Abstract
A current major challenge of acute myeloid leukemia research is to develop immunotherapeutic strategies that would be employable in all patients. We recently reported that appropriately stimulated γδ T cells are fully capable of mediating cytotoxicity against leukemic blasts.
Keywords: acute myeloid leukemia, antitumor immunity, immunosurveillance, γδ T cells
The immune response involves sentinels, such as circulating γδ T cells (Vγ9Vδ2 T cells), which are capable of recognizing and destroying abnormal cells. Vγ9Vδ2 T cells are T lymphocytes that operate at the interface between innate and adaptive immunity, exhibiting potent MHC-unrestricted cytotoxicity, high potential for cytokine release and a broad-spectrum recognition of cancer cells. Thus, Vγ9Vδ2 T cells are attractive effectors for cancer immunotherapy. Vγ9Vδ2 T cells have the natural ability to distinguish normal cells from “modified” cancer cells both in a TCR-dependent manner through recognition of natural phosphoantigens (PAgs) such as isopentenyl pyrophosphate (IPP, an intermediate of the mevalonate pathway, which is frequently dysregulated in cancer cells), and in a TCR-independent fashion, thanks to the expression of natural killer receptors (NKR) upon the recognition of target cells.
Acute myeloid leukemia (AML) is a heterogeneous disease with variable clinical outcomes. Induction chemotherapy given at diagnosis for the majority of patients has undergone little change in over 30 y. Allogeneic stem cell transplantation offers an example of a setting in which various immune effectors can contribute to the eradication of residual leukemic cells1 but its use is restricted to a minority of patients. Thus, the development of immunotherapeutic strategies that would be applicable to all patients appears highly desirable. We have recently reported that human Vγ9Vδ2 T cells specifically recognize and kill AML blasts.2
Phenotypic analyses of Vγ9Vδ2 T cells from AML patients at diagnosis showed that these cells exhibited a bias in their differentiation toward an effector memory phenotype, compared with Vγ9Vδ2 T cells from healthy volunteers (HV), in which the majority of circulating Vγ9Vδ2 T cells have a central memory phenotype.2 Effector memory cells are known to display higher functional capacities and a reduced (but existing) proliferation potential. Accordingly, we showed that Vγ9Vδ2 T cells from AML patients had a reduced potential of expansion compared with their counterparts from HVs. Interestingly, AML blasts appeared to be involved in the skewing toward this effector memory phenotype. Vγ9Vδ2 T cells do not seem the only cell compartment to suffer the influence of leukemic blasts. Indeed, a skewed effector profile was also observed in αβ CD3+CD8+ T cells from newly diagnosed AML patients.3 However, in the latter case, the effector functions of αβ CD3+CD8+ T cells were deficient due to impaired immunological synapses. When studying the cytotoxic potential of Vγ9Vδ2 T cells from AML patients, we found that these cells responded relatively well to AML blasts (compared with HV Vγ9Vδ2 T cells). This interaction was monitored both by trogocytosis (namely, the uptake of target-cell material following cell-cell contact) and by direct cytotoxicity. Nevertheless, we cannot exclude that the tumor might have acquired strategies to escape from, or impede, Vγ9Vδ2 T cells effector functions. For instance, the microenvironment generated by AML cells is known to prevent T-cell activation and proliferation4 and the direct contact between the chronic lymphocytic leukemia (CLL) cells and T cells has been reported to induce differential gene expression and impair the formation of the immune synapse.5 It is therefore conceivable that Vγ9Vδ2 T cells from AML patients may indeed encounter a certain inhibition from blast cells.
When we evaluated the expression of NKR ligands in AML patients at diagnosis to understand why immunity fails, we observed that except for a low expression of ULBP1, none of the NKG2D ligands were present on AML blasts. Conversely, DNAM-1 ligands, PVR and nectin 2, were expressed at the surface of leukemic cells.2 Of note, we found that DNAM-1 is partly involved in the recognition and killing of AML blasts, in line with a recent report on hepatocellular carcinoma6 and that the cytolytic activity of Vγ9Vδ2 T cells directly correlates with the surface expression of DNAM-1 ligands. In addition, we showed that stimulation with a synthetic PAg (BrHPP) drastically increases such the recognition of AML blast by Vγ9Vδ2 T cells, as previously described in other cancer settings such as follicular lymphoma.7
These results suggest that Vγ9Vδ2 T cells may require priming or co-activation for eliciting optimal antitumor responses. Moreover, these data further strengthen the rationale for the use of Vγ9Vδ2 T cells as therapeutic tools. Hence, the adoptive transfer of ex vivo expanded Vγ9Vδ2 T cells, or the in vivo stimulation with PAg or another Vγ9Vδ2 T cell agonist such as the aminobisphosphonate zoledronate, may turn out to improve classical therapies for AML. Supporting this hypothesis, two recent studies showed that zoledronate treatment improves in vitro Vγ9Vδ2 T cells functions in chronic myeloid leukemia.8,9 Moreover in the setting of allotransplantation for refractory acute lymphoblastic leukemia (ALL), γδ T cells were shown to exert a graft-vs.-leukemia effect without the appearance of graft-vs.-host disease (GvHD).10
Altogether, our work showed that appropriately stimulated γδ T cells are fully capable of recognizing and killing AML blasts (Fig. 1). Killing of leukemic cells is achieved by means of TCR- and DNAM-1-dependent activation. This study points out the requirements for the use of Vγ9Vδ2 T cells in therapies against AML. Furthermore, such a targeted cell-based therapy would present the major advantage to be available for most AML patients, and to be potentially devoid of adverse effects such as GvHD.
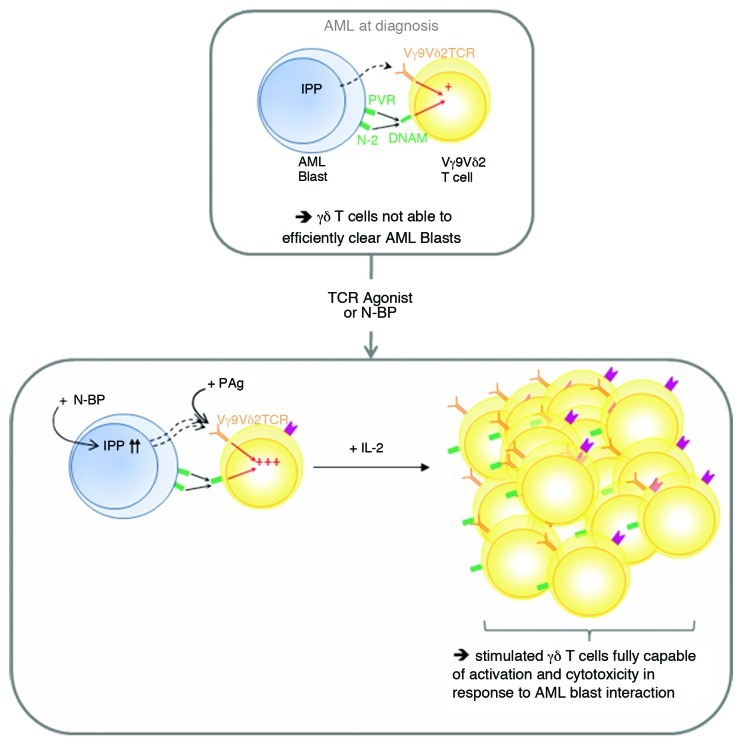
Figure 1. Appropriate stimulation allows an efficient Vγ9Vδ2 T cells anti-leukemic response. At diagnosis and without stimulation, Vγ9Vδ2 T cells recognize acute myeloid leukemia (AML) blasts in a TCR-dependent manner and in a TCR-independent manner, by binding to PVR and nectin 2 via DNAM-1. This induces the differentiation of Vγ9Vδ2 T cells toward an effector memory phenotype, but this is not sufficient to control disease progression. Vγ9Vδ2 T cells can be fully activated in vitro directly, by TCR agonists (synthetic phosphoantigens, PAgs), or indirectly, by using aminobisphosphonates, which lead to the accumulation of the natural PAg isopentenyl pyrophosphate (IPP). Activated Vγ9Vδ2 T cells can be subsequently expanded by an exogenous supply of interleukin-2 (IL-2) for subsequent adoptive transfer into cancer patients.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21512
References
- 1.Butturini A, Bortin MM, Gale RP. Graft-versus-leukemia following bone marrow transplantation. Bone Marrow Transplant. 1987;2:233–42. [PubMed] [Google Scholar]
- 2.Gertner-Dardenne J, Castellano R, Mamessier E, Garbit S, Kochbati E, Etienne A, et al. Human Vγ9Vδ2 T cells specifically recognize and kill acute myeloid leukemic blasts. J Immunol. 2012;188:4701–8. doi: 10.4049/jimmunol.1103710. [DOI] [PubMed] [Google Scholar]
- 3.Le Dieu R, Taussig DC, Ramsay AG, Mitter R, Miraki-Moud F, Fatah R, et al. Peripheral blood T cells in acute myeloid leukemia (AML) patients at diagnosis have abnormal phenotype and genotype and form defective immune synapses with AML blasts. Blood. 2009;114:3909–16. doi: 10.1182/blood-2009-02-206946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buggins AG, Milojkovic D, Arno MJ, Lea NC, Mufti GJ, Thomas NS, et al. Microenvironment produced by acute myeloid leukemia cells prevents T cell activation and proliferation by inhibition of NF-kappaB, c-Myc, and pRb pathways. J Immunol. 2001;167:6021–30. doi: 10.4049/jimmunol.167.10.6021. [DOI] [PubMed] [Google Scholar]
- 5.Ramsay AG, Johnson AJ, Lee AM, Gorgün G, Le Dieu R, Blum W, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118:2427–37. doi: 10.1172/JCI35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toutirais O, Cabillic F, Le Friec G, Salot S, Loyer P, Le Gallo M, et al. DNAX accessory molecule-1 (CD226) promotes human hepatocellular carcinoma cell lysis by Vgamma9Vdelta2 T cells. Eur J Immunol. 2009;39:1361–8. doi: 10.1002/eji.200838409. [DOI] [PubMed] [Google Scholar]
- 7.Gertner-Dardenne J, Bonnafous C, Bezombes C, Capietto AH, Scaglione V, Ingoure S, et al. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood. 2009;113:4875–84. doi: 10.1182/blood-2008-08-172296. [DOI] [PubMed] [Google Scholar]
- 8.D’Asaro M, La Mendola C, Di Liberto D, Orlando V, Todaro M, Spina M, et al. V gamma 9V delta 2 T lymphocytes efficiently recognize and kill zoledronate-sensitized, imatinib-sensitive, and imatinib-resistant chronic myelogenous leukemia cells. J Immunol. 2010;184:3260–8. doi: 10.4049/jimmunol.0903454. [DOI] [PubMed] [Google Scholar]
- 9.Siegers GM, Felizardo TC, Mathieson AM, Kosaka Y, Wang XH, Medin JA, et al. Anti-leukemia activity of in vitro-expanded human gamma delta T cells in a xenogeneic Ph+ leukemia model. PLoS One. 2011;6:e16700. doi: 10.1371/journal.pone.0016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamb LS, Jr., Musk P, Ye Z, van Rhee F, Geier SS, Tong JJ, et al. Human gammadelta(+) T lymphocytes have in vitro graft vs leukemia activity in the absence of an allogeneic response. Bone Marrow Transplant. 2001;27:601–6. doi: 10.1038/sj.bmt.1702830. [DOI] [PubMed] [Google Scholar]