Abstract
We recently reported a novel cooperative relationship between tumor-infiltrating B cells and CD8+ T cells in ovarian cancer, leading to increased patient survival. Here, we discuss the mechanisms whereby B cells might enhance cellular immunity, including serving as antigen-presenting cells, organizing tertiary lymphoid structures and secreting polarizing cytokines. The enhancement of both B and T-cell responses may result in more potent and sustained antitumor immunity.
Keywords: antigen-presenting cells, B cells, ovarian cancer, T cells, tumor-infiltrating lymphocytes
Numerous recent studies describe the strong association between tumor-infiltrating lymphocytes (TILs) and patient survival in human cancer.1 Although most studies focus on CD8+ T cells, other lymphocyte subsets also contribute to this effect. Here, we briefly describe the roles of CD20+ tumor-infiltrating B cells (CD20+ TILs), which are strongly associated with favorable outcomes in breast, lung, ovarian and cervical cancer.2 It is easy to rationalize the prognostic effect of CD8+ TILs, given their direct cytolytic activity against tumor cells. But how might CD20+ TILs promote tumor immunity? We recently investigated this issue in high-grade serous ovarian cancer (HGSC),3 a setting where we had previously shown that CD20+ TILs are strongly associated with survival.4
First, we demonstrated that CD20+ TILs display characteristics of antigen-experienced, oligoclonal B cells. This is in contrast with the polyclonal mixture of naïve and memory cells that would be expected if B cells simply were irrelevant bystanders in the tumor environment. Specifically, we showed that CD20+ TILs express cell surface IgG, indicating that they have undergone class switching. Moreover, we sequenced the CDR3 regions of immunoglobulin-coding mRNAs, which revealed that CD20+ TILs are oligoclonal and have undergone somatic hypermutation.3 Similar results have been reported in breast and germ cell tumors.2 Thus, CD20+ TILs have hallmarks of antigen-experienced, clonally expanded B cells.
Drawing from the transplantation and autoimmunity fields, we considered several mechanisms to explain how CD20+ TILs could increase patient survival. Initially, we asked whether CD20+ TILs might be a source of tumor-specific serum autoantibodies, which are commonly found in patients.5 Curiously, we found no association between CD20+ TILs and serum autoantibodies against two common tumor-associated antigens, NY-ESO-1 and p53.3 Therefore, it appears that CD20+ TILs are not the main source of tumor-specific serum autoantibodies.
As CD20+ TILs are not responsible for humoral antitumor immunity, we reasoned that they may play a role in cellular immunity. In autoimmunity and transplantation, infiltrating B cells have been associated with tissue destruction and appear to enhance T-cell responses in part by serving as antigen-presenting cells (APCs).6, 7 Consistent with this, we found that CD20+ TILs express molecules associated with APCs, including MHC Class I and II, CD80, CD86 and CD40.3 Moreover, by multicolor immunohistochemistry, we often found CD20+ TILs to localize with CD8+ T cells in loose aggregates within and adjacent to tumor islets.3 Similar “tertiary lymphoid structures” have been reported in autoimmunity, allograft rejection and chronic infection.8 Thus, CD8+ and CD20+ TILs co-localize in a pattern that is consistent with APC function.
These observations suggest that CD8+ and CD20+ TILs might work together to promote antitumor immunity and patient survival. We assessed this question by comparing survival in patients whose tumors contained CD8+ TILs with or without CD20+ TILs. Importantly, the presence of both CD8+ and CD20+ TILs was associated with markedly increased survival compared with CD8+ TILs alone or no TILs.3 Collectively, our study provides strong evidence, albeit indirect, that CD8+ and CD20+ TILs act cooperatively to promote antitumor immunity.
How might CD20+ TILs help promote superior antitumor immunity? We propose three possibilities (Fig. 1). First, by serving as APCs, CD20+ TILs might facilitate the persistence of CD8+ T cells for long periods.2 Whereas dendritic cells (DCs) may be well suited to initiate immune responses, protective antitumor immunity requires responses to persist for years. Perhaps CD20+ TILs provide ongoing stimulatory signals that inhibit the development of T-cell anergy or exhaustion. Moreover, B cells have the unique ability to take up specific antigen through their surface Ig molecules. This may allow concentration of low-level tumor antigens for processing and presentation to T cells. Second, B cells can produce cytokines such as lymphotoxin that promote the organization of local lymphoid structures.6 Indeed, in autoimmunity, B cell depletion with rituximab disrupts T-cell infiltrates in affected tissues.9 Third, B cells can produce cytokines that polarize T cells toward Th1, Th2 and maybe other functional phenotypes.10 In summary, several unique properties of B cells might make them ideally suited to promote potent T-cell responses over the time frames associated with human cancer.
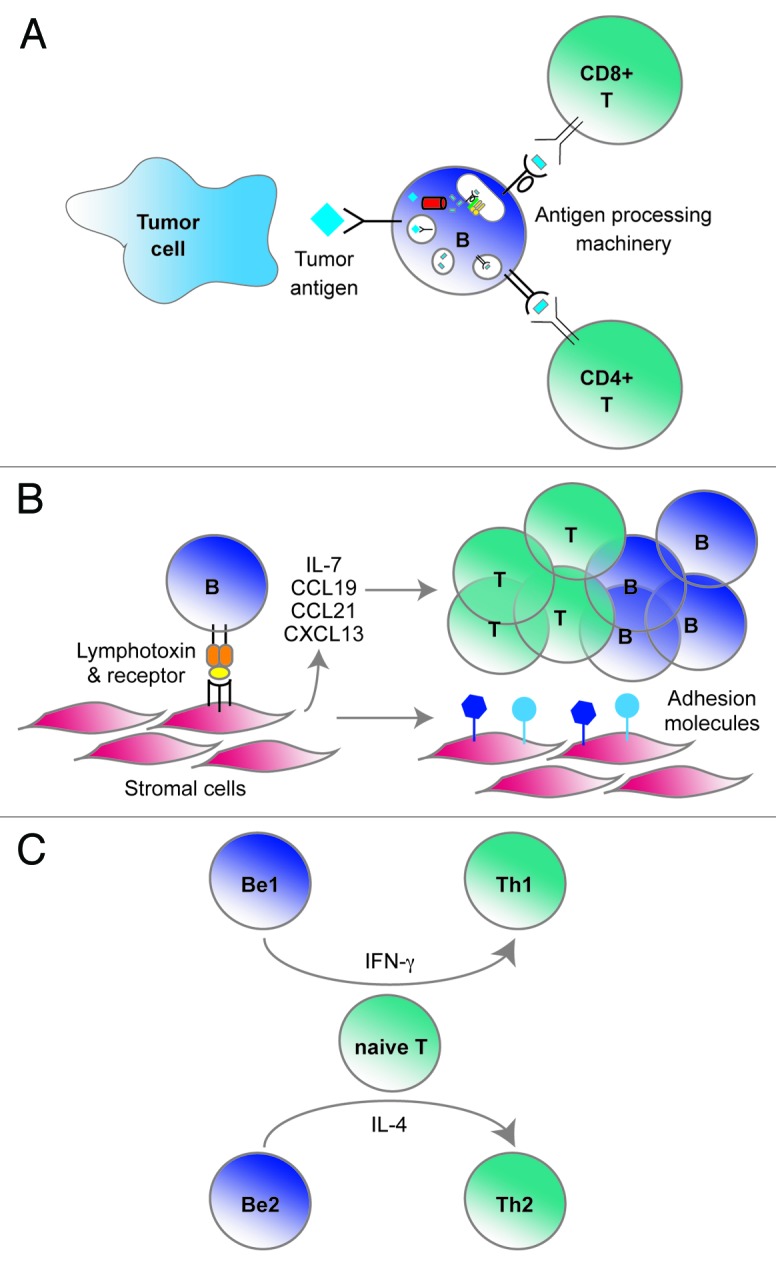
Figure 1. Three proposed roles for CD20+ tumor-infiltrating lymphocytes (TILs) in promoting anti-tumor immunity. (A) CD20+ TILs as antigen presenting cells. B cells can bind tumor antigens via surface Ig molecules, process them and then present peptides to CD8+ and CD4+ T cells via MHC Class I and Class II, respectively. (B) CD20+ TILs as lymphoid organizers. B cells are able to secrete lymphotoxin, which can induce stromal cells to express adhesion molecules, cytokines and chemokines. These factors, in turn, can recruit and retain other lymphocytes. (C) CD20+ TILs as polarizing cells. Type-I and Type-II B effector cells (Be1 and Be2) can secrete cytokines such as interferon γ (IFNγ) and interleukin-4 (IL-4), which can skew T-cell responses toward Th1, Th2 or other functional states.
Looking ahead, what are the key research questions concerning CD20+ TILs? To definitively demonstrate that CD20+ TILs serve as APCs to T cells, it is imperative to identify their cognate antigens. Although two antigens have been identified in breast cancer, the antigen repertoire of CD20+ TILs remains largely undefined.2 New high-throughput screening methods may now make antigen discovery more feasible. There is also an urgent need for understanding the functional profiles of CD20+ TILs. In HGSC, these cells show many of the hallmarks of memory B cells. However, they lack the canonical memory marker CD27,3 a B-cell phenotype that is also observed in other pathological conditions such as systemic lupus erythematosus. This suggests that CD20+ TILs might have unique functional properties that warrant further study. Of key importance will be to define factors that facilitate the development of coordinated CD8+ and CD20+ TIL responses. With an improved understanding of these issues, it should be possible to design immunotherapies that enhance not only CD8+ T cell immunity against cancer, but also the potent contributions of CD20+ TILs.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21650
References
- 1.Pagès F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 2.Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185:4977–82. doi: 10.4049/jimmunol.1001323. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, et al. CD20+ Tumor-Infiltrating Lymphocytes Have an Atypical CD27- Memory Phenotype and Together with CD8+ T Cells Promote Favorable Prognosis in Ovarian Cancer. Clin Cancer Res. 2012;18:3281–92. doi: 10.1158/1078-0432.CCR-12-0234. [DOI] [PubMed] [Google Scholar]
- 4.Milne K, Köbel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, et al. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One. 2009;4:e6412. doi: 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone B, Schummer M, Paley PJ, Thompson L, Stewart J, Ford M, et al. Serologic analysis of ovarian tumor antigens reveals a bias toward antigens encoded on 17q. Int J Cancer. 2003;104:73–84. doi: 10.1002/ijc.10900. [DOI] [PubMed] [Google Scholar]
- 6.Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–99. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 7.Martin F, Chan AC. B cell immunobiology in disease: evolving concepts from the clinic. Annu Rev Immunol. 2006;24:467–96. doi: 10.1146/annurev.immunol.24.021605.090517. [DOI] [PubMed] [Google Scholar]
- 8.Carragher DM, Rangel-Moreno J, Randall TD. Ectopic lymphoid tissues and local immunity. Semin Immunol. 2008;20:26–42. doi: 10.1016/j.smim.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takemura S, Klimiuk PA, Braun A, Goronzy JJ, Weyand CM. T cell activation in rheumatoid synovium is B cell dependent. J Immunol. 2001;167:4710–8. doi: 10.4049/jimmunol.167.8.4710. [DOI] [PubMed] [Google Scholar]
- 10.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–82. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]


