Abstract
The infiltration of melanoma lesions by dendritic cells (DCs) has been suggested to play a tumorigenic role due to the capacity of DCs to induce tumor tolerance and promote angiogenesis as well as metastasis. However, it has also been shown that tumor-infiltrating DCs (TIDCs) induce antitumor responses and hence may be targeted in cost-effective therapeutic approaches to obtain patient-specific DCs that present relevant tumor antigens, without the need for ex vivo DC expansion or tumor antigen identification. Unfortunately, little is known about the composition, nature and function of TIDCs found in human melanoma. The development of mouse melanoma models has greatly contributed to the molecular understanding of melanoma immunology in mice, but many questions on TIDCs remain unanswered. Here, we discuss current knowledge about melanoma TIDCs in various mouse models with regard to their translational potential and clinical relevance.
Keywords: dendritic cells, melanoma, mouse model
Introduction
The incidence of cutaneous malignant melanoma has been steadily increasing over the last decades. While complete surgical excision yields high 5-y survival for patients with localized tumors exhibiting a depth < 0.75 mm, the outcome is poor for patients with a greater depth of invasion or bearing metastases. The development of novel therapeutic approaches is therefore of great importance. Interestingly, melanomas are relatively immunogenic tumors and sensitive to cytotoxic T lymphocyte (CTL)-mediated lysis. As dendritic cells (DCs) are the main antigen-presenting cell (APC) population capable of inducing CTLs, DC transfer, DC targeting and in situ DC induction, recruitment and/or activation have been explored as promising immunotherapeutic strategies against melanoma. The topical or intratumoral administration of DC-activating agents—including interferon α (IFNα), bacillus Calmette-Guérin (BCG), or purified Toll-like receptor (TLR) ligands such as imiquimod—are recommended as treatment options for patients with in-transit melanoma metastasis.1-5 While this approach is relatively successful against cutaneous metastases, efficacy is limited for subcutaneous metastases. An improved understanding of the type, nature and functionality of TIDCs could lead to novel and more effective therapeutic approaches. To circumvent ethical issues and TIDC availability constraints associated with human research, various animal models for melanoma have been established in organisms including Xiphophorus, Danio rerio, guinea pigs, opossum and small rodents, all of which have unique advantages and disadvantages. The relevance of the model under examination depends on the questions to be answered and how closely the model mimics the histological, immunological and metastatic pattern observed in humans.6 To date, most work is performed in mice due to the availability of genetically modified animals, insights into mouse immunology, pathology and physiology and the plethora of mouse-specific research tools.
Here, we will briefly review the current knowledge of TIDCs obtained in the most common mouse melanoma models and the insight they have provided into the human disease.
Selection of Mouse Model for Melanoma
Melanoma models are generally divided into 3 different groups based on research focus: xenograft models, which allow for the study of tumor cell behavior; transplantation models, to study melanoma immunology; and genetically modified animal models, which focus on melanomagenesis. Pure chemical carcinogen-induced melanoma models have decreased in popularity as they have relatively low relevance to human disease and therefore will not be discussed further in this article.
Xenograft models consist of orthotopic or ectopic transplantation of human cancer cells or solid tumors into immunocompromised mice. The primary advantage of these models is the preservation of human cancer cell behavior, including metastatic potential and tissue preference. However, the absence of a functional immune system does not allow for the study of the interactions between tumors and immune cell subsets. While DC function is relatively normal in some immunocompromised mouse strains, various others—including those with a NOD.Cg-Prkdcscidil2rg background—exhibit defective DC development and function.7-9 In addition, human tumor-derived mediators might affect the recruitment, retention, development and function of mouse DCs in a different fashion than their mouse homologs. The more recent development of human melanoma models in humanized mice10-12 circumvents these issues and provides an intriguing platform for clinically relevant TIDC studies.
Syngenic transplantation models have been around since the identification of the Cloudman S91 melanoma in BDA/2 mice, Harding-Passey melanoma in BALB/c × DBA/2F1 mice and B16 melanoma in C57BL/6 mice.13-16 B16 is currently the most widely used melanoma model and has the advantages that it expresses at least 5 homologs of the best characterized human melanoma antigens (gp100/pmel17, MART-1/MelanA, tyrosinase, TRP-1/gp75 and TRP-2/DCT),17 it is immunogenic and it displays metastatic behavior. The main drawback of this model is the rapid growth of the primary tumor, resulting in problems related to vascularization, necrosis and swift mortality that preclude the assessment of prolonged tumor burden on TIDC behavior. Nevertheless, most TIDC studies have been performed in B16 melanoma models.
Genetically engineered models (GEMM). The identification of genetic and epigenetic abnormalities in human melanomas has led to the development of genetically engineered mice with a heritable predisposition to the development of melanoma. The (tissue-specific) expression of oncogenes including Ret, mutant forms of (N/K/H)-Ras and Braf and Hgf, coupled or not to backcrossing in susceptible genetic backgrounds (Ink4a/Arff/f, Tp53t/t, Cdkn2d−/−, Cdkn2a−/−, Cdk4R24C/R24C, etc.) has yielded melanoma models with different latency, penetrance and metastatic potential (reviewed in refs. 18, 19). Although the distribution of melanocytes differs between mice and humans, these models have great clinical relevance as they are based on genes known to be involved in the genesis and progression of human melanoma, and can be easily combined with relevant environmental triggers such as UV irradiation, to accelerate melanoma incidence. Only recently the field has begun to use these models for TIDC studies.
Dendritic Cells
DCs are a heterogeneous population in terms of origin, morphology, phenotype and function. DCs are derived from common myeloid and lymphoid precursors and rely heavily on FLT3 ligand (FLT3L) and/or granulocyte macrophage colony-stimulating factor (GM-CSF) for their development.20-22 DCs express on their surface MHC class I and class II molecules together with a wide variety of positive (CD40, CD80, CD86, CD137L, CD70) and negative (PDL1, PDL2) co-stimulatory molecules. In addition, DCs can produce a broad range of soluble pro-and anti-inflammatory mediators, including multiple cytokines and chemokines. T cells interacting with DCs via cognate TCR-peptide-MHC complexes will undergo apoptosis, anergy, or develop a regulatory phenotype if the balance of co-stimulation is tilted on the negative side.23 Conversely, if positive signals surpass an intrinsic threshold, T cells will undergo proliferation, differentiate and acquire effector functions. Immature DCs display great phagocytic functions, relatively poor antigen-presenting capacity and low levels of positive co-stimulatory molecules. Upon activation via innate receptors such as TLRs or NOD-like receptors (NLRs), pro-inflammatory cytokines or cross-linking of CD40, DCs mature, reduce their phagocytic potential, increase antigen-presenting capacity, upregulate co-stimulatory molecules, change their cytokine production profile (qualitatively and quantitatively) and migrate to draining (lymphoid) areas, where they interact with T cells.24-26
Cells with DC characteristics have been repeatedly described in human melanoma samples. Depending on study, markers used, localization and maturation status, DC infiltration has been linked to a positive27-30 or negative31 prognostic outcome. The discrepancy in outcomes can be attributed to differences in clinical stages, the use of primary vs. metastatic lesions, as well as use of markers that are relatively non-specific or restrictive to a subpopulation of DCs.32
Studies in several other tumor systems indicate that malignant cells inhibit dendropoiesis, decelerate DC differentiation and maturation, induce functional DC deficiencies and accelerate cell death in DCs or their precursors.33,34 The maintenance of an immature phenotype or the promotion of a tolerogenic one could lead to anergy/deletion of tumor-specific T cells and the induction of cells with immunosuppressive functions such as FOXP3+ regulatory T cells (Tregs). An inhibited DC differentiation might also contribute to the accumulation of myeloid-derived suppressor cells, as the latter generate from precursors that under physiological conditions would differentiate into DCs, macrophages and neutrophils.35 In addition, immature DCs and pre-DCs have been suggested to promote angiogenesis through the secretion of growth factors (i.e., vascular endothelial growth factor, VEGF) that directly act on the endothelium, or the production of mediators that enhance the sensitivity of endothelial cells to growth factors.36,37 Some studies suggest that DC precursors might even undergo endothelial transdifferentiation or provide a scaffold for subsequent lining by endothelial cells.38
Murine and Human DC Populations
Recent genomic and proteomic approaches have discovered significant similarities between human and mouse DC populations,39-43 thereby strengthening the relevance of TIDC research in mouse melanoma models. While several aspects of localization, surface marker and TLR expression, phagocytic potential and antigen presenting capacity are relatively comparable between some mouse and human DC subsets, these are not perfect matches and in some cases the equivalent populations are absent. We will briefly describe the mouse and human DC populations in the following sections.
Mouse DC populations
Under steady-state conditions, mouse DCs express Cd11c as well as MHC class II molecules and can be subdivided into plasmacytoid DC (pDCs) and conventional DCs (cDCs).21 pDCs express intermediate levels of Cd11c as well as high levels of Cd45ra (B220), Pdca1 (Cd317), Tlr1, Tlr2, Tlr4, Tlr7 and Tlr9, and play an important role in infection due to their capacity to produce large amounts of type I IFNs.44 Antigen presentation by pDCs is thought to be relatively poor.45 cDCs can be further subdivided into blood-derived resident cDCs and migratory cDCs.
Blood-derived resident cDCs are present in lymphoid tissues and encompass: (1) Cd11chighMHCII+Cd8α+CD205+Sirpα-Cd11b- (Cd8α DCs), which express Xcl1, Clec9A and often Cd103. Cd8α DCs express mRNAs coding for most TLRs except Tlr5 and Tlr7, and are characterized by high Tlr3 expression.44 These DCs have the greatest potential to prime CTLs against cell-associated antigens via cross-presentation, but have relatively low CD4+ T-cell activation potential46; (2) Cd11chighMHCII+Cd8α-33D1+Sirpα+Cd11b+ (Cd11b DCs), which predominantly activate CD4+ T cells, have poor cross-presentation capacity and express most TLRs except Tlr3; (3) Cd11chighMHCII+ cells that lack Cd8α, Cd4 and Cd11b (generally termed “double” or “triple” negative DCs), which may or may not express Xcl1, Clec9A, Tlr3 and Cd103. DC subsets in this population have been shown to potently prime both CD4+ and CD8+ T cells against cell-associated antigens.47-49
Migratory DCs can be found in many organs and migrate upon activation into draining lymphoid areas.22,50,51 As this review focuses on melanoma we will limit our description to skin-resident DCs. Various populations of DCs have been described in non-inflamed mouse skin. Langerhans cells (LCs), Cd11b+Cd207+Cd103- DCs reside in the epidermis and express Tlr2, Tlr4 and Tlr9 but not Tlr7.52 The cross-presentation of cell-associated antigens by LCs has not been demonstrated, but LCs have the capacity to cross-present antigen associated with TLR ligands.53,54 In the dermis Cd11b+Cd207- dermal DC (dDCs) represent the major DC subset, whereas Cd207+Cd103+ dDCs and Cd207-Cd11b- dDCs represent ~20% of the entire dDC population. dDCs express most TLRs and the Cd103+ dDC population has been associated with the cross-presentation of cell-associated antigens.52,55,56
The fact that distinct DC subsets share several surface markers and that their expression levels change upon activation complicate the identification of DC subsets. Environmental cues associated with inflammation or tumors can change the surface characteristics of DCs as well as their functional properties, adding another layer of complexity to identification of DCs.
Human DC populations
Like mouse DCs, human DCs are generally divided into pDCs and cDCs. pDCs are lineage negative (lin-) CD11c-HLA-DR+CD123+BDCA2/4+ and express high levels of TLR7 and TLR9.57,58 In contrast to mouse pDCs, human pDCs have been shown to cross-present cell-associated antigens.45,59 Human cDCs can be further divided based on their expression of BDCA-1 (CD1c) and BDCA-3 (CD141). On one hand, BDCA-1+ DCs are similar to mouse Cd11b DCs as they express SIRPα and CD11b,39 strongly respond to TLR1 and TLR6 agonists and promote CD4+ T-cell responses. On the other hand, BDCA-3+ DCs exhibit strong similarities with mouse Cd8α DCs and express CLEC9A, XCR1 and high levels of TLR3.60,61 It has recently been shown that BDCA-3 DCs have the greatest capacity for cross-presentation of all human DC subsets.60-62
LCs are the only DCs found in healthy human epidermis and comprise 2–8% of all epidermal cells. LCs express high levels of CD1a, MHC class II molecules, CD207 and EpCAM, and low levels of CD205 and the DC immunoreceptor (DCIR).63 Both DCIR and CD205 are associated with antigen uptake and induction of antigen-specific T-cell responses.64 LCs express mRNA coding for TLR1, TLR2, TLR5, TLR6 and TLR9 (but not for TLR4, TLR7 and TLR8).65 The number of dDCs populations described in humans has recently been expanded. The major dDC population is BDCA-1+, and most of these cells express CD11c while only about 50% of the BDCA-1+ population express CD1a.66 CD1c-BDCA-3+ dDCs represent about 10% of all CD11c+ dDCs and demonstrate superior cross-presentation of soluble antigens as compared with other DC populations.66 Most dDCs express mRNA coding for TLR1, TLR2, TLR4, TLR8 and TLR10 but the exact distribution of these TLRs among specific DC subsets needs further delineation.63
Human melanoma TIDCs
Melanoma-infiltrating DCs have been found in primary and metastatic lesions and encompass a broad spectrum of DC-like cells, including CD207+ LCs, pDCs and CD1a+ DCs (Table 1).27,28,31,67-69 Due to differences in patient material, the relatively low frequency of TIDCs, the use of ambiguous analytical markers, and approaches that limit the number of available analytical markers analytical markers, there is little consensus on the exact composition of the TIDC population.32 However, there is a general agreement on the fact that the frequency of TIDCs is higher in the peritumoral area than within neoplastic lesions and that TIDCs with the most mature phenotype (DC-LAMP+CD83+fascin+) tend to reside in the peritumoral area.27,31,67,68 It is thought that immature DCs enter tumors via the vasculature and — following further differentiation and activation — migrate toward the tumor edge. There, DCs either locally form T-cell clusters or continue to migrate toward the draining lymph node, where they interact with T cells. The relationship between the presence and location of different TIDC subsets and clinical outcome remains a puzzle, as it not only depends on the type of TIDCs, but also on their activity as well as on functional interactions with other cells, all aspects that remain poorly understood.
Table 1. Human melanoma TIDC.
| Study | DC marker | DC specifics |
|---|---|---|
| Garcia-Plata69 |
S100, CD1a, HLA-DR |
S100+CD1a+ (LC) increased in peritumoral infiltrate compared with overlying epidermis. HLA-DR levels variable. |
| Movassagh28 |
CD123, DC-LAMP, fascin, CD1a, CD207 |
CD1a+ and CD207+ cells in epidermis of regressing lesion infiltration; fascin+/DC-LAMP+ cells accumulation around microvessels within tumor area (tumor regression) |
| Salio102 |
CD123, BDCA2, CLA |
Observed in majority of melanomas; numbers higher in infiltrating and metastatic samples. Numbers increase with severity of disease |
| Vermi68 |
CD1a, CD123, CD207, DC-Sign DC-LAMP, MR |
Increase in dermal myeloid and pDC compared with healthy skin. Intratumoral immature: MR+/DC-SIGN+/CD1a- and CD1a+/CD207- cells Peritumoral immature: CD1a+/CD207+LC; MR+/DC-SIGN+/CD1a-; CD1a+/CD207-; CD123+/BDCA-2+; Peritumoral mature: CD83+DC-LAMP+ |
| Ladanyi27 |
CD1a, DC-LAMP |
CD1a+ in melanoma cell nests and stroma, DC-LAMP+ in peritumoral area: inverse correlation CD1a+ and DC-LAMP+ cells with melanoma thickness |
| Simonetti67 |
CD83, CD207 |
Inverse correlation langerin+ cells with tumor depth; lower density of CD83(+) DC in thick melanomas |
| Charles103 |
BDCA-2 |
Observed in 37% of cases. Located close to the tumor within the peritumoral leukocyte infiltrate, representing 2–5% of these cells |
| Jensen31 |
CD123, DC-LAMP |
CD123 infiltration: tumor stroma (~30%), tumor nest (~15%) of samples DC-LAMP+ infiltration: tumor stroma (~30%), peritumoral (~50%) of samples |
| Erdag30 |
DC-LAMP, CD163neg |
> 1% of CD45 cells: Metastasis to LN contain higher number of LAMP+ cells compared with metastasis to skin/soft tissue peritoneum, small intestine |
| Martinet104 | DC-LAMP, fascin | DC-LAMP+ cells frequently associated with tumor HEV; Density of DC-LAMP+ cells correlates with density of tumor HEV |
Mouse melanoma TIDCs
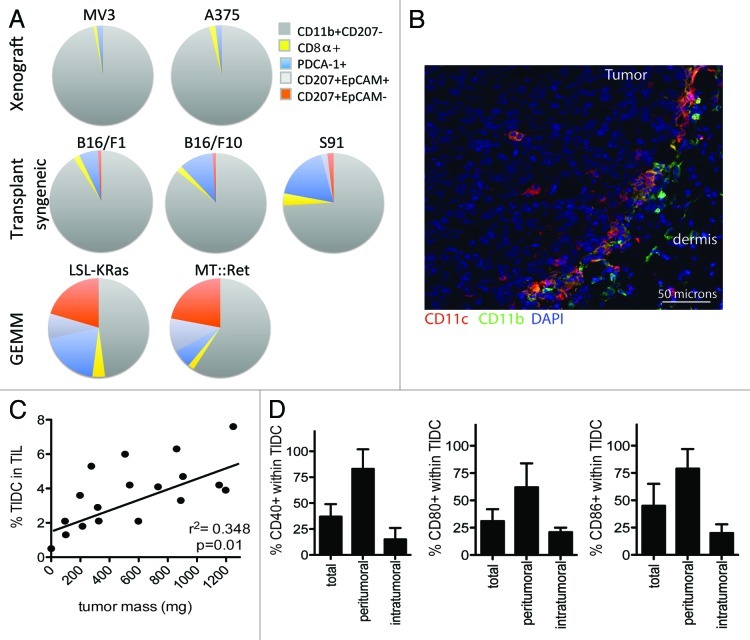
While mouse models have the advantage of providing abundant tumor material, which allows for an easy selection of tumors at different developmental stages, there is surprisingly little consensus in the field about mouse TIDC frequency, composition and function. Some of these discrepancies result from the use of different model systems or genetic backgrounds. When we compared two xenograft models, 3 syngenic transplantation models and 2 GEMMs, we observed significant differences in TIDC frequency (data not shown) and composition between models (Fig. 1A). The highest frequency of TIDCs was seen in syngenic transplant models, while GEMMs exhibited significantly less TIDCs. However, GEMMs showed a greater diversity of TIDCs, with marked infiltration by pDCs, LCs and dDCs. Xenografts showed the least diverse variety, completely lacking LCs and dDCs, while in syngenic transplant models an occasional dDC (CD207+EpCAM-) subset was found. Although a full comparison is hard to make as not all studies used the same set of markers, a review of the current literature on mouse melanoma revealed similar findings in different model systems (Table 2).70-75
Figure 1. Composition, location and maturation of tumor-infiltrating dendritic cells. (A) Composition of CD45+Lin-CD11c+MHCII+ tumor-infiltrating dendritic cells (TIDCs) in different melanoma models. Tumors (400–600mm2) were harvested from Nu/J nude mice (MV3, A375; n = 6 mice per group), BDA/2 mice (CloudmanS91; n = 5 mice per group) and C57BL/6 mice (B16F1 and B16/F10; n = 9 mice per group), digested according to standard protocols,106,107 and analyzed by multicolor flow cytometry. β-actin::LSL-KRAS mice crossed onto a Tyr::CreERT2 background108 were repeatedly treated with tamoxifen between 1 and 2 mo of age. Tumors were harvested 4–6 mo later (1–2 melanomas per mouse, n = 3 mice). MT::Ret transgenic mice109 were aged and spontaneous melanomas were harvested when their surface reached 200–300 mm2 (1–3 melanomas per mouse, n = 4 mice). (B) Representative localization of TIDCs in a snap-frozen B16/F10 tumor seven days after the subcutaneous injection of 2 × 106 tumor cells in C57Bl/6 mice, as observed by confocal microscopy. Red, CD11c; Green, CD11b; Blue, nuclei (4',6-diamidino-2-phenylindole, DAPI). (C) Relationship between the frequency of TIDCs among tumor-infiltrating lymphocytes (TILs) and the size of B16/F10 melanomas growing in C57Bl/6 mice, as determined by flow cytometry. (D) Differential expression of maturation markers on peritumoral and intratumoral TIDCs. B16/F10 tumors (≈600 mg, n = 4–5 tumors per group) were harvested and the peritumoral area was collected using ophthalmic blades, followed by the processing of peritumoral and intratumoral tissues according to standard protocols.106,107 CD40, CD80 and CD86 expression were determined among live CD45+Lin-CD11c+MHCII+ cells by multicolor flow cytometry.
Table 2. Mouse melanoma TIDC characteristics.
| Model | DC marker | Frequency | DC subpopulation | Characteristics |
|---|---|---|---|---|
|
Scid-MV3 xenograft105 |
BSA-I binding |
~35/5 high power fields |
- |
- |
| B16/F10. s.c.73 |
CD11c+, MHCII+ |
- |
All CD11b+; further negative for EpCAM, PDCA-1, CD4, CD8α |
Partially activated; reduced capacity for protein uptake and subsequent MHC II presentation; less sensitive to TLR stim. |
| B16/F10. s.c.75 |
CD11c+, MHCII+ |
- |
All CD11b+; most F4/80+ ~23.5%, GR1+; few pDC |
GR1+ less mature populations, fails to stimulate MLR, produce more IL-10; protein pulsed Gr1+ DC poorly activate OVA-specific CD4 and CD8 T cells in vivo |
| B16/F10 sec.c.77 |
CD11c+, MHCII+ |
|
|
Pre-DC (Lin-CD11c+MHCII-Flt3+) cells are recruited in the tumor, differentiate and activated CD8 T cells in vitro upon peptide pulsing |
| B16/F10 sec.c.92 |
CD11c+ |
- |
- |
TIDC express high levels of SOCS3 and have reduced M2-PK activity. |
| B16-OVA s.c.74 |
CD11c+, MHCII+ |
~30% of TIL |
Mostly CD11b+, ~5% pDC, hardly CD207+ |
Immature phenotype; fail to activate OVA-specific CD4 and CD8 T cells ex vivo |
| B16-OVA s.c.70 |
CD11c+ |
~20% of TIL |
~33% CD11b+MHCIIhigh, rest CD11b- MHCIImedium |
Partially mature; no in vitro activation of OVA-specific CD4 and poor activation of CD8 T cells |
| B16/F10 sec.c71,81 |
CD11c+, MHCII+ |
0.13 ± 0.07% of total cells |
~3% pDC, ~2.25% CD8αDC, > 95% non-pDC non CD8α |
Decreased number compared with skin; Immature phenotype, particle uptake in vivo normal; protective upon transfer. |
| K17–3571 |
CD11c+, MHCII+ |
4.0 ± 0.22% of total cells |
~15% pDC, ~12% CD8αDC |
Increased number compared with skin; immature phenotype; particle uptake in vivo normal |
| Tyr:N-RasQ61K+ DMBA/C3H6O71 |
CD11c+, MHCII+ |
0.02 ± 0.004 of total cells |
~58% pDC, ~40% non pDC non CD8αDC |
Decreased number compared with skin; immature phenotype |
| MT/ret72 | CD11c+, MHCII+ | 3–10% of TIL | - | Increasingly immature phenotype upon melanoma progression |
The differences in the composition of TIDCs across models and species highlight the importance of model validation for each type of study. While all models have significantly contributed to the current understanding of melanoma immunology, pre-clinical DC targeting studies would benefit from models that more accurately resemble the TIDC composition seen in patients.
Mouse TIDC activation status
As in human melanoma, mature mouse TIDCs tend to reside in the peritumoral areas and total TIDCs seem to increase upon disease progression (Fig. 1B and C).72 Most studies assessing mouse TIDC activation and maturation status were based on the flowcytometric analysis of CD11c+ cells from the entire tumor. Consequently, most reports show a biphasic distribution of the maturation markers CD40/CD80 and CD86.70,71,73,74 The differential analysis of the peritumoral and intratumoral zones of B16/F10 melanomas replicate histological observations, showing significantly more mature TIDCs in the peritumoral area as compared with the intratumoral one (Fig. 1D). It is thought that the tumor environment promotes the recruitment of DC precursors and immature DCs, but little is known on the ability of melanomas to support in situ DC differentiation.76 Diao, et al. showed that adoptively transferred immediate cDC precursors (Lin-CD11c+MHCII- cells) are recruited to B16/F10 tumors, where they proliferate and differentiate into cells with T-cell priming capacity in vitro, suggesting at least a partial acquisition of DC-like functions.77 On the other hand, in vivo data from Fainaru, et al. demonstrate that the recruitment of immature DCs promotes angiogenesis and tumor growth by enhancing endothelial cell migration and the subsequent formation of vascular networks.78 Moreover, the depletion of CD11c+ cells in CD11c-diphtheria toxin receptor (DTR) transgenic mice has been shown to significantly reduce the tumor mass of intraperitoneally injected B16/F10 melanoma cells.78 While other models suggest a role for an endothelial-like differentiation of DC precursors, VEGFA, β defensin, basic fibroblast growth factor (bFGF) and transformin growth factor β1 (TGFβ1) in this process, the mechanism underpinning DC-supported vasculogenesis in melanoma has not been clearly established.79,80
Mouse TIDC functionality
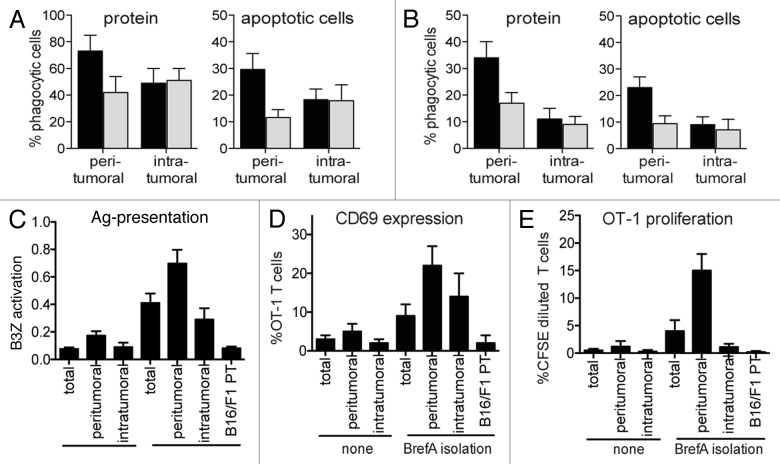
In order to operate as bona fide APCs, DCs need to acquire antigens through one of the phagocytic pathways, process and present them and communicate with T cells locally or upon migration to draining areas. Studies injecting beads into tumors revealed that a sizable fraction of TIDCs acquire one or more beads, indicating that that particulate uptake mechanisms is relatively intact.71,73 However, Gerner, et al. showed that TIDCs manifest a defect in the uptake of intratumorally injected proteins as compared with dDCs from healthy tissue.73 Separating peritumoral and intratumoral TIDCs, we found that the in vitro uptake of proteins and apoptotic cell material was higher for peritumoral, as compared with intratumoral, TIDCs (Fig. 2A). Similar observations were made when peritumoral and intratumoral TIDCs were analyzed 4 h after the intratumoral injection of proteins in vivo. Interestingly, the co-administration of lipopolysaccharide (LPS) appears to decrease the phagocytic uptake by peritumoral TIDCs, but not by their intratumoral counterparts (Fig. 2B).
Figure 2. Functionality of tumor-infiltrating dendritic cells. (A) In vitro phagocytic capacity of tumor-infiltrating dendritic cells (TIDCs). Peritumoral and intratumoral TIDCs were isolated from B16/F10 tumors growing in C57Bl/6 mice (as described in the legend of Figure 1) and cultured for 4 h with CFSE-labeled apoptotic splenocytes (1:3 ratio) or 100 µg/mL fluorochrome-conjugated ovalbumin (OVA) in the presence (black bar) or absence (gray bar) of 0.1 µg/mL lipopolysaccharide (LPS, from Salmonella minnesota R595) (n = 4–5 tumors per group). (B) In vivo phagocytic capacity of TIDCs. B16/F10 tumors (≈600 mm2) were injected with 1 × 106 CFSE-labeled apoptotic cells or 200 µg fluorochrome-conjugated OVA in the presence or absence of 10 µg LPS. Four hours later, peritumoral and intratumoral TIDCs were isolated and analyzed by flow cytometry (n = 4–5 tumors per group). (C) Effect of brefeldin A (BrefA) employed during TIDC isolation from B16-OVA melanomas on endogenous tumor-antigen presentation. BrefA was added during digestion, incubations and sorting at a concentration of 40 µg/mL.82 Peritumoral and intratumoral TIDCs from OVA-expressing B16/F10 tumors were cultured with OVA257–264-specific B3Z hybridoma cells and hybridoma activation was determined 20 h later by chlorophenol red-β-D-galactopyranoside (CPRG) conversion (n = 5–6 tumors per group).110 TIDC derived from B16/F10 parental tumors were used as negative control. (D,E) Peritumoral and intratumoral TIDCs (isolated in the presence or absence of BrefA) were co-cultured with CFSE-labeled OVA257–264-specific OT-1 T cells. After 24 h, activation was determined by CD69 expression in 7-AAD-CD8+Vα2+Vβ5+ cells. OT-1 T-cell proliferation was assessed by CFSE dilution after 72 h of culture with the indicated TIDCs (n = 5–6 tumors per group).
Most studies reveal a decreased CD4+ and CD8+ T-cell activating capacity of TIDCs isolated from antigen-expressing tumors or upon antigen pulsing in vivo.70,73,74 However, other studies indicate potent T-cell priming capacity of TIDCs, both in vitro or in vivo.71,77,81 This discrepancy can be partly explained by the fact that these studies differed relative to TIDC composition, TIDC localization, TIDC maturation state, TIDC isolation methods and in vitro functional assessment protocols. By separating TIDCs based on GR1 expression, Diao, et al. showed that GR1+ expressing TIDCs produce more interleukin (IL-10) and exhibit lower CD8+ and CD4+ T-cell priming capacity than GR1- TIDCs when loaded with antigens in vitro.75 In addition, CD8+ T cells primed by GR1+ TIDCs demonstrated significantly reduced cytokine production compared with CD8+ T cells primed by GR1- TIDCs. Gerner, et al. suggested that the decreased TIDC capacity for CD4+ T-cell activation results predominantly from reduced antigen uptake as they found antigen processing and presentation to be unaltered.73 To further dissect the antigen presenting and T-cell priming/activating potential of TIDCs, we isolated peritumoral and intratumoral TIDCs from ovalbumin (OVA)-expressing B16 tumor-bearing mice and cultured them with an OVA257–264-specific reporter cell line (B3Z) and CFSE-labeled OVA257–264-specific OT-1 T cells. We included brefeldin A in the isolation procedure to prevent the turnover of MHC-I-peptide complexes while preserving the TIDC maturation state.82 Importantly, significant antigen presentation was observed only when brefeldin A was present during the isolation period, illustrating the importance of optimizing and standardizing TIDC isolation protocols. The total TIDC fraction poorly activated B3Z cells (Fig. 2C), suggesting a low frequency of OVA257–264-MHC complexes. Consequently, total TIDC-mediated OT-1 T-cell activation and proliferation, as determined by CD69 upregulation and CFSE dilution assays, was relatively poor (Fig. 2D and E). However, peritumoral TIDCs displayed a comparatively higher frequency of OVA257–264-MHC complexes and activated (while inducing the proliferation) of a sizable fraction of OT-1 T cells (Fig. 2C-E). Intratumoral TIDCs exhibited less OVA257–264-MHC complexes and activated OT-1 T cells without inducing proliferation. This lack of proliferation could be restored by the addition of IL-2 but not upon the blockade of IL-10 or TGFβ, suggesting the induction of T-cell non-responsiveness. Importantly, the treatment of peritumoral TIDCs with TLR4 or TLR9 ligands significantly increased their potential to induce T-cell proliferation, while the same treatment did not improve the functionality of intratumoral TIDCs (data not shown). Altogether, these observations show that differences in isolation protocols, TIDC subsets, and functional assays significantly complicate the comparison between studies and the extrapolative value of their findings.
While many studies indicate a decrease in the maturation and functionality of melanoma TIDCs, the mechanisms that underpin such changes in APC functions are still unclear. Increased expression of immunosuppressive cytokines and membrane-associated molecules by TIDCs has been implicated in TIDC dysfunction.72,83 Other models suggest that tumor-derived cytokines or a reduction in the sensitivity of TIDCs to innate signals prevents maturation, migration and thereby impair TIDC function.84-88 However, prolonged TIDC retention and the maintenance of an immature phenotype has recently been linked to lipid accumulation following increased scavenger receptor A expression89 and LXRα mediated CCR7 downregulation.90 Norian, et al. have linked TIDC dysfunction to increased l-arginine metabolism in a spontaneous model of mammary carcinoma.91 More importantly, Zhang, et al. have correlated the reduced functionality of B16 melanoma TIDCs to a decreased metabolic proficiency, resulting from increased SOCS3-pyruvate kinase M2 interactions.92 These observations clearly exemplify that the focus on basic immunological assays and parameters has become too restricted to determine the mechanisms of TIDC dysfunction. For a full appreciation of the developmental and functional defects in TIDC, research disciplines beyond classical immunology will have to be incorporated into the experimental approaches.
Scientific and Therapeutic Considerations
Mouse models have been extensively used to test topical therapeutic therapies. Comparable to human melanoma, the injection of GM-CSF, IFNα, imiquimod, or BCG has been shown to result in various degrees of therapeutic success in mice.5,93-96 In many of these approaches, either increased numbers of DCs or enhanced DC maturation was observed in the tumor or tumor-draining lymph node.93-96 In addition, other purified TLR ligands including poly(I:C), CpG oligonucleotides, LPS, alone or coupled to additional immunomodulatory therapies have been used successfully.97-99 The intratumoral administration of crude bacterial products, cytokines and stimulatory molecules delivered by viral vectors, microspheres or nanoparticles is well established in mouse models but has not been translated to the human system.5,100,101 While all these therapeutic approaches were suggested to target TIDCs or support TIDC functions, it is likely that they only partly activate TIDCs, as (1) some specifically targeted DC populations are absent or poorly represented in the tumor, and (2) some specifically targeted receptors are poorly expressed by TIDCs or rendered non-functional by the tumor microenvironment. In these cases, it is more likely that other cells in the tumor environment are stimulated to promote a DC activating/restoring microenvironment.
In order to improve the clinical relevance and translational potential of mouse melanoma models for the design, optimization, and identification of novel therapeutic interventions that target TIDCs we will have to overcome several hurdles. An improved identification and characterization of human TIDCs will be critical to identify and validate the best mouse models for each type of study. Eventually, the panel of DC specific markers used in human and mouse studies will have to be standardized, even as investigators continue to discover new markers and DC populations.32 Furthermore, the optimization and standardization of protocols for TIDC isolation and functional assessments will be essential for allowing study-to-study comparisons and the extrapolation of data across species as well as laboratories.
This said, a great gain might be made by an increased collaboration between different research disciplines. This could result, for instance, in the generation of better mouse models, such as humanized mice for xenograft transplantation studies and GEMMs with TIDC patterns that resemble human TIDC profiles at different stages of disease, as well as new analytical platforms for extended TIDC analyses.
Although it is unlikely that mouse melanoma models will ever completely recapitulate the complexity of human melanoma in clinical situations, so far we have only scratched the surface of the true potential of mouse models for the analysis of TIDC dysfunction and the development of therapeutic interventions. Combining and integrating current models, standardizing analytical methods and expanding the disciplines of research will be instrumental for significantly improving the clinical relevance of mouse models and the identification of novel therapeutic targets.
Acknowledgments
This work is supported by NIH grant CA138617 (NCI) and AI079545 (NIAID) to E.M.J.
Ethical Statement
All animal experiments were performed in strict accordance with animal protocols approved by the Institutional IACUC at CCHMC and LIAI that operate according the guidelines by the Association for Assessment and Accreditation of Laboratory Animal Care International and the recommendations in the Care and Use of Laboratory Animals of the National Institute of Health.
Glossary
Abbreviations:
- APC
antigen presenting cell
- CCR7
chemokine receptor 7
- cDC
conventional DC
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- dDC
dermal DC
- IFN
interferon
- GEMM
genetically engineered mouse model
- LC
Langerhans cell
- LXRα
liver X receptor alpha
- NLR
NOD-like receptor
- pDC
plasmacytoid DC
- SOCS3
suppressor of cytokine signaling 3
- TIDC
tumor-infiltrating DC
- TLR
Toll-like receptor
- VEGF
vascular endothelial growth factor
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22660
References
- 1.Triozzi PL, Tuthill RJ, Borden E. Re-inventing intratumoral immunotherapy for melanoma. Immunotherapy. 2011;3:653–71. doi: 10.2217/imt.11.46. [DOI] [PubMed] [Google Scholar]
- 2.Good LM, Miller MD, High WA. Intralesional agents in the management of cutaneous malignancy: a review. J Am Acad Dermatol. 2011;64:413–22. doi: 10.1016/j.jaad.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Nicholas C, Lesinski GB. Immunomodulatory cytokines as therapeutic agents for melanoma. Immunotherapy. 2011;3:673–90. doi: 10.2217/imt.11.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molenkamp BG, Sluijter BJ, van Leeuwen PA, Santegoets SJ, Meijer S, Wijnands PG, et al. Local administration of PF-3512676 CpG-B instigates tumor-specific CD8+ T-cell reactivity in melanoma patients. Clin Cancer Res. 2008;14:4532–42. doi: 10.1158/1078-0432.CCR-07-4711. [DOI] [PubMed] [Google Scholar]
- 5.Rigel DS, Carucci JA. Malignant melanoma: prevention, early detection, and treatment in the 21st century. CA Cancer J Clin. 2000;50:215–36, quiz 237-40. doi: 10.3322/canjclin.50.4.215. [DOI] [PubMed] [Google Scholar]
- 6.Roadmap for new opportunities in melanoma reseach. http://wwwsocietymelanomaresearchorg 2011.
- 7.Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180–91. [PubMed] [Google Scholar]
- 8.Ohteki T, Fukao T, Suzue K, Maki C, Ito M, Nakamura M, et al. Interleukin 12-dependent interferon gamma production by CD8alpha+ lymphoid dendritic cells. J Exp Med. 1999;189:1981–6. doi: 10.1084/jem.189.12.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–82. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 10.Carreno BM, Garbow JR, Kolar GR, Jackson EN, Engelbach JA, Becker-Hapak M, et al. Immunodeficient mouse strains display marked variability in growth of human melanoma lung metastases. Clin Cancer Res. 2009;15:3277–86. doi: 10.1158/1078-0432.CCR-08-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–7. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vatakis D. Engineering hematopoietic stem cells to create melanoma specific CTL. Oncoimmunology. 2012;1:539–40. doi: 10.4161/onci.19408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fidler IJ, Nicolson GL. Organ selectivity for implantation survival and growth of B16 melanoma variant tumor lines. J Natl Cancer Inst. 1976;57:1199–202. doi: 10.1093/jnci/57.5.1199. [DOI] [PubMed] [Google Scholar]
- 14.Fidler IJ, Darnell JH, Budmen MB. Tumoricidal properties of mouse macrophages activated with mediators from rat lymphocytes stimulated with concanavalin A. Cancer Res. 1976;36:3608–15. [PubMed] [Google Scholar]
- 15.Nordlund JJ. Clinical appearance of cutaneous melanomas. Yale J Biol Med. 1975;48:403–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Maguire HC., Jr. Tumor immunology with particular reference to malignant melanoma. Int J Dermatol. 1975;14:3–11. doi: 10.1111/j.1365-4362.1975.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhai Y, Yang JC, Spiess P, Nishimura MI, Overwijk WW, Roberts B, et al. Cloning and characterization of the genes encoding the murine homologues of the human melanoma antigens MART1 and gp100. J Immunother. 1997;20:15–25. doi: 10.1097/00002371-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damsky WE, Jr., Bosenberg M. Mouse melanoma models and cell lines. Pigment Cell Melanoma Res. 2010;23:853–9. doi: 10.1111/j.1755-148X.2010.00777.x. [DOI] [PubMed] [Google Scholar]
- 19.McKinney AJ, Holmen SL. Animal models of melanoma: a somatic cell gene delivery mouse model allows rapid evaluation of genes implicated in human melanoma. Chin J Cancer. 2011;30:153–62. doi: 10.5732/cjc.011.10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 2010;234:45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- 21.Steinman RM, Idoyaga J. Features of the dendritic cell lineage. Immunol Rev. 2010;234:5–17. doi: 10.1111/j.0105-2896.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- 22.Helft J, Ginhoux F, Bogunovic M, Merad M. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol Rev. 2010;234:55–75. doi: 10.1111/j.0105-2896.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 23.Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunol Rev. 2011;241:206–27. doi: 10.1111/j.1600-065X.2011.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watts C, West MA, Zaru R. TLR signalling regulated antigen presentation in dendritic cells. Curr Opin Immunol. 2010;22:124–30. doi: 10.1016/j.coi.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Coquerelle C, Moser M. DC subsets in positive and negative regulation of immunity. Immunol Rev. 2010;234:317–34. doi: 10.1111/j.0105-2896.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- 26.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 27.Ladányi A, Kiss J, Somlai B, Gilde K, Fejos Z, Mohos A, et al. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother. 2007;56:1459–69. doi: 10.1007/s00262-007-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Movassagh M, Spatz A, Davoust J, Lebecque S, Romero P, Pittet M, et al. Selective accumulation of mature DC-Lamp+ dendritic cells in tumor sites is associated with efficient T-cell-mediated antitumor response and control of metastatic dissemination in melanoma. Cancer Res. 2004;64:2192–8. doi: 10.1158/0008-5472.CAN-03-2969. [DOI] [PubMed] [Google Scholar]
- 29.Fridman WH, Galon J, Pagès F, Tartour E, Sautès-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011;71:5601–5. doi: 10.1158/0008-5472.CAN-11-1316. [DOI] [PubMed] [Google Scholar]
- 30.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72:1070–80. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen TO, Schmidt H, Møller HJ, Donskov F, Høyer M, Sjoegren P, et al. Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer. 2012;118:2476–85. doi: 10.1002/cncr.26511. [DOI] [PubMed] [Google Scholar]
- 32.Karthaus N, Torensma R, Tel J. Deciphering the message broadcast by tumor-infiltrating dendritic cells. Am J Pathol. 2012;181:733–42. doi: 10.1016/j.ajpath.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Pinzon-Charry A, Maxwell T, López JA. Dendritic cell dysfunction in cancer: a mechanism for immunosuppression. Immunol Cell Biol. 2005;83:451–61. doi: 10.1111/j.1440-1711.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 34.Farren MR, Carlson LM, Lee KP. Tumor-mediated inhibition of dendritic cell differentiation is mediated by down regulation of protein kinase C beta II expression. Immunol Res. 2010;46:165–76. doi: 10.1007/s12026-009-8118-5. [DOI] [PubMed] [Google Scholar]
- 35.Schmid MC, Varner JA. Myeloid cells in the tumor microenvironment: modulation of tumor angiogenesis and tumor inflammation. J Oncol. 2010;2010:201026. doi: 10.1155/2010/201026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coukos G, Benencia F, Buckanovich RJ, Conejo-Garcia JR. The role of dendritic cell precursors in tumour vasculogenesis. Br J Cancer. 2005;92:1182–7. doi: 10.1038/sj.bjc.6602476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sprague L, Muccioli M, Pate M, Meles E, McGinty J, Nandigam H, et al. The interplay between surfaces and soluble factors define the immunologic and angiogenic properties of myeloid dendritic cells. BMC Immunol. 2011;12:35. doi: 10.1186/1471-2172-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gottfried E, Kreutz M, Haffner S, Holler E, Iacobelli M, Andreesen R, et al. Differentiation of human tumour-associated dendritic cells into endothelial-like cells: an alternative pathway of tumour angiogenesis. Scand J Immunol. 2007;65:329–35. doi: 10.1111/j.1365-3083.2007.01903.x. [DOI] [PubMed] [Google Scholar]
- 39.Crozat K, Guiton R, Guilliams M, Henri S, Baranek T, Schwartz-Cornil I, et al. Comparative genomics as a tool to reveal functional equivalences between human and mouse dendritic cell subsets. Immunol Rev. 2010;234:177–98. doi: 10.1111/j.0105-2896.2009.00868.x. [DOI] [PubMed] [Google Scholar]
- 40.Segura E, Kapp E, Gupta N, Wong J, Lim J, Ji H, et al. Differential expression of pathogen-recognition molecules between dendritic cell subsets revealed by plasma membrane proteomic analysis. Mol Immunol. 2010;47:1765–73. doi: 10.1016/j.molimm.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 41.Chow A, Brown BD, Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol. 2011;11:788–98. doi: 10.1038/nri3087. [DOI] [PubMed] [Google Scholar]
- 42.Reizis B. Classical dendritic cells as a unique immune cell lineage. J Exp Med. 2012;209:1053–6. doi: 10.1084/jem.20121038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robbins SH, Walzer T, Dembélé D, Thibault C, Defays A, Bessou G, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, et al. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 2003;33:827–33. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 45.Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29:352–61. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 47.Katz JD, Ondr JK, Opoka RJ, Garcia Z, Janssen EM. Cutting edge: merocytic dendritic cells break T cell tolerance to beta cell antigens in nonobese diabetic mouse diabetes. J Immunol. 2010;185:1999–2003. doi: 10.4049/jimmunol.1001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reboulet RA, Hennies CM, Garcia Z, Nierkens S, Janssen EM. Prolonged antigen storage endows merocytic dendritic cells with enhanced capacity to prime anti-tumor responses in tumor-bearing mice. J Immunol. 2010;185:3337–47. doi: 10.4049/jimmunol.1001619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bedoui S, Prato S, Mintern J, Gebhardt T, Zhan Y, Lew AM, et al. Characterization of an immediate splenic precursor of CD8+ dendritic cells capable of inducing antiviral T cell responses. J Immunol. 2009;182:4200–7. doi: 10.4049/jimmunol.0802286. [DOI] [PubMed] [Google Scholar]
- 50.Milling S, Yrlid U, Cerovic V, MacPherson G. Subsets of migrating intestinal dendritic cells. Immunol Rev. 2010;234:259–67. doi: 10.1111/j.0105-2896.2009.00866.x. [DOI] [PubMed] [Google Scholar]
- 51.Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206:3115–30. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev. 2010;234:120–41. doi: 10.1111/j.0105-2896.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaplan DH. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010;31:446–51. doi: 10.1016/j.it.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh JZ, Kurche JS, Burchill MA, Kedl RM. TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway. Blood. 2011;118:3028–38. doi: 10.1182/blood-2011-04-348839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henri S, Poulin LF, Tamoutounour S, Ardouin L, Guilliams M, de Bovis B, et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med. 2010;207:189–206. doi: 10.1084/jem.20091964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–95. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 57.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdörfer B, Giese T, et al. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 58.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–93. doi: 10.1002/1521-4141(200111)31:11<3388::AID-IMMU3388>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 59.Hoeffel G, Ripoche AC, Matheoud D, Nascimbeni M, Escriou N, Lebon P, et al. Antigen crosspresentation by human plasmacytoid dendritic cells. Immunity. 2007;27:481–92. doi: 10.1016/j.immuni.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 60.Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre CA, Ventre E, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1283–92. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1261–71. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–60. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toebak MJ, Gibbs S, Bruynzeel DP, Scheper RJ, Rustemeyer T. Dendritic cells: biology of the skin. Contact Dermatitis. 2009;60:2–20. doi: 10.1111/j.1600-0536.2008.01443.x. [DOI] [PubMed] [Google Scholar]
- 64.Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Flacher V, Bouschbacher M, Verronèse E, Massacrier C, Sisirak V, Berthier-Vergnes O, et al. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J Immunol. 2006;177:7959–67. doi: 10.4049/jimmunol.177.11.7959. [DOI] [PubMed] [Google Scholar]
- 66.Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity. 2012;37:60–73. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simonetti O, Goteri G, Lucarini G, Rubini C, Stramazzotti D, Lo Muzio L, et al. In melanoma changes of immature and mature dendritic cell expression correlate with tumor thickness:an immunohistochemical study. Int J Immunopathol Pharmacol. 2007;20:325–33. doi: 10.1177/039463200702000212. [DOI] [PubMed] [Google Scholar]
- 68.Vermi W, Bonecchi R, Facchetti F, Bianchi D, Sozzani S, Festa S, et al. Recruitment of immature plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas. J Pathol. 2003;200:255–68. doi: 10.1002/path.1344. [DOI] [PubMed] [Google Scholar]
- 69.García-Plata D, Lessana-Leibowitch M, Palangie A, Gulliemette J, Sedel D, Méndez L, et al. Immunophenotype analysis of dendritic cells and lymphocytes associated with cutaneous malignant melanomas. Invasion Metastasis. 1995;15:125–34. [PubMed] [Google Scholar]
- 70.Ataera H, Hyde E, Price KM, Stoitzner P, Ronchese F. Murine melanoma-infiltrating dendritic cells are defective in antigen presenting function regardless of the presence of CD4CD25 regulatory T cells. PLoS One. 2011;6:e17515. doi: 10.1371/journal.pone.0017515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Preynat-Seauve O, Schuler P, Contassot E, Beermann F, Huard B, French LE. Tumor-infiltrating dendritic cells are potent antigen-presenting cells able to activate T cells and mediate tumor rejection. J Immunol. 2006;176:61–7. doi: 10.4049/jimmunol.176.1.61. [DOI] [PubMed] [Google Scholar]
- 72.Zhao F, Falk C, Osen W, Kato M, Schadendorf D, Umansky V. Activation of p38 mitogen-activated protein kinase drives dendritic cells to become tolerogenic in ret transgenic mice spontaneously developing melanoma. Clin Cancer Res. 2009;15:4382–90. doi: 10.1158/1078-0432.CCR-09-0399. [DOI] [PubMed] [Google Scholar]
- 73.Gerner MY, Mescher MF. Antigen processing and MHC-II presentation by dermal and tumor-infiltrating dendritic cells. J Immunol. 2009;182:2726–37. doi: 10.4049/jimmunol.0803479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stoitzner P, Green LK, Jung JY, Price KM, Atarea H, Kivell B, et al. Inefficient presentation of tumor-derived antigen by tumor-infiltrating dendritic cells. Cancer Immunol Immunother. 2008;57:1665–73. doi: 10.1007/s00262-008-0487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diao J, Zhao J, Winter E, Cattral MS. Tumors suppress in situ proliferation of cytotoxic T cells by promoting differentiation of Gr-1(+) conventional dendritic cells through IL-6. J Immunol. 2011;186:5058–67. doi: 10.4049/jimmunol.1004125. [DOI] [PubMed] [Google Scholar]
- 76.Vicari AP, Treilleux I, Lebecque S. Regulation of the trafficking of tumour-infiltrating dendritic cells by chemokines. Semin Cancer Biol. 2004;14:161–9. doi: 10.1016/j.semcancer.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 77.Diao J, Zhao J, Winter E, Cattral MS. Recruitment and differentiation of conventional dendritic cell precursors in tumors. J Immunol. 2010;184:1261–7. doi: 10.4049/jimmunol.0903050. [DOI] [PubMed] [Google Scholar]
- 78.Fainaru O, Almog N, Yung CW, Nakai K, Montoya-Zavala M, Abdollahi A, et al. Tumor growth and angiogenesis are dependent on the presence of immature dendritic cells. FASEB J. 2010;24:1411–8. doi: 10.1096/fj.09-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, et al. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10:950–8. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 80.Huarte E, Cubillos-Ruiz JR, Nesbeth YC, Scarlett UK, Martinez DG, Buckanovich RJ, et al. Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Res. 2008;68:7684–91. doi: 10.1158/0008-5472.CAN-08-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Preynat-Seauve O, Contassot E, Schuler P, French LE, Huard B. Melanoma-infiltrating dendritic cells induce protective antitumor responses mediated by T cells. Melanoma Res. 2007;17:169–76. doi: 10.1097/CMR.0b013e3281844531. [DOI] [PubMed] [Google Scholar]
- 82.Benke D, Krüger T, Lang A, Hamilton-Williams EE, Kurts C. Inclusion of Brefeldin A during dendritic cell isolation allows in vitro detection of cross-presented self-antigens. J Immunol Methods. 2006;310:12–9. doi: 10.1016/j.jim.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 83.Krempski J, Karyampudi L, Behrens MD, Erskine CL, Hartmann L, Dong H, et al. Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer. J Immunol. 2011;186:6905–13. doi: 10.4049/jimmunol.1100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gerlini G, Tun-Kyi A, Dudli C, Burg G, Pimpinelli N, Nestle FO. Metastatic melanoma secreted IL-10 down-regulates CD1 molecules on dendritic cells in metastatic tumor lesions. Am J Pathol. 2004;165:1853–63. doi: 10.1016/S0002-9440(10)63238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012;13:832–42. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shurin MR, Shurin GV, Lokshin A, Yurkovetsky ZR, Gutkin DW, Chatta G, et al. Intratumoral cytokines/chemokines/growth factors and tumor infiltrating dendritic cells: friends or enemies? Cancer Metastasis Rev. 2006;25:333–56. doi: 10.1007/s10555-006-9010-6. [DOI] [PubMed] [Google Scholar]
- 87.O’Hayre M, Salanga CL, Handel TM, Allen SJ. Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. Biochem J. 2008;409:635–49. doi: 10.1042/BJ20071493. [DOI] [PubMed] [Google Scholar]
- 88.Umansky V, Sevko A. Melanoma-induced immunosuppression and its neutralization. Semin Cancer Biol. 2012;22:319–26. doi: 10.1016/j.semcancer.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 89.Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880–6. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Villablanca EJ, Raccosta L, Zhou D, Fontana R, Maggioni D, Negro A, et al. Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat Med. 2010;16:98–105. doi: 10.1038/nm.2074. [DOI] [PubMed] [Google Scholar]
- 91.Norian LA, Rodriguez PC, O’Mara LA, Zabaleta J, Ochoa AC, Cella M, et al. Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via L-arginine metabolism. Cancer Res. 2009;69:3086–94. doi: 10.1158/0008-5472.CAN-08-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Z, Liu Q, Che Y, Yuan X, Dai L, Zeng B, et al. Antigen presentation by dendritic cells in tumors is disrupted by altered metabolism that involves pyruvate kinase M2 and its interaction with SOCS3. Cancer Res. 2010;70:89–98. doi: 10.1158/0008-5472.CAN-09-2970. [DOI] [PubMed] [Google Scholar]
- 93.Vuylsteke RJ, Molenkamp BG, van Leeuwen PA, Meijer S, Wijnands PG, Haanen JB, et al. Tumor-specific CD8+ T cell reactivity in the sentinel lymph node of GM-CSF-treated stage I melanoma patients is associated with high myeloid dendritic cell content. Clin Cancer Res. 2006;12:2826–33. doi: 10.1158/1078-0432.CCR-05-2431. [DOI] [PubMed] [Google Scholar]
- 94.Vuylsteke RJ, Molenkamp BG, Gietema HA, van Leeuwen PA, Wijnands PG, Vos W, et al. Local administration of granulocyte/macrophage colony-stimulating factor increases the number and activation state of dendritic cells in the sentinel lymph node of early-stage melanoma. Cancer Res. 2004;64:8456–60. doi: 10.1158/0008-5472.CAN-03-3251. [DOI] [PubMed] [Google Scholar]
- 95.Kirkwood JM, Tarhini AA, Panelli MC, Moschos SJ, Zarour HM, Butterfield LH, et al. Next generation of immunotherapy for melanoma. J Clin Oncol. 2008;26:3445–55. doi: 10.1200/JCO.2007.14.6423. [DOI] [PubMed] [Google Scholar]
- 96.Pan PY, Li Y, Li Q, Gu P, Martinet O, Thung S, et al. In situ recruitment of antigen-presenting cells by intratumoral GM-CSF gene delivery. Cancer Immunol Immunother. 2004;53:17–25. doi: 10.1007/s00262-003-0417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13:552–9. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 98.Furumoto K, Soares L, Engleman EG, Merad M. Induction of potent antitumor immunity by in situ targeting of intratumoral DCs. J Clin Invest. 2004;113:774–83. doi: 10.1172/JCI19762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guha M. Anticancer TLR agonists on the ropes. Nat Rev Drug Discov. 2012;11:503–5. doi: 10.1038/nrd3775. [DOI] [PubMed] [Google Scholar]
- 100.Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nat Mater. 2009;8:151–8. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ali OA, Emerich D, Dranoff G, Mooney DJ. In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci Transl Med. 2009;1:8ra19. doi: 10.1126/scitranslmed.3000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Salio M, Cella M, Vermi W, Facchetti F, Palmowski MJ, Smith CL, et al. Plasmacytoid dendritic cells prime IFN-gamma-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. Eur J Immunol. 2003;33:1052–62. doi: 10.1002/eji.200323676. [DOI] [PubMed] [Google Scholar]
- 103.Charles J, Di Domizio J, Salameire D, Bendriss-Vermare N, Aspord C, Muhammad R, et al. Characterization of circulating dendritic cells in melanoma: role of CCR6 in plasmacytoid dendritic cell recruitment to the tumor. J Invest Dermatol. 2010;130:1646–56. doi: 10.1038/jid.2010.24. [DOI] [PubMed] [Google Scholar]
- 104.Martinet L, Le Guellec S, Filleron T, Lamant L, Meyer N, Rochaix P, et al. High endotherlial venules (HEVs) in human melanoma lesions. Major gateways for tumor-infiltrating lymphocytes. OncoImmunology. 2012;1:829–39. doi: 10.4161/onci.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thies A, Dautel P, Meyer A, Pfüller U, Schumacher U. Low-dose mistletoe lectin-I reduces melanoma growth and spread in a scid mouse xenograft model. Br J Cancer. 2008;98:106–12. doi: 10.1038/sj.bjc.6604106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McBride S, Hoebe K, Georgel P, Janssen E. Cell-associated double-stranded RNA enhances antitumor activity through the production of type I IFN. J Immunol. 2006;177:6122–8. doi: 10.4049/jimmunol.177.9.6122. [DOI] [PubMed] [Google Scholar]
- 107.Radoja S, Saio M, Frey AB. CD8+ tumor-infiltrating lymphocytes are primed for Fas-mediated activation-induced cell death but are not apoptotic in situ. J Immunol. 2001;166:6074–83. doi: 10.4049/jimmunol.166.10.6074. [DOI] [PubMed] [Google Scholar]
- 108.Milagre C, Dhomen N, Geyer FC, Hayward R, Lambros M, Reis-Filho JS, et al. A mouse model of melanoma driven by oncogenic KRAS. Cancer Res. 2010;70:5549–57. doi: 10.1158/0008-5472.CAN-09-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kato M, Takahashi M, Akhand AA, Liu W, Dai Y, Shimizu S, et al. Transgenic mouse model for skin malignant melanoma. Oncogene. 1998;17:1885–8. doi: 10.1038/sj.onc.1202077. [DOI] [PubMed] [Google Scholar]
- 110.Shastri N, Gonzalez F. Endogenous generation and presentation of the ovalbumin peptide/Kb complex to T cells. J Immunol. 1993;150:2724–36. [PubMed] [Google Scholar]