/c hepatocyte and C7 clone ( Table 2 , group A).
Table 2. Specificity of activation of C7 clone and protection.
| Treatment | ||||||
| TAP−/− mouse group | Anka wt SPZ | BALB/c hepatocytes | C7 clone | Mice protected/total injected | % of protection | |
| A | None | SPZ infected | yes | 5/5 | 100 | p-value |
| B | None | SPZ infected | none | 0/5 | 0 | A versus B = 0.008 |
| C | Yes | Naïve | yes | 1/7 | 14 | A versus C = 0.015 |
| D | Yes | none | yes | 0/3 | 0 | A versus D = 0.018 |
| E | Yes | none | none | 0/3 | 0 | A versus E = 0.018 |
Mice were first injected (iv) or not with 103 live wild-type (wt) ANKA PbSPZ 10 h before they received or not 7×105 naïve-BALB/c hepatocytes and C7 clone as indicated above. Infected hepatocytes were isolated from BALB/c mice injected with 106 ANKA wt PbSPZ 2 h earlier; and naïve BALB/c hepatocytes were isolated from naïve BALB/c mice. Mice were considered protected if they remained parasite negative 2 weeks after infection.
Infected Hepatocytes Prime PbCSP-specific CD8+ T-cells and Protect Mice against SPZ Challenge
Since the infected hepatocytes can reactivate resting CSP-specific CD8+ T-cells and induce protective immunity ( Table 1 and 2 ), the next step was to determine if they could also prime naïve T-cells to protect mice against live SPZ challenge. To this effect, we injected iSPZ-infected BALB/c hepatocytes into naive BALB/c mice before challenging with live SPZ. Considering that the immunization procedure may give rise to a sub-optimal immunity (about 700 infected hepatocytes injected IS if the overall infection efficacy is estimated to be 10%), mice were challenged with a sub-optimal or optimal dose of live SPZ (2×103 and 5×103) that led to 60% and 100% infection in naïve mice, respectively, with an overall protection of 13% ( Table 3 ). In contrast, mice receiving iSPZ-infected hepatocytes were protected at 64% (7/11; p = 0.014) ( Table 3 ), suggesting that the infected hepatocytes could contribute to the priming of naïve T-cells and protection of mice against infection.
Table 3. BALB/c mice injected with iSPZ-loaded hepatocytes are protected against SPZ challenge.
| 2 weeks after challenge | ||||
| Mouse group (n of SPZ for challenge) | Treatment | Mice protected/total challenged | % of protection | p-value: IS versus naive |
| A (2000) | IS | 5/5 | 100 | 0.167 |
| Naive | 2/5 | 40 | ||
| B (5000) | IS | 2/6 | 33 | 0.125 |
| Naive | 0/10 | 0 | ||
| Total | IS | 7/11 | 64 | 0.014 |
| Naive | 2/15 | 13 | ||
BALB/c mice were injected (IS transfer) with 7×105 PbiSPZ-infected or naïve BALB/c hepatocytes. Infected hepatocytes were obtained from BALB/c mice immunized with 106 ANKA wt iSPZ 2 h earlier. Recipient mice were then challenged with two different doses (2×103 and 5×103, respectively, A and B) of live PbSPZ one week later. Mice were considered protected if they remained parasite negative 2 weeks after challenge.
To further corroborate that hepatocytes could prime naïve T-cells and induce protection, BALB/c mice were treated with cyt c to delete cross-presenting DCs before and during iSPZ immunization. This protocol was established according to previous studies which showed that cross-presenting DCs can be largely depleted after in vivo cyt c treatment [16], [26]. The first experiment (as pilot) showed a significant reduction of 60% of PbCSP245–253 specific CD8+ T-cells in PBL after cyt c treatment (data not shown). In the second experiment, the analysis was extended to other organs (LN, spleen and liver) ( Fig. 1 ). Thus, FACS analysis showed that induction of PbCSP-specific CD8+ T-cells was highly reduced in the cyt c- compared to the PBS -treated mice in PBL and liver (86% and 67%, respectively), while this reduction was about 50% in LN and spleen ( Fig. 1 , inserted panel). Taking the PBL data from the two experiments or from all organs in the second experiment, normalizing and combining them, we obtained p values of 0.003 (PBL) and 0.001 (organs) for the cyt c-treated compared to PBS groups. In spite of these differences, both groups were protected after SPZ challenge ( Table 4 ).
Figure 1. Level of PbCSP-specific CD8+ T-cells in different organs of BALB/C mice after treatment with either cyt c or PBS and immunization with PbiSPZ.
Two groups of BALB/c mouse were pre-treated (iv through the tail vein) with 5 mg/mouse of cyt c per day for 3 days in 100 µl of PBS or with 100 µl of PBS alone. Immediately after the last treatment, mice were immunized with 1×105 PbiSPZ in 100 µl of RPMI. One-week later, PbCSP epitope-specific CD8+ T-cell frequency (2 mice per group of treatment) was evaluated in peripheral blood lymphocytes (PBL), liver, lymph nodes (LN) and spleen. Naïve (receiving neither cyt c, PBS nor iSPZ) mice were used as negative control. Inserted panel shows the fold change in frequency and the percentage of inhibition of PbCSP-specific CD8+ T-cells in cyt c-treated compared to PBS alone-treated groups.
Table 4. Protection from infection in cyt c- and PBS- treated mice.
| Treatment | Mice protected/total challenged | % of protection |
| Cyt c | 10/10 | 100 |
| PBS | 10/10 | 100 |
| Naive | 1/10 | 10 |
Protection of mice after cyt c or PBS treatment from live SPZ challenge of two independent experiments (5 mice per group in each experiment). Mice were either pre-treated iv for 3 days with 5 mg/mouse of horse cyt c per day in 100 µl of PBS or with 100 µl of PBS alone. On the last day of treatment, mice were immunized iv with 1×105 PbiSPZ in 100 µl of RPMI. One week later, mice were challenged with 5×103 live PbSPZ. Parasitemia was checked at 1 and 2 weeks after challenge. Mice were considered protected if they remained parasite negative 2 weeks after challenge.
Protective Immune Response Induced in BALB/c Mice Immunized with spect (−) iSPZ
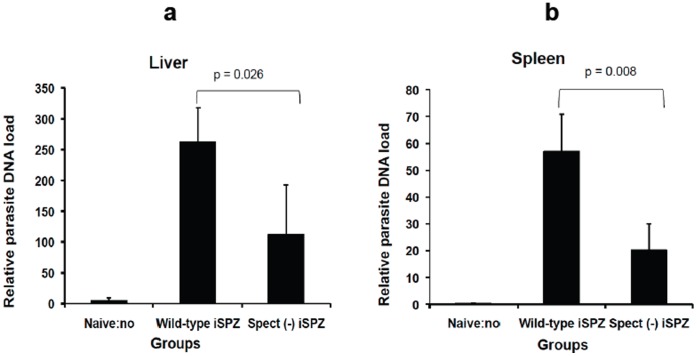
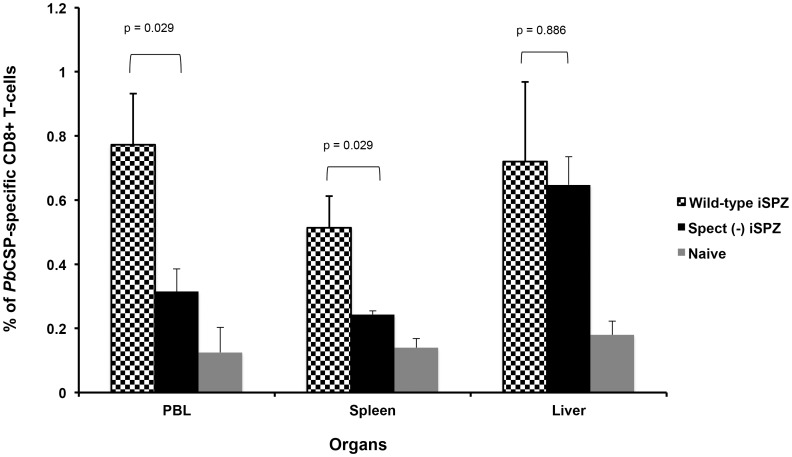
Wild-type SPZs are known to be able to cross several cells (leaving a trail of the CSP behind) before infecting a single hepatocyte, unlike spect (−) SPZ that infect hepatocytes without cell traversal. It has been shown that both infected and traversed hepatocytes are able to process and present PbSPZ CSP to primed CD8+ T-cells to induce IFN-γ secretion in vitro but only the infected hepatocytes were responsible for their own elimination [6], [20]. Similar experiments were then performed in vivo. Thus, it was expected that spect (−) SPZ would activate a lower number of the PbCSP-specific CD8+ T-cells in the periphery and present a lower parasite load in the liver. RT-PCR clearly showed that the relative parasite DNA load in liver and spleen was significantly higher in mice receiving wt iSPZ compared to the spect (−) iSPZ parasite group (p = 0.026 and 0.008, respectively) ( Fig. 2a and b ). In addition, FACS analysis showed that the level of the PbCSP-specific CD8+ T-cell response was significantly higher in the wt compared to the spect (−) group in PBL and spleen (p = 0.029) suggesting a role of cross-presenting DCs in these compartments ( Fig. 3 ). In contrast, in the liver, the frequency of CD8+ T-cells was similar (p = 0.886) for the two kinds of iSPZ ( Fig. 3 ) in spite of a lower parasite load for spect (−). Both groups of mice were protected against live SPZ challenge ( Table 5 ). Together, these data indicate that T-cell traversal by SPZ induces a higher level of immune response in PBL and spleen, but not in the liver, further supporting the key role of infected hepatocytes in antigen presentation and induction of protective immunity.
Figure 2. Relative comparative wt and spect (−) parasite DNA load in spleen and liver of immunized mice.
To compare relative load of wt and spect (−) parasite DNA in liver and spleen, BALB/c (3 mice per group) were immunized iv on tail with 1×105 iSPZ (wt or spect (−)) in 500 µl of RPMI. Two hours later, a real-time PCR was performed following extraction of respective parasite RNA. Every sample was done in duplicate. To avoid any contamination by eventual parasite from blood, the liver and spleen were perfused with PBS before performing the RT-PCR. Figures a and b represent wt and spect (−) parasite DNA load, respectively, in the liver and spleen 2 h after iSPZ injection (iv). Naïve mice (receiving only 500 µl iv of DMEM) were used as a control.
Figure 3. Frequency of PbCSP-specific CD8+ T-cells in different organs of BALB/c mice immunized with wt or spect (−) iSPZ.
BALB/c mice (4 mice per group) were immunized (iv) with wt or spect (−) ANKA PbiSPZ (one dose of 1×105 iSPZ). Seven (7) days later, PBL, liver and spleen cells of mice were isolated to determine frequency of PbCSP-specific CD8+ T-cells by flow cytometry (FACScan). The CD8+ T-cells were double stained with PE-conjugated PbCSP epitope tetramer and FITC-conjugated anti-mouse CD8b antibody. The naive group received neither wt nor spect (−) iSPZ. p-value compares statistically significant mean of CD8+ T-cell frequency between wt and spect (−) iSPZ-immunized groups.
Table 5. Both wt and spect (−) PbiSPZ immunization protect mice against SPZ challenge.
| Immunization (number of PbISPZ) | Challenge with PbSPZ | Mice protected/total challenged | % of protection |
| Wild-type (1×105) | 1×104 | 5/5 | 100 |
| Spect (−) (1×105) | 1×104 | 5/5 | 100 |
| Naive (0) | 1×104 | 0/5 | 0 |
One week after 1×105 PbiSPZ (wt or spect (−)) immunization, BALB/c mice were challenged with 1×104 live ANKA wt PbSPZ. Parasitemia was checked at 1 week and 2 weeks post challenge and mice were considered protected when they remained parasite negative 2 weeks after challenge.
Discussion
Data presented in this manuscript provide further evidence for the role of Plasmodium infected hepatocytes in the stimulation of protective secondary CD8+ T-cells leading to the elimination of P. berghei pre-erythrocytic stages. In addition, they indicate that hepatocytes can indeed prime sporozoite-specific, protective naïve T-cells. While the antigen-presenting role of infected hepatocytes in the secondary CD8+ T-cell response seems to be accepted, the mode of priming naïve CD8+ T-cells is still, in our opinion, not yet established. With regard to the first point, the in vivo data presented here are fully consistent with our previous in vitro results [6], [20] and with in vivo data published by Zavala and collaborators [7] via bone marrow cell transfer experiments. In our case, we transferred either BALB/c infected hepatocytes or a CS specific T-cell clone or both to TAP-deficient H-2kb mice. Tap−/−mice were chosen to bypass the possibility that antigen presentation is mediated by professional antigen presenting cells which might have ingested apoptotic infected hepatocytes [8] or live sporozoites possibly externally associated with BALB/c hepatocytes, and minimize host vs graft immune responses. In addition, we have determined protection as lack of infection in mice 14 days post-challenge, which represents a stringent, but the only significant standard for protection for pre-erythrocytic vaccines. Our results show that protection from infection is antigen-specific since mice are not protected if an irrelevant CD8+ T-cell clone is used. In addition, as observed in vitro [6], our results indicate that infected hepatocytes are directly killed by the antigen- specific T cells and not by a bystander effect through continuous secretion of IFN-γ or other lymphokines by the injected T-cell clones or host vs graft immune response since concomitant infection of TAP-deficient mice does not lead to protection after treatment with the CS specific T-cell clone and/or naïve BALB/c hepatocytes. These and the previous data [6], [7] clearly establish the central role of infected hepatocytes in the total clearance of Plasmodium infection in vivo once an immune response has been induced (secondary response). However, in our opinion, the role of hepatocytes in priming naïve, T-cells is not yet elucidated. Zavala and collaborators [7], [16] claim that peripheral dendritic cells prime CD8+ T-cells, while Leiriao et al. [8] suggest that apoptotic infected hepatocytes provide antigens to liver dendritic cells. In addition, these claims seem to be supported by the notion that hepatocytes act as tolerizing cells [27], [28]. On the other hand, given the large number of hepatocyte genes affected by sporozoites and salivary gland components, including some related to antigen processing and presentation and chemokine production it is not far fetched to hypothesize that infected hepatocytes become full-fledged antigen presenting cells upon infection [29], [30]. This would allow the activation of T-cells specific to sporozoite and late liver stage antigens (14). This would optimize the balance between infection and immune responses that parasites and hosts have developed through co-evolution. Evidence that the liver is central to obtaining an optimal immune response was provided early on by Renia et al [10] in which similar results as obtained here were presented, where immunization with non-parenchymal cells did not result in protection. Recent data by Epstein et al. [12] in which a better immune response was obtained by immunization of mice with irradiated sporozoites via iv than id or sc injection also point to the antigen-presenting role of the liver. In this study, we specifically target the role of infected hepatocytes in the immune response by immunizing (iv) mice with spect (−) iSPZ that infect hepatocytes without prior traversal. We show that, in spite of a significant decrease in parasite DNA load, the liver CSP-specific CD8+ T-cell response was similar to that in wt iSPZ-immunized groups. This and cyt c treatment of mice prior to immunization with iSPZs further support the key role of infected hepatocytes in T-cell priming to provide protective immune responses.
In conclusion, the data provided show that hepatocytes can indeed present Plasmodium berghei CSP epitopes to primed CD8+ T-cells and strongly suggest that they could also prime parasite-specific naïve T-cells to fully protect mice against a live parasite challenge. But in our opinion, formal proof of the role of hepatocytes in antigen presentation can only be obtained by isolating malaria parasite infected hepatocytes for in vivo and in vitro experiments.
Acknowledgments
We thank HR McDonald for the gift of TAP −/− mice, I Lüscher for help in preparing the tetramers, R. Menard for providing the spect (−) parasites and the University of Lausanne for in-house support.
Funding Statement
The authors have no support or funding to report.
References
- 1. Jobe O, Lumsden J, Mueller AK, Williams J, Silva-Rivera H, et al. (2007) Genetically Attenuated Plasmodium berghei Liver Stages Induce Sterile Protracted Protection That Is Mediated by Major Histocompatibility Complex Class I–Dependent Interferon-g–Producing CD8+ T-Cells. . J Infect Dis 196: 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scheller LF, Azad AF (1995) Maintenance of protective immunity against malaria by persistent hepatic parasites derived from irradiated sporozoites. . Proc Natl Acad Sci U S A 92: 4066–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Romero P, Maryanski JL, Corradin G, Nussenzweig RS, Nussenzweig V, et al. (1989) Cloned cytotoxic T-cells recognize an epitope in the circumsporozoite protein and protect against malaria. . Nature 341: 323–326. [DOI] [PubMed] [Google Scholar]
- 4. Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, et al. (1987) Gamma interferon, CD8+ T-cells and antibodies required for immunity to malaria sporozoites. . Nature 330: 664–666. [DOI] [PubMed] [Google Scholar]
- 5. Grüner AC, Mauduit M, Tewari R, Romero JF, Depinay N, et al. (2007) Sterile Protection against Malaria Is Independent of Immune Responses to the Circumsporozoite Protein. . PLoSOne 2: e1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bongfen SE, Torgler R, Romero JF, Renia L, Corradin G (2007) Plasmodium berghei-infected primary hepatocytes process and present the circumsporozoite protein to specific CD8+T-cells in vitro. . J Immunol 178: 7054–7063. [DOI] [PubMed] [Google Scholar]
- 7. Chakravarty S, Cockburn IA, Kuk S, Overstreet MG, Sacci JB, et al. (2007) CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. . Nat Med 13: 1035–1041. [DOI] [PubMed] [Google Scholar]
- 8. Leiriao P, Mota MM, Rodriguez A (2005) Apoptotic Plasmodium-Infected Hepatocytes Provide Antigens to Liver Dendritic Cells. . J Infect Dis 191: 1576–81. [DOI] [PubMed] [Google Scholar]
- 9. Renia L, Maranon C, Hosmalin A, Gruner AC, Silvie O, et al. (2006) Do apoptotic Plasmodium-infected hepatocytes initiate protective immune responses? . J Infect Dis 193: 163–4. [DOI] [PubMed] [Google Scholar]
- 10. Renia L, Rodrigues MM, Nussenzweig V (1994) Intrasplenic immunization with infected hepatocytes: a mouse model for studying protective immunity against malaria pre-erythrocytic stage. . Immunology 82: 1164–168. [PMC free article] [PubMed] [Google Scholar]
- 11. Mota MM, Pradel G, Vanderberg JP, Hafalla JC, Frevert U, et al. (2001) Migration of Plasmodium sporozoites through cells before infection. . Science 291: 141–144. [DOI] [PubMed] [Google Scholar]
- 12. Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, et al. (2011) Live attenuated malaria vaccine designed to protect through hepatic CD8+ T-cell immunity. . Science 334: 475–80. [DOI] [PubMed] [Google Scholar]
- 13. Voza T, Kebaier C, Vanderberg JP (2010) Intradermal immunization of mice with radiation-attenuated sporozoites of Plasmodium yoelii induces effective protective immunity. . Malar J 9: 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Douradinha B, van Dijk M, van Gemert GJ, Khan SM, Janse CJ (2011) Immunization with genetically attenuated P52-deficient Plasmodium berghei sporozoites induces a long-lasting effector memory CD8+ T-cell response in the liver. . J Immune Based Ther Vaccines 9: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frevert U, Engelmann S, Zougbédé S, Stange J, Ng B, et al. (2005) Intravital observation of Plasmodium berghei sporozoite infection of the liver. . PLoS Biol 3: e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cockburn IA, Tse SW, Radtke AJ, Srinivasan P, Chen YC, et al. (2011) Dendritic Cells and Hepatocytes Use Distinct Pathways to Process Protective Antigen from Plasmodium in vivo. . PLoS Pathog 7: e1001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Kaer L, Ashton-Rickardt PG, Ploegh HL, Tonegawa S (1992) TAP1 Mutant are deficient in Antigen presentation, Surface Class I Molecules, and CD4–8+ T cells. . Cell press 71: 1205–1214. [DOI] [PubMed] [Google Scholar]
- 18. Chefalo PJ, Grandea AG 3rd, Van Kaer L, Harding CV (2003) Tapasin −/− and TAP1−/− Macrophages Are Deficient in Vacuolar Alternate Class I MHC (MHC-I) Processing due to Decreased MHC-I Stability at Phagolysosomal pH1. . J Immunol 170: 5825–5833. [DOI] [PubMed] [Google Scholar]
- 19. Ishino T, Yano K, Chinzei Y, Yuda M (2004) Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. . PloS Biol 2: E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bongfen SE, Balam S, Torgler R, Romero JF, Corradin G (2008) Processing of the circumsporozoite protein in infected hepatocytes is not dependent on aspartic proteases. . Parasite Immunol 30: 375–378. [DOI] [PubMed] [Google Scholar]
- 21. Luescher IF, Anjuère F, Peitsch MC, Jongeneel CV, Cerottini JC, et al. (1995) Structural analysis of TCR-ligand interactions studied on H-2Kd-restricted cloned CTL specific for a photoreactive peptide derivative. . Immunity 3: 51–63. [DOI] [PubMed] [Google Scholar]
- 22. Seglen PO (1976) Preparation of isolated rat liver cells. . Methods Cell Biol 13: 29–83. [DOI] [PubMed] [Google Scholar]
- 23. Meis JF, Verhave JP, Jap PH, Meuwissen JH (1983) An ultrastructural study on the role of Kupffer cells in the process of infection by Plasmodium berghei sporozoites in rats. . Parasitology 86: 231–242. [DOI] [PubMed] [Google Scholar]
- 24. Seguin MC, Ballou WR, Nacy CA (1989) Interaction of Plasmodium berghei sporozoites and murine kupffur cells. . J Immunol 143: 1716. [PubMed] [Google Scholar]
- 25. Torgler R, Bongfen SE, Romero JC, Tardivel A, Thome M, et al. (2008) Sporozoite-mediated hepatocyte wounding limits plasmodium parasite development via MyD88-mediated NF- κB cctivation and inducible NO synthase expression. . J Immunol 180: 3990–3999. [DOI] [PubMed] [Google Scholar]
- 26. Lin ML, Zhan Y, Proietto AI, Prato S, Wu L, et al. (2008) Selective suicide of cross-presenting CD8+ dendritic cells by cytochrome c injection shows functional heterogeneity within this subset. . Proc Natl Acad Sci U S A 105: 3029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mehal WZ, Azzaroli F, Crispe IN (2001) Antigen presentation by liver cells controls intrahepatic t cell trapping, whereas bone marrow-derived cells preferentially promote intrahepatic T cell apoptosis. . J Immunol 167: 667–673. [DOI] [PubMed] [Google Scholar]
- 28. Crispe IN, Giannandrea M, Klein I, John B, Sampson B, et al. (2006) Cellular and molecular mechanisms of liver tolerance. . Immunol Rev 213: 101–118. [DOI] [PubMed] [Google Scholar]
- 29. Chattopadhyay R, de la Vega P, Paik SH, Murata Y, Ferguson EW, et al. (2011) Early transcriptional responses of HepG2-A16 liver cells to infection by Plasmodium falciparum sporozoites. . J Biol Chem 286: 26396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Albuquerque SS, Carret C, Grosso AR, Tarun AS, Peng X, et al. (2009) Host cell transcriptional profiling during malaria liver stage infection reveals a coordinated and sequential set of biological events. . BMC Genomics 10: 270. [DOI] [PMC free article] [PubMed] [Google Scholar]