Abstract
Genetic studies were performed in a French series of hepatocellular carcinomas. New oncogenes (NFE2L2) and tumor suppressor genes (IRF2, ARID1A and RPS6K3) were found to be recurrently altered. Moreover, a genotoxic signature was identified, raising the possible implication of a genotoxic exposure in the etiology of HCC, which remains to be characterized.
Keywords: hepatocellular carcinoma, exome sequencing, oncogene, tumor suppressor gene, IRF2
Hepatocellular carcinoma (HCC) accounts for the vast majority of primary liver tumors that usually develop in the cirrhotic liver. HBV or HCV infection, high alcohol intake and non-alcoholic fatty liver disease are major risk factors associated with the occurrence of HCC, HCC being the third most frequent cause of cancer-related mortality worldwide. Recently, framed by the International Cancer Genome Consortium (ICGC) project,1 we performed genomic analyses, including next-generation sequencing, to identify gene alterations involved in liver carcinogenesis. To this aim, we sequenced the entire coding genome of 24 HCCs as well as that of 24 paired non-transformed liver tissues. We also determined chromosome gains and losses using high-density comparative genomic hybridization in a cohort of 125 HCCs related to various etiologies, which were used to validate the most frequently mutated genes identified in the first HCC series.2-5 Using this approach, we identified about 1,000 different genes affected by somatic mutations that are predicted to have functional consequence at the protein level.
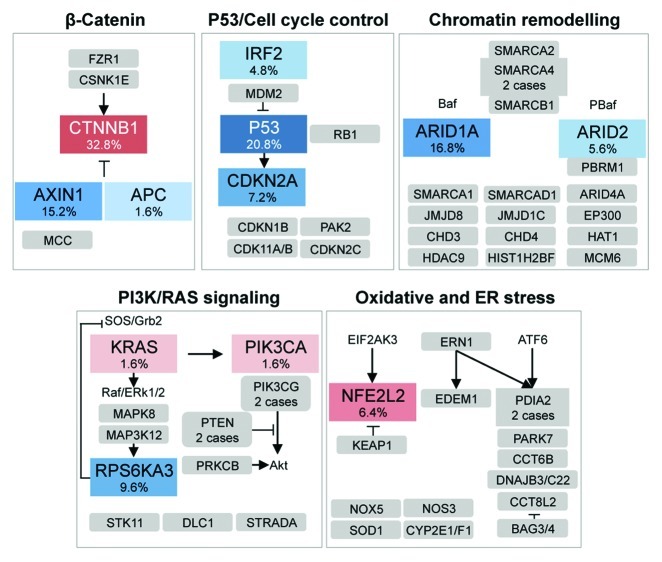
Analyzing cell signaling pathways recurrently altered by gene mutations and/or homozygous deletions in tumors, we identified several signaling pathways (Fig. 1) that are recurrently deregulated in HCC. The WNT/β-catenin pathway was the most frequently activated signal transduction altered among 125 HCC samples, mainly due to mutations in CTNNB1, AXIN1 or APC (Fig. 1). Because these 3 genes belong to the same pathway, their mutations were observed in a mutually exclusive pattern.
Figure 1. Major pathways that are commonly altered by somatic mutations or homozygous gene deletions in hepatocellular carcinoma. Alteration frequencies are expressed as a percentage mutation and/or homozygous deletion in the validation series of 125 (red or blue when activated or inactivated, respectively) or 24 exome-sequenced (gray) hepatocellular carcinomas (HCCs). For unique gene mutations, no frequency is indicated. Arrows represent positive interactions and lines are inhibitory interactions.
The p53/cell cycle signaling pathway was the second most commonly altered cascades, with TP53 inactivating mutations or the inactivation of CDKN2A (Fig. 1). TP53 alterations were mostly exclusive from CTNNB1 mutations (p = 0.0001) and associated with an elevated number of chromosomal rearrangements (p = 0.003), which is consistent with the well-known function of p53 in the maintenance of chromosome stability. In addition, we identified for the first time a recurrent inactivation of IRF2 (the gene coding for the interferon regulatory factor 2), which was observed in almost 5% of HBV-related HCCs (p = 0.0003). IRF2 is a transcriptional regulator that plays a major role in the regulation of cell growth and immune responses.6 We further investigated the functional consequences of IRF2 inactivation in HCC cell lines, demonstrating that IRF2 acts as a tumor suppressor gene. Thus, IRF2 silencing resulted in increased cell proliferation while its overexpression led to a dramatic cell death response by apoptosis. IRF2-silenced cell lines that were subcutaneously xenografted in nude CD1 mice grew much faster than their control counterparts. As it has been reported that IRF2 bind MDM2, we hypothesized that the lack of IRF2 expression could impair p53 function by increasing its proteasomal degradation.7 Indeed, we found that IRF2 silencing downregulates p53 and that there is a strong correlation between IRF2 and p53 expression at the protein level (R2 = 0.72; p = 0.004). Consistent with this hypothesis, we also observed that, in human HCC samples, IRF2 and TP53 mutations were mutually exclusive whereas IRF2- and TP53-mutated tumors belonged to the same transcriptomic subclass.8 Therefore, we identified IRF2 as a new tumor suppressor gene in HBV-related HCC, and we demonstrated that its inactivation leads to functional inactivation of p53.
Chromatin remodeling was the third most frequently altered pathway in HCC samples, as resulting from ARID1A and ARID2 mutations (Fig. 1). Consistent with our findings, Fujimoto and collaborators found that genes coding for chromatin regulators including ARID1A, ARID1B, ARID2, MLL and MLL3, were mutated in 50% of a Japanese cohort of HCC mainly related to viral infection.9 Consequently, a large number of HCCs worldwide seems to be linked to alteration in chromatin remodeling.
Among genes that were less frequently mutated in our French series of HCCs, we identified, for the first time in solid tumors, recurrent mutations in RPS6KA3 (9.6%), encoding the ribosomal S6 protein kinase 2 (RSK2) that is involved in RAS/MAPK signaling pathway. Because RSK2 is a known inhibitor of the RAS/MAPK pathway, RSK2 may act as a tumor suppressor and its inactivation might result in the activation of the RAS pathway. We also identified activating mutation of NFE2L2 in 6.4% of HCCs. NFE2L2 encodes NRF2, a transcription factor that has a pivotal role in the response to oxidative stress. This gene is affected by activating mutations in lung as well as head and neck cancer, and it is now appears as a new oncogene in HCC. When we analyzed the spectrum of mutations in the series of 125 HCCs, we identified a significant association between mutations in ARID1A, RPSK6KA3 or NFE2L2 and mutations in CTNNB1 or AXIN1, suggesting that the Wnt/β-catenin signaling might cooperate with either oxidative stress responses, chromatin remodeling and the RAS/MAPK pathway to promote liver carcinogenesis.
Finally, analyzing the spectrum of somatic mutations, we identified an over-representation of G > T transversions, which accumulated preferentially in the non-transcribed DNA strand. This type of mutation usually results from the exposure to genotoxics that induce DNA adducts.10 In HCC, such a genotoxic signature was most frequently found among tumors that developed in non-cirrhotic livers. The identification of the underlying genotoxic requires further epidemiological studies.
In conclusion, this study is a first step toward the identification of the somatic mutations that occur in HCC. New oncogenes and tumor suppressor genes have already been identified in this setting, but other genes will presumably be discovered in the future. There’s a huge diversity in the combinations of genetic defects that underlie tumorigenesis, raising the need for the functional characterization of the function of each gene involved in this process. An important issue is now to identify the genes that are driver or passenger for HCC tumorigenesis, as well as to clarify the putative interplay between somatic gene alterations and the tumor microenvironment.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21480
References
- 1.Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44:694–8. doi: 10.1038/ng.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nault JC, Zucman-Rossi J. Genetics of hepatobiliary carcinogenesis. Semin Liver Dis. 2011;31:173–87. doi: 10.1055/s-0031-1276646. [DOI] [PubMed] [Google Scholar]
- 3.Rebouissou S, Amessou M, Couchy G, Poussin K, Imbeaud S, Pilati C, et al. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature. 2009;457:200–4. doi: 10.1038/nature07475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rebouissou S, Imbeaud S, Balabaud C, Boulanger V, Bertrand-Michel J, Tercé F, et al. HNF1alpha inactivation promotes lipogenesis in human hepatocellular adenoma independently of SREBP-1 and carbohydrate-response element-binding protein (ChREBP) activation. J Biol Chem. 2007;282:14437–46. doi: 10.1074/jbc.M610725200. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Zhao H, Zhang X, Wood LD, Anders RA, Choti MA, et al. Inactivating mutations of the chromatin remodeling gene ARID2 in hepatocellular carcinoma. Nat Genet. 2011;43:828–9. doi: 10.1038/ng.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol. 2008;26:535–84. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- 7.Pettersson S, Kelleher M, Pion E, Wallace M, Ball KL. Role of Mdm2 acid domain interactions in recognition and ubiquitination of the transcription factor IRF-2. Biochem J. 2009;418:575–85. doi: 10.1042/BJ20082087. [DOI] [PubMed] [Google Scholar]
- 8.Boyault S, Rickman DS, de Reyniès A, Balabaud C, Rebouissou S, Jeannot E, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto A, Totoki Y, Abe T, Boroevich KA, Hosoda F, Nguyen HH, et al. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat Genet. 2012;44:760–4. doi: 10.1038/ng.2291. [DOI] [PubMed] [Google Scholar]
- 10.Hainaut P, Pfeifer GP. Patterns of p53 G-->T transversions in lung cancers reflect the primary mutagenic signature of DNA-damage by tobacco smoke. Carcinogenesis. 2001;22:367–74. doi: 10.1093/carcin/22.3.367. [DOI] [PubMed] [Google Scholar]