Abstract
Dendritic cells (DCs) are powerful activators of primary and secondary immune responses and have promising activity as anticancer vaccines. However, various populations of immune cells, including natural killer cells, regulatory T cells and especially cytotoxic T lymphocytes (CTLs), can inhibit DC function through cytotoxic clearance. Spontaneous tumor-specific CTL responses are frequently observed in patients before immunotherapy, and it is unclear how such pre-existing responses may affect DC vaccines. We used an adoptive transfer model to show that DC vaccination fail to induce the expansion of pre-existing CTLs or increase their production of interferon γ (IFNγ). The expansion and effector differentiation of naïve host CD8+ T cells was also suppressed in the presence of CTLs of the same specificity. Suppression was caused by the cytotoxic functions of the adoptively transferred CTLs, as perforin-deficient CTLs could respond to DC vaccination by expanding and increasing IFNγ production. Proliferation and effector differentiation of host CD8+ T cells as well as resistance to tumor challenge were also significantly increased. Expression of perforin by antitumor CTLs was critical in regulating the survival of vaccine DCs, while FAS/FASL and TRAIL/DR5 had a significant, but comparatively smaller, effect. We conclude that perforin-expressing CTLs can suppress the activity of DC-based vaccines and prevent the expansion of naïve and memory CD8+ T cells as well as antitumor immune responses. We suggest that, paradoxically, temporarily blocking the cytotoxic functions of CTLs at the time of DC vaccination should result in improved vaccine efficiency and enhanced antitumor immunity.
Keywords: cytotoxicity, dendritic cells, perforin, rodent, T cells, tumor immunotherapy
Introduction
Dendritic cells (DCs) are powerful antigen-presenting cells whose role in the initiation of primary CD4+ and CD8+ T-cell immune responses, as well as in the reactivation of memory CD8+ T-cell responses, is well recognized.1 Because of their high immunogenicity, DCs loaded with different types of antigenic tumor material, including peptides, tumor lysates or apoptotic tumor cells, have been used as vaccines in several studies of tumor immunotherapy (reviewed in ref. 2). The results of these studies have confirmed that DC-based vaccines can successfully elicit antitumor immune responses and, in some cases, generate objective clinical responses.
Tumor progression is the result of an ongoing reciprocal interaction between malignant cells and the immune system.3 As a consequence, patients frequently show spontaneous immune response to antigens expressed by tumor cells.4 Tumor-specific CD8+ cytotoxic T lymphocytes (CTLs) can be observed in the peripheral blood, secondary lymphoid organs and at the tumor site. These T cells may show an activated phenotype5 but their characterization has often revealed various degrees of functional impairment6 including signaling defects,7 expression of markers associated with inactivation,8,9 and altered cytokine responses.10,11 In contrast, the cytotoxic activity of tumor-specific CD8+ T cells is mostly preserved.12 Immunotherapy aims at re-programming the immune response from a status of functional unresponsiveness to the activation of effector functions.13
A few studies have examined the impact of existing antitumor immune responses on the success of subsequent immunotherapies. In the case of DC-based vaccines, it is known that T cells have the capacity to clear antigen-presenting DCs,14,15 but the impact of such killing on antitumor T-cell responses has not been fully examined.
In this study, we examine the effect of CTL-mediated cytotoxicity on the induction of antitumor T cell responses by DC-based vaccines. We used mutant CTLs and/or DCs to characterize the contribution of different cytotoxic mechanisms to DC killing. We also show that wild-type (WT) CTL, but not CTL that are defective in cytotoxic functions, strongly inhibit the induction of primary and memory T-cell responses by DC-based vaccines as well as the boosting of antitumor immune responses. Our results suggest that existing antitumor immune responses can have a substantial effect on the impact of DC-based immunotherapy and predict that temporary inhibition of cytotoxic functions may result in improved therapeutic effect of DC vaccination.
Results
Multiple cytotoxic mechanisms contribute to the clearance of DC-based vaccines by CTLs in vivo
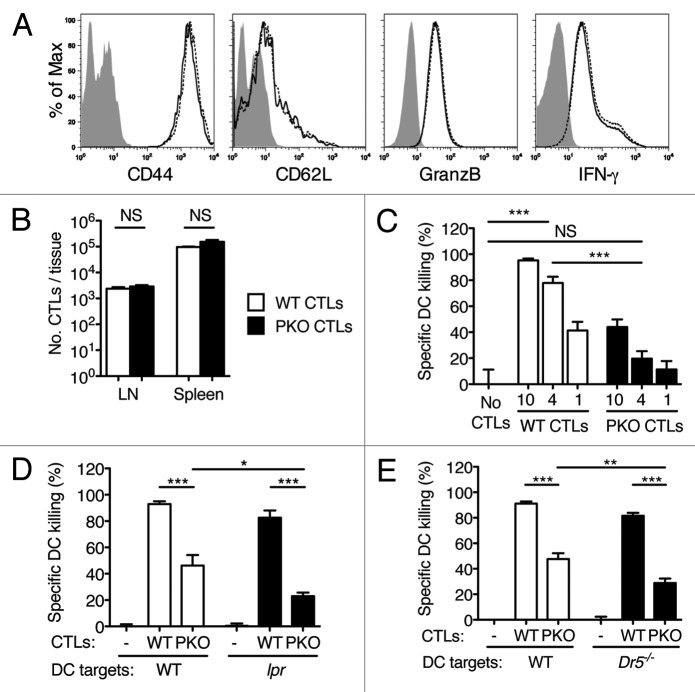
We wished to examine the effect of CTL- and non CTL-mediated cytotoxicity on the ability of DC-based vaccines to re-stimulate antitumor immune responses. We used an adoptive transfer model in which T-cell receptor TCR-transgenic WT or perforin knockout (PKO) CTLs were activated in vitro and injected i.v. into C57BL/6 hosts. In these mice, specific CTLs differentially expressed perforin, while all other cell populations including natural killer (NK) cell sand regulatory T cells (Tregs) were perforin sufficient. WT and PKO CTLs were recovered from in vitro cultures in similar numbers (not shown), and expressed similar levels of activation markers and of the cytotoxic granule protein granzyme B (Fig. 1A). Antigen (Ag)-dependent interferon γ (IFNγ) production (Fig. 1A), FASL expression and granule exocytosis (data not shown) by the two populations were also similar. After adoptive transfer, WT and PKO CTLs were recovered from both lymphoid (Fig. 1B) and non-lymphoid (not shown) tissues in similar numbers. Thus, WT and PKO CTLs did not differ to notable extents in terms of activation status or in vivo distribution.
Figure 1. CTL-mediated killing of DCs in vivo is mainly perforin-dependent and is modestly affected by inactivation of the FAS/FASL or TRAIL/DR5 pathways. (A-E) WT and PKO CTL were generated in vitro by culturing TCR-transgenic T cells with DCs and Ag. Activated T cells were expanded in IL-2 for 1–2 d, and transferred i.v. into naïve syngeneic recipients. One day later recipient mice were injected s.c. with DCs loaded or not with Ag. DC survival was determined in the draining LN 48 h after DC injection. (A) CTL phenotype before transfer. WT and PKO L318 CTLs were generated as described, re-stimulated with specific peptide (IFNγ only) and examined for the expression of the indicated activation markers by flow cytometry. Filled histograms: unstained CTLs; solid line, WT CTLs; dashed line, PKO CTLs. (B) Recovery of WT and PKO L318 CTLs from the spleen and LNs of naïve recipients one day after transfer. The bar graph shows means + SEM for 3 mice/group. (C) In vivo killing of Ag-loaded WT DCs by WT or PKO L318 CTLs. Recipient mice were injected with 10, 4 or 1 million CTLs as indicated; DC killing was examined in draining LN 48h after DC challenge. The bar graph shows means + SEM for 3 mice/group. (D, E) In vivo killing of Ag-loaded WT, Faslpr or Dr5−/− DCs by WT and PKO OT-I CTLs. Recipient mice were injected with 5 × 106 CTLs and 0.5 × 106 DCs; DC killing was examined in draining LN 48 h after DC challenge. Bar graphs show means + SEM for groups of 3–6 mice, and are representative of 2–8 experiments that gave similar results. NS: not significant; *: 0.01 < p < 0.05; **: 0.001 < p < 0.01; ***: p < 0.001 by one-way ANOVA with Tukey’s post-test.
We examined the survival of vaccine DCs in C57BL/6 mice injected with WT or PKO CTLs. WT CTLs effectively killed Ag-loaded WT DCs injected s.c., and prevented their accumulation in draining lymph nodes (LNs), while PKO CTLs were about 10-fold less effective in doing so (Fig. 1C). To examine cytotoxic mechanisms other than those mediated by perforin, mice were injected with WT or PKO CTLs and then challenged with WT DCs or DCs lacking death receptors. This setup enabled us to test the combined effect of multiple defects in cytotoxic function. As shown in Figure 1D, Ag-loaded WT DCs were almost completely eliminated by WT CTLs. Perforin deficiency reduced the effectiveness of DC killing by half, whereas FAS inactivation had a more modest effect. When both perforin and FAS were simultaneously inactivated, DC killing was significantly reduced as compared with the inactivation of perforin alone, but was not completely abolished. Similar results were obtained using DCs from Dr5−/− mice (Fig. 1E). Thus, inactivating DR5 had a small but significant effect on the susceptibility of DCs to killing by PKO CTLs, but the simultaneous inactivation of both perforin and the DR5/TRAIL pathway was not sufficient to abolish killing. Thus, perforin is the main effector mechanism underlying the killing of DCs by CTLs, while FASL and TRAIL have a measurable, but less prominent, role.
PKO CTLs respond more vigorously than WT CTLs to s.c. vaccination with DCs loaded with Ag
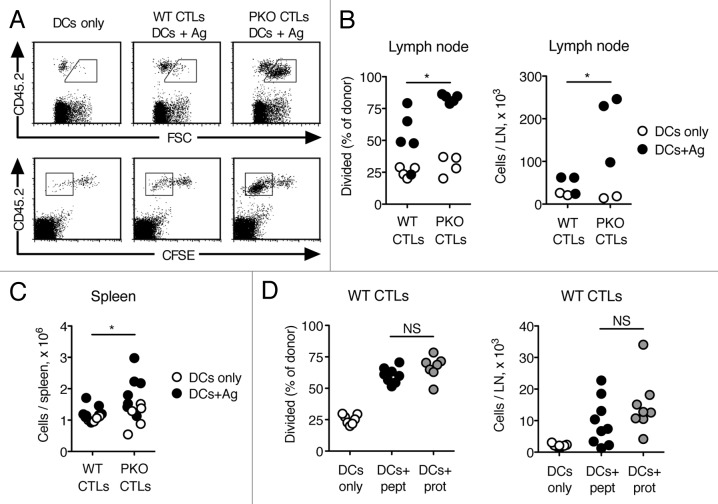
We compared the response of WT and PKO CTLs to DC vaccination. Vaccination with DCs and no Ag induced some expansion of CTLs in the LN draining the vaccination site. However, the size of these cells remained relatively small (Fig. 2A). Immunization with DCs loaded with Ag led to a modest increase in the cell size and the division of WT CTLs (Fig. 2A and B). In contrast, PKO CTLs increased in size and proliferated vigorously (Fig. 2A and B). Similarly, the numbers of WT CTLs in LN were only slightly increased in mice injected with DC loaded with Ag as compared with animals receiving DCs only, while the numbers of PKO CTLs were significantly higher (Fig. 2B). The numbers of WT CTLs found in the spleen on day 7 after vaccination (Fig. 2C), and their frequency in blood (not shown) were also consistently lower than those of PKO CTLs in similarly vaccinated mice. Thus, the response of PKO CTLs to DC vaccines is stronger than the response of WT CTLs.
Figure 2. PKO CTLs respond more vigorously than WT CTLs to vaccination with Ag-loaded DCs. (A-D) WT CTLs and PKO CTLs were generated as described in the legend to Figure 1, labeled with CFSE and transferred i.v. into CD45-congenic recipients. One day later recipient mice were vaccinated s.c. with Ag-loaded DCs or DCs only, and CTL responses were determined as detailed below. (A) Representative flow plots of WT and PKO L318 CTLs recovered from draining LN 3 d after immunization with DCs only or Ag-loaded DCs. (B) Expansion of WT and PKO L318 CTLs in draining LNs was determined 3 d after vaccination with Ag-loaded DCs or DCs only. Data are from the same experiment shown in (A). (C) Numbers of WT and PKO L318 CTLs were determined in the spleen 7 d after s.c. vaccination with Ag-loaded DCs or DC only. (D) Expansion of WT OT-I CTLs in draining LNs was determined 3 d after vaccination with DCs loaded with peptide Ag (DCs+pept), DCs loaded with protein Ag (DCs+prot), or DCs only. Each dot represents one mouse. Data are from one of 2–6 experiments that gave similar results (A, B), or combined from two independent experiments (C, D). Statistical analysis refers to the comparison between mice that received DCs loaded with Ag. NS: not significant; *: 0.01 < p < 0.05; **: 0.001 < p < 0.01 by Student's t-test (B, C) or one-way ANOVA with Tukey’s post-test (D).
The experiments described above used DCs loaded with peptide Ag. We wished to determine whether DCs loaded with whole protein Ag would show a superior ability to induce the proliferation of WT CTLs in vivo. We used OT-I CTLs and DCs loaded with ovalbumin (OVA) at 2 mg/mL, after preliminary experiments showed that this dose of OVA is sufficient for efficient cross-presentation and results in Ag presentation in LNs. As shown in Figure 2D, immunization with DCs loaded with peptide or protein Ag induced comparable extents of division of WT CTLs as well as comparable numbers of CD8+ T cells in the draining LN. Therefore, loading DCs with protein vs. peptide Ag does not change the response of WT CTLs to DC vaccines.
The increased response of PKO CTLs to DC vaccination is cell extrinsic
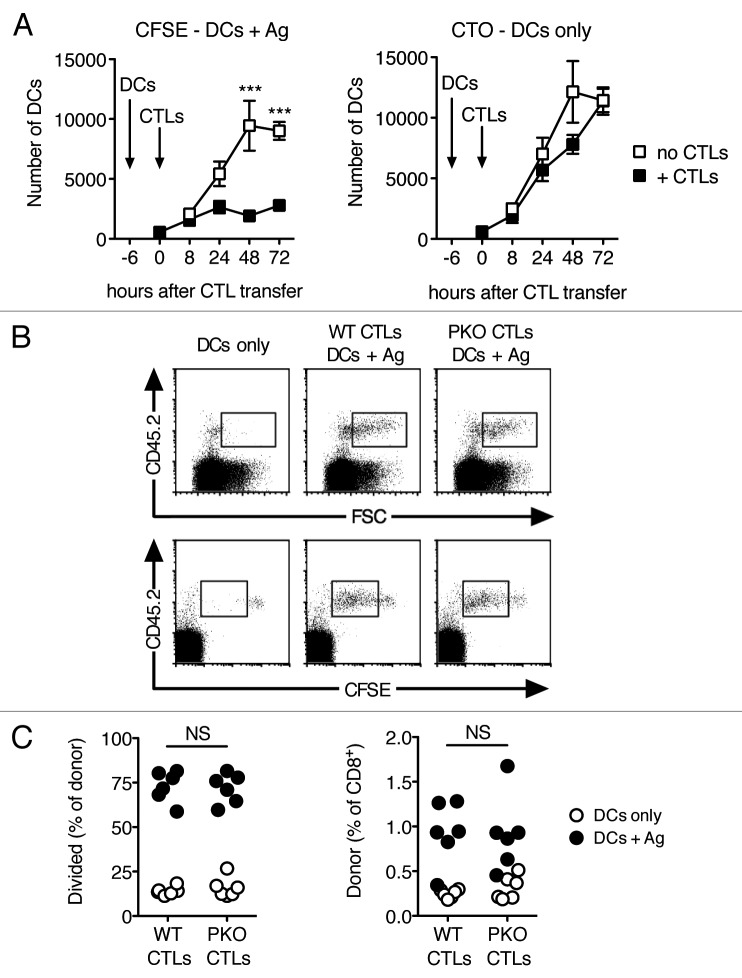
The stronger proliferation of PKO CTLs compared with that of WT CTLs might be due to the intrinsic toxicity of perforin,16 or to extrinsic factors. To address this possibility, we designed an experiment in which WT and PKO CTLs were exposed to similar numbers of Ag-loaded DCs in the LN, as cell-intrinsic effects of perforin should still affect the response in this scenario. Naïve recipient mice were injected with Ag-loaded DCs first, and then with CTLs at a later time point. In these conditions, CTLs can stop the accumulation of DCs in the LN, but have little effect on the DCs that are already in the LN or its close proximity (Fig. 3A).
Figure 3. WT and PKO CTLs respond equally well to Ag presented in the LN. (A) Naïve mice were injected s.c. with a 1:1 mix of CFSE-labeled Ag-loaded DCs, and CTO-labeled DCs with no Ag. Six h later, half of the mice were injected i.v. with OT-I CTLs activated in vitro as described in Figure 1. At the indicated time points, mice were sacrificed and the numbers of CFSE+ and CTO+ DCs in draining LNs were evaluated by flow cytometry. Each symbol shows mean ± SEM for a group of 3–6 mice; data are from one of two experiments that gave similar results. (B, C) WT and PKO L318 CTLs were generated as described, labeled with CFSE and transferred i.v. into CD45-congenic recipients that had been injected s.c. one day earlier with Ag-loaded DCs or DCs only. CTL responses were examined in draining LNs 66 h after CTL transfer. (B) Representative flow plots of live CD8+ cells recovered from the draining LNs of mice adoptively transferred with WT or PKO CTLs and immunized with DCs only or Ag-loaded DCs. Gates highlight large activated cells (top row) or divided cells (lower row). (C) Expansion of WT and PKO CTLs in draining LNs of mice vaccinated with DCs only or Ag-loaded DCs. Each dot represents one mouse, gating was as in (B). Combined data from two independent experiments are shown. NS: not significant.
Mice were injected with Ag-loaded DCs, and then given CFSE-labeled WT or PKO CTLs 24 h later. CTL proliferation was evaluated in the draining LN 66 h after the transfer of CTLs. As shown in Figure 3B, both WT and PKO CTL populations increased in cell size and divided in response to DCs loaded with Ag, with the responses of these CTL populations being similar to each other. The percentages of WT and PKO CTLs were also comparably increased (Fig. 3C), suggesting a similar expansion of the two populations. We conclude that WT and PKO CTLs have a similar ability to respond to Ag when this is appropriately presented in the LN.
WT, but not PKO, CTLs inhibit the response of naïve CD8+ and CD4+ T cells to DC vaccination
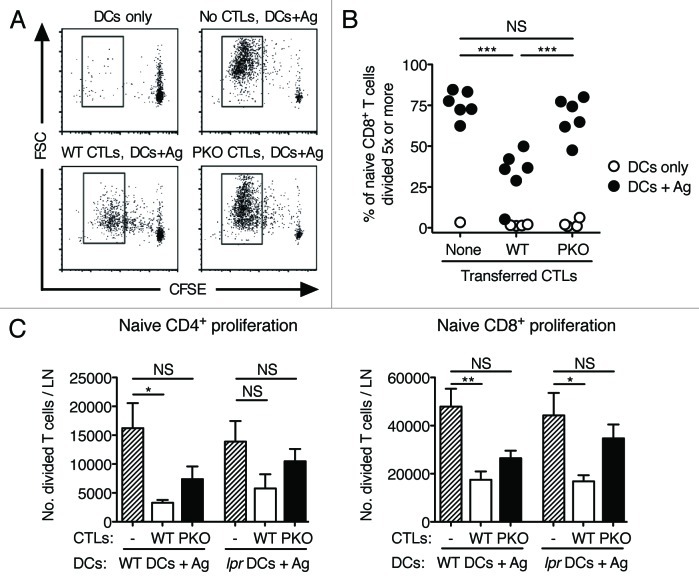
We next asked whether WT and PKO CTLs would differentially affect the proliferation of naïve T cells of the same specificity. Naïve C57BL/6 mice were injected i.v. with CFSE-labeled CD45.1+ naive CD8+ T cells together with CD45.1- WT CTLs, PKO CTLs, or no CTLs. Mice were vaccinated with DCs loaded with Ag or DCs only, and CD8+ T-cell proliferation was evaluated in the draining LN 3 d later. As shown in Figure 4A, proliferation was observed only in mice vaccinated with DCs loaded with Ag. In the absence of CTLs, naïve CD8+ T cells increased in cell size and underwent several cycles of division. When WT CTLs were present, CD8+ T cells underwent fewer divisions, and their cell size was only modestly increased. In contrast, PKO CTLs had almost no effect on the response of CD8+ T cells, which proliferated and increased in cell size almost as vigorously as in the absence of CTLs. The results of this experiment are summarized in Figure 4B, which shows the percentage of CD45.1+CD8+ cells that divided 5 times or more. Similarly, Figure 4C shows that, in mice vaccinated with DCs loaded with MHC Class I and Class II-restricted peptides, the number of CD8+ and CD4+ T cells, respectively, that underwent division in the draining LN was significantly lower in the presence of WT CTLs. Inactivation of perforin-dependent killing in CTLs partly reversed this decrease, while inactivation of FAS-dependent cytotoxicity had a small, non-significant effect. When perforin-dependent and FAS-dependent killing were simultaneously inactivated by using PKO CTLs and lpr DCs, the effect of CTLs on the division of naïve CD4+ and CD8+ T cells was mostly reversed.
Figure 4. The proliferation of naïve CD4+ and CD8+ cells after vaccination with Ag-loaded DCs is inhibited by WT, but not PKO, CTLs. (A-C) WT and PKO CTLs were generated as described in the legend to Figure 1 and transferred i.v. into syngeneic recipients together with CD45-congenic, CFSE-labeled naïve T cells. One day later, mice were vaccinated with Ag-loaded DCs or DCs only. Proliferation of the naïve T-cell population was assessed in draining LNs 3 d after vaccination. (A) Representative flow plots of naive L318 CD8+ T cells recovered from the draining LNs of mice adoptively transferred with L318 CTLs and immunized with DCs. (B) Percent naïve L318 CD8+ T cells divided 5 times or more, recovered from the draining LNs of the indicated experimental groups. Data are from one of two experiments that gave similar results and are from the same experiment shown in (A). Each symbol corresponds to one mouse. (C) Numbers of divided, naïve OT-I CD8+ T cells and OT-II CD4+ T cells recovered from the draining LNs of mice that were adoptively transferred with 5 × 106 WT or PKO OT-I CTLs, and immunized with 0.5 × 106 WT or Faslpr DCs. Bar graphs show means + SEM for groups of 4–10 mice. NS: not significant; *: 0.01 < p < 0.05; **: 0.001 < p < 0.01; ***: p < 0.001 by one-way ANOVA with Tukey’s post-test.
Increased cytokine production by CD8+ T cells after DC vaccination is inhibited by WT but not PKO CTLs
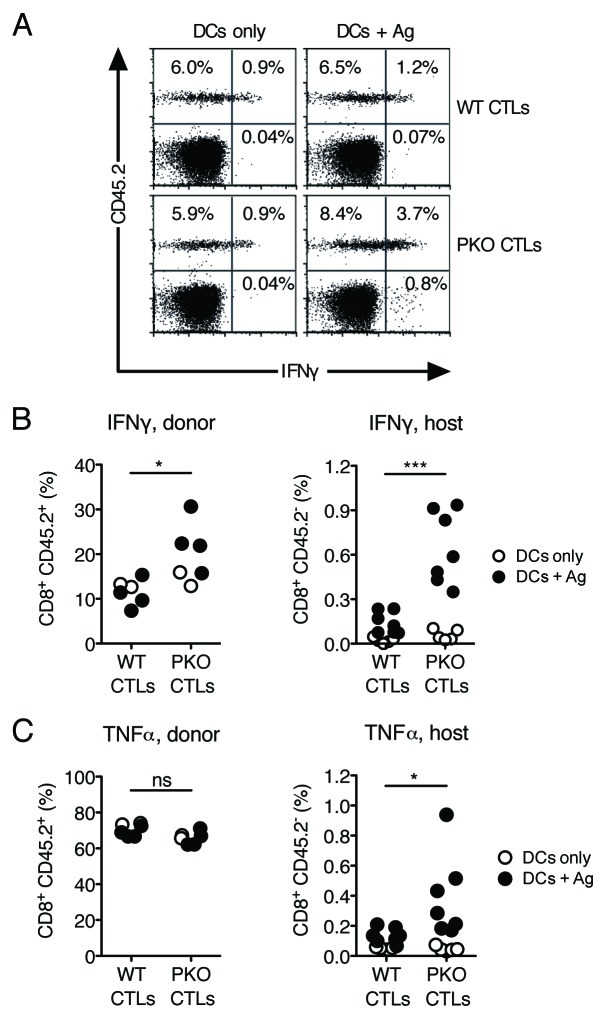
We wished to determine whether differential CD8+ T-cell proliferation in the presence of WT or PKO CTLs is also associated with differences in cytokine production. Mice were injected with CTLs and vaccinated with DCs as described above, and intracellular cytokine production was examined in the spleen 7 d later. As shown in Figure 5A and B, WT and PKO CTLs from mice vaccinated with DCs only were able to produce IFNγ upon in vitro re-stimulation, as would be expected for pre-activated memory cells, while cytokine production by host CD8+ T cells was undetectable. Vaccination with DCs loaded with Ag did not detectably increase IFNγ production, either by WT CTLs, or by the host CD8+ T-cell population, suggesting that DC vaccination had little impact on immune responses in these mice. Production of tumor necrosis factor α (TNFα) by WT and PKO CTLs was already very high (Fig. 5C), and did not further increase upon DC vaccination. In contrast, the production of IFNγ by PKO CTLs was substantially increased after vaccination with Ag-loaded DCs, and was significantly higher than that by WT CTLs (Fig. 5B). Priming of host CD8+ T cells to IFNγ and TNFα production (Fig. 5B and C) was also observed, suggesting that in these mice vaccination with DCs loaded with Ag induces the activation of naïve cells. We conclude that WT CTLs inhibit the ability of DC vaccines to prime naïve CD8+ T cells to IFNγ and TNFα production.
Figure 5. The increase in frequency of IFNγ-producing CD8+ T cells after vaccination with Ag-loaded DCs is inhibited by WT, but not PKO, CTLs. (A-C) CD45.1 mice were injected with CD45.2+ WT or PKO L318 CTLs and vaccinated with Ag-loaded DCs or DCs only as described in the legend to Figure 2. Cytokine production by transferred CTLs and by the host naïve T-cell population was assessed in the spleen 7 d after DC immunization. (A) Representative flow plots of total CD8+ T cells from spleens in the indicated treatment groups. Spleen cells were re-stimulated in vitro in the presence of specific Ag and stained for intracellular IFNγ. (B) Percentages of adoptively transferred CTLs (left) and host resident CD8+ T cells (right) that were IFNγ+ by in vitro re-stimulation and intracellular staining. (C) Percentages of adoptively transferred CTLs (left) and host resident CD8+ T cells (right) that were TNFα+ by in vitro re-stimulation and intracellular staining. Each dot plot shows combined data from two independent experiments. Each symbol corresponds to one mouse. Statistics refer to the comparison between mice adoptively transferred with WT or PKO CTLs, and immunized with Ag-loaded DCs. NS: not significant; *: 0.01 < p < 0.05; ***: p < 0.001 by a two-tailed unpaired Student’s t-test.
DC vaccines fail to boost antitumor immunity in mice adoptively transferred with WT CTLs
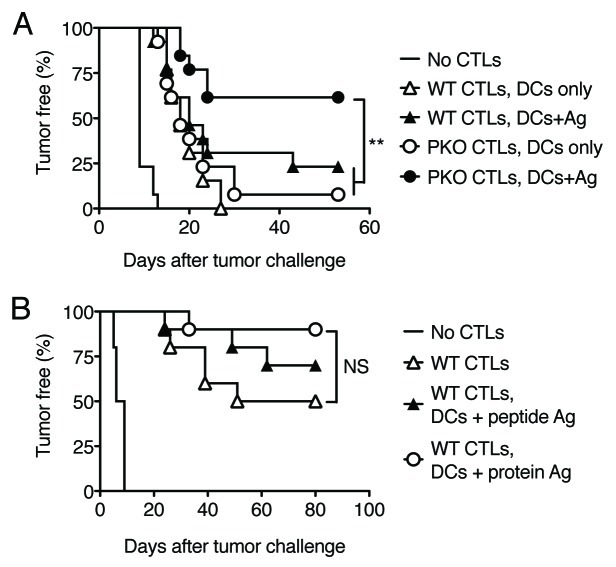
We wished to determine whether increased responses to DC vaccination in mice receiving PKO vs. WT CTLs might also correlate with improved tumor resistance. We used a Lewis lung carcinoma (LL-LCMV) model, as our previous work indicates that IFNγ is critical for rejection of this tumor,17 while perforin is not required (data not shown). Mice received WT or PKO CTLs at a dose that confers only partial tumor protection, and they were vaccinated on the next day with DCs without Ag or Ag-loaded DCs to boost the response. As shown in Figure 6A, mice injected with WT or PKO CTLs and vaccinated with DCs without Ag developed tumors at similar rates, which were slower than the rates observed in naïve mice. Vaccination with DCs loaded with Ag modestly delayed tumor growth in mice injected with WT CTLs, but significantly improved survival in mice injected with PKO CTLs. Thus, the vaccine activity of Ag-loaded DCs is inhibited by CTLs via their cytotoxic function.
Figure 6. Vaccination Ag-loaded DCs boosts tumor protection in mice adoptively transferred with PKO, but not WT, CTLs. (A, B) WT CTL and PKO CTLs were generated as described in the legend to Figure 1 and transferred i.v. into naive recipients. One day later recipient mice were vaccinated s.c. with DC loaded with peptide Ags, DC loaded with protein Ags, or DC only, and challenged with tumor cells 5 d later. Tumor appearance was monitored every 2–3 d and mice were scored as tumor positive when tumor size was in excess of 4 mm2. (A) Mice were injected with 5 × 106 WT or PKO L318 CTLs and challenged with LL-LCMV tumors. The graph shows combined data from two independent experiments with a total of 13 mice/group. (B) Mice were injected with 1 × 106 WT OT-I CTLs and challenged with B16-OVA tumor cells. The graph shows combined data from two independent experiments with a total of 10 mice/group. NS: not significant; **: 0.001 < p < 0.01 by a log-rank test.
We then asked whether vaccination with DCs loaded with protein, instead of peptide, Ag might be more effective at improving the response of WT CTLs to an OVA-expressing B16 melanoma. PKO CTLs were not used in this experiment, since their ability to control the growth of B16-OVA tumors is impaired by perforin deficiency (data not shown). As shown in Figure 6B, vaccination with DCs loaded with peptide Ag or with protein Ag did not significantly boost the ability of WT CTLs to delay the growth of B16-OVA tumors. In addition, DCs loaded with protein Ag were not superior to DC loaded with peptide Ag in boosting the response of WT CTLs.
Discussion
In this paper, we demonstrate that pre-existing cytotoxic antitumor immunity has a substantial inhibitory effect on the efficacy of DC vaccines. Our studies were based on an adoptive transfer model that enabled us to carefully control the activation status and numbers of T cells transferred in vivo, as well as to select experimental conditions whereby transferred CTLs exactly reproduced the findings in immunized mice,18 hence generating results that are relevant to physiological responses. This experimental setting enabled us to closely examine the mechanism by which CTLs inhibit immune responses, by eliminating the contribution of other immune cells such as NK or Tregs, and by allowing for the discrimination between the responses of the CTLs themselves and those of naïve host cells. Using this model, we demonstrate that CTLs can suppress primary and secondary immune responses as induced by DC vaccination, leading to impaired boosting of antitumor immunity. Importantly, we also find that reducing the cytotoxic function of CTLs through the genetic inactivation of perforin results in a significantly improved ability to generate strong primary and memory immune responses to DC vaccines, as well as in improved tumor rejection. Thus, perforin expressed by CTLs mediates a powerful immunoregulatory role that limits the efficacy of tumor immunotherapy.
We considered the possibility that DCs loaded with peptide Ag, as used in several of our experiments, might be especially sensitive to CTL-mediated killing due to the high expression of MHC class I-peptide complexes at their surface.19 To assess this possibility, we compared DCs loaded with peptide Ag to DCs loaded with protein Ag, which requires processing and generates lower numbers of surface MHC Class I-peptide complexes.19 Our previous experiments using DCs loaded with protein Ag confirmed that these DCs are indeed less susceptible to CTL-mediated killing than peptide-loaded DCs and are able to access the LN in detectable numbers.20 Nonetheless, the ability of protein-loaded DCs to re-stimulate CTL proliferation, or boost antitumor immune responses, was not improved, suggesting that the Ag reaching the LN was not sufficiently immunogenic.
The relevance of our findings is 2-fold. First, our data are relevant in the context of DC-based immunotherapy. As shown in Figure 6, the cytotoxic ability of tumor-specific CTLs essentially prevented a DC vaccine from boosting tumor-protective immunity, presumably by preventing the priming of host CD8+ T cells as well as the acquisition of multifunctional effector activity by memory T cells that is normally observed after repeated antigenic stimulations.21 DCs loaded with protein Ag were not significantly more effective than DCs loaded with peptide, suggesting that the failure of DC vaccination to boost antitumor immunity is not due to the method of antigen loading. Indeed, results from control experiments indicated that DCs loaded with OVA protein or an OVA-derived peptide were equally effective at inducing primary antitumor immune responses in naïve mice (data not shown). We did not investigate whether other types of immunotherapies, such as the administration of Ag together with adjuvant, are affected by CTL cytotoxic function. Given the central role of DCs in antigen presentation in vivo, we would expect most immunotherapies to be affected by CTL activity, at least to some extent. As tumor-specific CTLs are often observed in cancer patients even before treatment, our findings are likely to be relevant to a considerable proportion of patients before and/or during immunotherapy.
Second, our data provide a mechanism that potentially underlies increased immune responses as observed in perforin-deficient mice and humans.22,23 For example, PKO mice generate increased immune responses upon repeated immunization or viral infection,18,22,24 and may succumb to severe inflammation when vaccinated.25 The interpretation of some of these studies is complicated by the use of infection models in which perforin-expressing cells also participate in the clearance of infectious agents.22,26 The resulting increased Ag load makes it difficult to establish the precise cause of increased immune responses.26-28 In addition, perforin has been reported to be necessary for the function of Tregs,29,30 suggesting that defective Treg function may contribute to enhanced immune responses. Our study used a non-replicating Ag to exclude effects of varying Ag load, and an adoptive transfer model in which only CD8+ T cells differed in perforin expression. This simplified model nonetheless recapitulated several characteristics of perforin deficiency22,26 such as increased expansion of CD4+ and CD8+ T cells as well as an increased capacity of T cells to produce cytokines. Thus, a selective deficiency of perforin in the CTL population combined with Ag presentation by DCs is sufficient to reproduce the main features of disregulated immune responses observed in PKO hosts. Other authors have suggested that CD8+ T cells regulate Ag presentation in vitro31 and in vivo32,33 and that perforin27 and/or FAS34 are critical mediators of this process. Some of these studies used naïve WT and PKO T cells, while our experiments used activated CTLs that were already cytotoxic before transfer, and expressed FASL (not shown) and presumably also perforin and TRAIL.35 In these conditions, perforin was clearly the major mediator to regulate DC killing and T-cell responses, while FAS and DR5 played a smaller, but significant, role.
One additional mechanism that might explain increased T-cell responses in PKO mice is T-cell suicide or fratricide, whereby WT CTLs succumb to the toxic effect of endogenously expressed cytotoxic mediators,16,36 or kill each other upon recognition of Ags acquired from antigen-presenting cells through trogocytosis.37 To examine this possibility we performed a simple experiment in which mice were injected with DCs first, to enable DC migration to LNs, and then adoptively transferred with WT or PKO CTLs. If perforin was involved in T-cell suicide or fratricide, in such setting PKO T cells should still survive better than WT cells, and accumulate in LNs in larger numbers. However, this was not the case. The similar response of WT and PKO CTLs strongly suggests that the effect of perforin is not cell-intrinsic, but mediated via a second population of cells, most likely DCs.
In conclusion, our data describe a perforin-dependent mechanism of regulation of the immune response, and provide evidence that this mechanism significantly affects the efficacy of DC-based vaccines. We propose that this mechanism acts as an additional “immune checkpoint”38 that counteracts DC-based immunotherapy and may be co-opted by tumors to escape the immune response.39 We also present evidence that, in the context of DC immunotherapy, this perforin-dependent checkpoint operates during a brief period soon after DC administration. Therefore, our results suggest that, paradoxically, a temporary blockade of T cell-mediated cytotoxic function at the time of DC vaccination, as would be rendered possible by the development of perforin inhibitors,40 may maximize the efficacy of DC-based immunotherapy.
Materials and Methods
Mice
All mice were maintained at the Malaghan Institute Biomedical Research Unit, Victoria University of Wellington. Experimental procedures were approved by the Victoria University Animal Ethics Committee and conducted in accordance with Institutional guidelines. C57BL/6 mice were originally from Jackson Laboratory, Bar Harbor, ME; CD45-congenic B6.SJL-Ptprca were from Animal Resources Centre, Perth, Australia; C57BL/6-Faslpr mice were from the Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia. The TCR transgenic strain Line31841 was crossed to PKO mice42 (Jackson Laboratories) for two generations to obtain PKO-Line318 mice. Line318, OT-I43 and OT-II44 mice were crossed to B6.SJL-Ptprca mice for one generation to obtain CD45-heterozygous Line318, OT-I and OT-II mice, respectively. Dr5−/− mice45 were bred at the Penn State Hershey Cancer Institute and used according to Institutional Animal Care and Use Committee guidelines.
In vitro culture media and reagents
All cultures were in complete Iscove-modified Dulbecco's medium (IMDM), consisting of IMDM supplemented with 5% fetal calf serum (FCS), 100 U/mL penicillin, 100 μg/mL streptomycin and 55 μM 2-mercaptoethanol (2-ME) (all from Invitrogen Corp., Auckland, NZ). The gp33 (LCMV33–41 KAVYNFATM), OVA257–264 (SIINFEKL) and OVA323–339 (ISQAVHAAHAEINEAGR) peptides were purchased from Mimotopes Pty Ltd. OVA protein fraction V was from Sigma Aldrich Co, St Louis MO.
DC injection and immunization
DCs were prepared from the bone marrow of C57BL/6 mice by culture in 10 ng/mL mouse recombinant granulocyte macrophage colony-stimulating factor (rGM-CSF) and 20 ng/mL mouse recombinant interleukin-4 (rIL-4), as previously described.46 DCs were activated by adding 100 ng/mL of lipopolysaccharide (LPS, Sigma Aldrich Co.) on day 6, and loosely adherent cells were harvested by gentle pipetting on day 7.
For immunizations, DCs were harvested and re-suspended at 1 × 106 cells/mL. Peptides were added to the cells at a final concentration of 1–10 μM and cells were incubated at 37°C for 2–4 h. For OVA protein loading, OVA was added to DCs in culture at a final concentration of 2 mg/mL for 48 h, LPS was added during the final 18–24 h. Cells were washed to remove excess Ag, and 1–5 × 105 cells were injected s.c. in 100 µL.
Generation of specific CTLs in vitro
Total LN cells from L318, PKO L318, or OT-I mice were cultured with LPS-treated DCs and Ag as described,47,48 and expanded in human rIL-2 for 1–2 d before transfer. This protocol routinely yielded cell populations that were 90–95% TCR Vα+Vβ+ CD62Llow by flow cytometry and highly cytotoxic.
In vivo DC cytotoxicity assays
LPS-treated DCs were labeled with fluorescent dyes so that their survival in vivo could be tracked.14 One population of DCs was loaded with 1 µM gp33 peptide or 10 µM OVA257–264 and labeled with the green fluorescent dye CFSE (Molecular Probes) while the second population of DC was left unloaded and labeled with the orange dye CTO (Molecular Probes), as described. Each mouse was injected intradermally into the distal forelimb or ear pinna with 1 × 106 CFSE-labeled DCs and 1 × 106 CTO-labeled DCs. At various times after DC injection, draining LNs were harvested, incubated for 1 h at 37°C in an enzyme cocktail containing 2.4 mg/mL collagenase II (Invitrogen) and 0.1 mg/mL DNase I (Sigma Aldrich), and passed several times through a 21G needle and then a gauze before flow cytometry analysis. The percent killed DC in individual LNs was calculated according to the formula: 100 – [(no. Ag-loaded DCs/ no. non Ag-loaded DCs in immune LN) × 100 / (no. Ag-loaded DCs/ no. non Ag-loaded DCs in control LN)].
Adoptive transfer experiments
L318, OT-I and OT-II T cells were prepared from naïve LN and spleen cell suspensions, positively selected using anti-CD8 and anti-CD4 magnetic beads (Miltenyi Biotech GmBH), respectively, and labeled with 1 µM CFSE for 10 min at 37°C. Labeling was stopped by adding FCS and IMDM, and cells were washed extensively before injection. CTL populations generated in vitro as described above were labeled with CFSE using the same conditions. Cells were injected i.v. at the following doses: 5 × 106 L318 or PKO L318 CTLs (or as otherwise indicated), 1–5 × 106 OT-I CTLs, and 1 × 106 naïve L318, OT-I or OT-II cells. In each experiment, the CFSE-labeled cells were also CD45 congenic with respect to the host and other adoptively transferred cells. Recipient mice were immunized with DCs with or without Ag, and/or challenged with tumor cells as indicated.
Flow cytometry
Cell suspensions were incubated with the appropriate concentration of antibodies in PBS containing 2% FCS and 0.01% sodium azide. The anti-FcγRII mAb 2.4G2 was used at 10 µg/mL. All antibodies were from BD PharMingen, with the exception of anti-CD45.2, anti-granzyme B, anti-TNFα and appropriate isotype control antibodies, which were from eBioscience. Anti-CD8 (clone 2.43) was purified in-house from hybridoma supernatants. Samples were analyzed using a FACSCalibur (Becton-Dickinson) and FlowJo software (Tree Star). Propidium Iodide (BD PharMingen) was used to exclude dead cells.
Intracellular cytokine staining
Cell suspensions were cultured in complete IMDM containing 1 µM gp33 and Golgistop® (BD Bioscience) for 4 h. Cells were harvested, surface stained, fixed and then permeabilized. Intracellular cytokines were detected using the BD PharMingen intracellular staining kit and anti-IFNγ, anti-TNFα, or appropriate isotype control antibodies as per manufacturer’s specifications.
Tumor challenge experiments
The gp33-expressing Lewis lung carcinoma (LL-LCMV)49 and OVA-expressing B16 melanoma (B16-OVA)50 were maintained in complete IMDM containing 0.5 mg/mL Geneticin® (Invitrogen). Mice were challenged with 1 × 105 LL-LCMV or 1 × 106 B16-OVA cells injected s.c. in the left flank, and examined every 2–3 d to monitor tumor growth as described.49 Mice were scored as tumor-free if the tumor mass did not exceed 4 mm2.
Statistical analysis
Statistical analyses were performed using the Prism GraphPad software. Statistical tests and significance thresholds employed are indicated in each figure legend.
Acknowledgments
This work was funded by research grants from the Marsden Fund, Health Research Council of NZ and Wellington Medical Research Foundation to F.R. J.Z.M. was funded by a Lottery Health PhD Scholarship and the Marsden Fund. R.P. was funded by a University of Otago PhD Scholarship. We thank all staff of the Malaghan Institute for helpful discussions. We also thank the staff of the Biomedical Research Unit for expert animal breeding.
Glossary
Abbreviations:
- Ag
antigen
- CFSE
5-(and 6)-Carboxyfluorescein diacetate succinimidyl ester
- CTL
cytotoxic T lymphocyte
- CTO
CellTracker™ Orange
- CMTMR
[5-(and-6)-(((4-Chloromethyl)Benzoyl) Amino)Tetramethyl-rhodamine]
- DC
dendritic cell
- DR5
death receptor 5
- IMDM
Iscove’s modified Dulbecco medium
- LN
lymph node
- NK
natural killer
- PKO
perforin knockout
- TCR
T-cell receptor
- TNF
tumor necrosis factor
- TRAIL
TNF-related apoptosis-inducing ligand
- Treg
regulatory T cell
- WT
wild-type
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22128
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–77. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 4.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 5.Romero P, Dunbar PR, Valmori D, Pittet M, Ogg GS, Rimoldi D, et al. Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T lymphocytes. J Exp Med. 1998;188:1641–50. doi: 10.1084/jem.188.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–85. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 7.Demotte N, Stroobant V, Courtoy PJ, Van Der Smissen P, Colau D, Luescher IF, et al. Restoring the association of the T cell receptor with CD8 reverses anergy in human tumor-infiltrating lymphocytes. Immunity. 2008;28:414–24. doi: 10.1016/j.immuni.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–61. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 10.Ulchaker J, Panuto J, Rayman P, Novick A, Elson P, Tubbs R, et al. Interferon-gamma production by T lymphocytes from renal cell carcinoma patients: evidence of impaired secretion in response to interleukin-12. J Immunother. 1999;22:71–9. doi: 10.1097/00002371-199901000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Zippelius A, Batard P, Rubio-Godoy V, Bioley G, Liénard D, Lejeune F, et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 2004;64:2865–73. doi: 10.1158/0008-5472.CAN-03-3066. [DOI] [PubMed] [Google Scholar]
- 12.Mahnke YD, Devevre E, Baumgaertner P, Matter M, Rufer N, Romero P, et al. Human melanoma-specific CD8(+) T-cells from metastases are capable of antigen-specific degranulation and cytolysis directly ex vivo. Oncoimmunology. 2012;1:467–530. doi: 10.4161/onci.19856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–15. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 14.Hermans IF, Ritchie DS, Yang J, Roberts JM, Ronchese F. CD8+ T cell-dependent elimination of dendritic cells in vivo limits the induction of antitumor immunity. J Immunol. 2000;164:3095–101. doi: 10.4049/jimmunol.164.6.3095. [DOI] [PubMed] [Google Scholar]
- 15.Loyer V, Fontaine P, Pion S, Hétu F, Roy DC, Perreault C. The in vivo fate of APCs displaying minor H antigen and/or MHC differences is regulated by CTLs specific for immunodominant class I-associated epitopes. J Immunol. 1999;163:6462–7. [PubMed] [Google Scholar]
- 16.Opferman JT, Ober BT, Narayanan R, Ashton-Rickardt PG. Suicide induced by cytolytic activity controls the differentiation of memory CD8(+) T lymphocytes. Int Immunol. 2001;13:411–9. doi: 10.1093/intimm/13.4.411. [DOI] [PubMed] [Google Scholar]
- 17.Kemp RA, Ronchese F. Tumor-specific Tc1, but not Tc2, cells deliver protective antitumor immunity. J Immunol. 2001;167:6497–502. doi: 10.4049/jimmunol.167.11.6497. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Huck SP, McHugh RS, Hermans IF, Ronchese F. Perforin-dependent elimination of dendritic cells regulates the expansion of antigen-specific CD8+ T cells in vivo. Proc Natl Acad Sci U S A. 2006;103:147–52. doi: 10.1073/pnas.0509054103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–26. doi: 10.1016/S1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 20.Ma JZ, Lim SN, Qin JS, Yang J, Enomoto N, Ruedl C, et al. Murine CD4+ T cell responses are inhibited by cytotoxic T cell-mediated killing of dendritic cells and are restored by antigen transfer. PLoS One. 2012;7:e37481. doi: 10.1371/journal.pone.0037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naïve and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000;1:47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- 22.Matloubian M, Suresh M, Glass A, Galvan M, Chow K, Whitmire JK, et al. A role for perforin in downregulating T-cell responses during chronic viral infection. J Virol. 1999;73:2527–36. doi: 10.1128/jvi.73.3.2527-2536.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957–9. doi: 10.1126/science.286.5446.1957. [DOI] [PubMed] [Google Scholar]
- 24.Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2000;290:1354–8. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- 25.Badovinac VP, Hamilton SE, Harty JT. Viral infection results in massive CD8+ T cell expansion and mortality in vaccinated perforin-deficient mice. Immunity. 2003;18:463–74. doi: 10.1016/S1074-7613(03)00079-7. [DOI] [PubMed] [Google Scholar]
- 26.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104:735–43. doi: 10.1182/blood-2003-10-3413. [DOI] [PubMed] [Google Scholar]
- 27.Belz GT, Zhang L, Lay MD, Kupresanin F, Davenport MP. Killer T cells regulate antigen presentation for early expansion of memory, but not naive, CD8+ T cell. Proc Natl Acad Sci U S A. 2007;104:6341–6. doi: 10.1073/pnas.0609990104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lykens JE, Terrell CE, Zoller EE, Risma K, Jordan MB. Perforin is a critical physiologic regulator of T-cell activation. Blood. 2011;118:618–26. doi: 10.1182/blood-2010-12-324533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boissonnas A, Scholer-Dahirel A, Simon-Blancal V, Pace L, Valet F, Kissenpfennig A, et al. Foxp3+ T cells induce perforin-dependent dendritic cell death in tumor-draining lymph nodes. Immunity. 2010;32:266–78. doi: 10.1016/j.immuni.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–46. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Sad S, Kägi D, Mosmann TR. Perforin and Fas killing by CD8+ T cells limits their cytokine synthesis and proliferation. J Exp Med. 1996;184:1543–7. doi: 10.1084/jem.184.4.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hafalla JC, Morrot A, Sano G, Milon G, Lafaille JJ, Zavala F. Early self-regulatory mechanisms control the magnitude of CD8+ T cell responses against liver stages of murine malaria. J Immunol. 2003;171:964–70. doi: 10.4049/jimmunol.171.2.964. [DOI] [PubMed] [Google Scholar]
- 33.Wong P, Pamer EG. Feedback regulation of pathogen-specific T cell priming. Immunity. 2003;18:499–511. doi: 10.1016/S1074-7613(03)00081-5. [DOI] [PubMed] [Google Scholar]
- 34.Chen M, Felix K, Wang J. Critical role for perforin and Fas-dependent killing of dendritic cells in the control of inflammation. Blood. 2012;119:127–36. doi: 10.1182/blood-2011-06-363994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brincks EL, Katewa A, Kucaba TA, Griffith TS, Legge KL. CD8 T cells utilize TRAIL to control influenza virus infection. J Immunol. 2008;181:4918–25. doi: 10.4049/jimmunol.181.7.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang M, Park SM, Wang Y, Shah R, Liu N, Murmann AE, et al. Serine protease inhibitor 6 protects cytotoxic T cells from self-inflicted injury by ensuring the integrity of cytotoxic granules. Immunity. 2006;24:451–61. doi: 10.1016/j.immuni.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Hudrisier D, Riond J, Mazarguil H, Gairin JE, Joly E. Cutting edge: CTLs rapidly capture membrane fragments from target cells in a TCR signaling-dependent manner. J Immunol. 2001;166:3645–9. doi: 10.4049/jimmunol.166.6.3645. [DOI] [PubMed] [Google Scholar]
- 38.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matter M, Pavelic V, Pinschewer DD, Mumprecht S, Eschli B, Giroglou T, et al. Decreased tumor surveillance after adoptive T-cell therapy. Cancer Res. 2007;67:7467–76. doi: 10.1158/0008-5472.CAN-06-4372. [DOI] [PubMed] [Google Scholar]
- 40.Lena G, Trapani JA, Sutton VR, Ciccone A, Browne KA, Smyth MJ, et al. Dihydrofuro[3,4-c]pyridinones as inhibitors of the cytolytic effects of the pore-forming glycoprotein perforin. J Med Chem. 2008;51:7614–24. doi: 10.1021/jm801063n. [DOI] [PubMed] [Google Scholar]
- 41.Pircher H, Bürki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–61. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 42.Kägi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen KJ, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–7. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 43.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 44.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 45.Finnberg N, Gruber JJ, Fei P, Rudolph D, Bric A, Kim SH, et al. DR5 knockout mice are compromised in radiation-induced apoptosis. Mol Cell Biol. 2005;25:2000–13. doi: 10.1128/MCB.25.5.2000-2013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garrigan K, Moroni-Rawson P, McMurray C, Hermans I, Abernethy N, Watson J, et al. Functional comparison of spleen dendritic cells and dendritic cells cultured in vitro from bone marrow precursors. Blood. 1996;88:3508–12. [PubMed] [Google Scholar]
- 47.Perret R, Ronchese F. Effector CD8+ T cells activated in vitro confer immediate and long-term tumor protection in vivo. Eur J Immunol. 2008;38:2886–95. doi: 10.1002/eji.200838483. [DOI] [PubMed] [Google Scholar]
- 48.Robinson MJ, Ronchese F, Miller JH, La Flamme AC. Paclitaxel inhibits killing by murine cytotoxic T lymphocytes in vivo but not in vitro. Immunol Cell Biol. 2010;88:291–6. doi: 10.1038/icb.2009.96. [DOI] [PubMed] [Google Scholar]
- 49.Hermans IF, Daish A, Moroni-Rawson P, Ronchese F. Tumor-peptide-pulsed dendritic cells isolated from spleen or cultured in vitro from bone marrow precursors can provide protection against tumor challenge. Cancer Immunol Immunother. 1997;44:341–7. doi: 10.1007/s002620050392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]