Abstract
We identified novel mechanisms whereby TIM-3 suppresses innate immunity as induced by nucleic acids. Interaction of TIM-3 with HMGB1 inhibits the recruitment of nucleic acids to the endosomal compartment of dendritic cells, impairing the transduction of innate immune signals. Thus, TIM-3 is an effective target for enhancing the immunogenicity of nucleic acids in the context of cancer therapy.
Keywords: Dendritic cells, HMGB1, innate immunity, nucleic acids, TIM-3
Innate immunity serves as a well-organized pattern recognition system for infectious and stress-induced components within inflammatory microenvironments. In particular, nucleic acids derived from infectious agents or dying host cells are detected by pattern-recognition receptors such as Toll-like receptors (TLRs) and cytosolic sensors for DNA and RNA, leading to the induction of cytokines and other proinflammatory mediators that are essential for innate immune responses.1
Discrimination between foreign and self nucleic acids serves as a key mechanism protecting the host from pathogens while maintaining tissue homeostasis, as exposure of self-nucleic acids to the innate immune system frequently results in severe inflammation and autoimmune reactions.2 However, several studies have unveiled mechanisms whereby self nucleic acids gain access to components of the innate immune system. Endogenous proteins released from inflammatory environments, such as danger-associated molecular patterns (DAMPs), preferentially interact with self nucleic acids. Thus, the formation of complexes with DAMPs enables nucleic acids to gain access to the endosomal compartment, in which receptors from the innate immune system recognize nucleic acids and orchestrate proinflammatory responses.3 In addition, recent studies reveal that exogenous nucleic acids mediate immunogenic activities by interacting with pattern recognition systems. For example, DNA released from dying host cells has a key role in triggering the adjuvant effects of aluminum, thus facilitating dendritic cell (DC) migration and antigen-specific T-cell responses.4 Moreover, cellular damage as induced by UV irradiation results in the structural modification of self RNAs and hence in the delivery of innate immune signals via TLR3.5 Thus, nucleic acids generated from host cells have the potential to activate innate immunity under various pathological situations.
Transformed cells are assumed to be a mixture of self and “non-self” nucleic acids, the latter being a result of tumor-associated mutations. In addition, the tumor microenvironment consists of fibroblasts and myeloid cells, which produce multiple inflammatory mediators including DAMPs.6 However, it remains largely unclear whether inflammatory mediators activate innate immunity by releasing ‘immunogenic” nucleic acids in the tumor microenvironment. Thus, it is critical to address the molecular mechanisms by which tumor microenvironments can affect the ability of nucleic acids to interact with the pro-inflammatory signal machinery and activate the innate immune system.
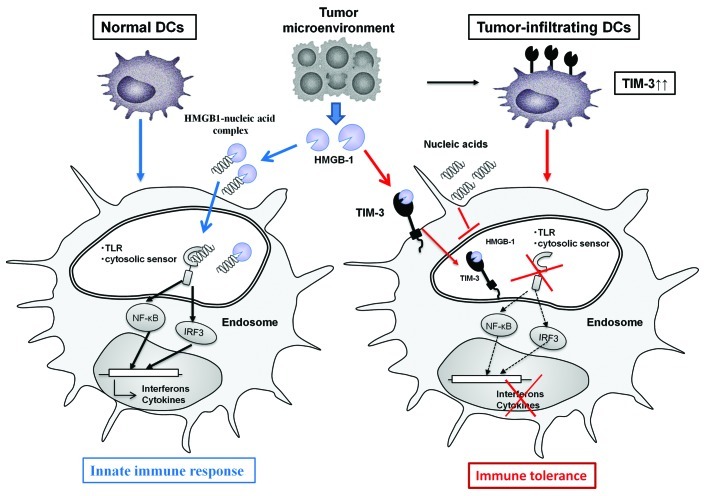
TIM-3 is upregulated on Type 1 T helper CD8+ T lymphocytes during the chronic phase of infection as well as during oncogenesis and can trigger their apoptotic demise following the ligation of galectin 9.7 We identified an unexpected function of TIM-3 in negatively regulating nucleic acid-mediated innate immune responses from DCs found in the tumor microenvironment.8 TIM-3 is expressed on tumor-infiltrating DCs at much higher levels than on DCs in normal tissues, and preferentially binds with the major DAMP high mobility group box-1 (HMGB1), which has a critical role in stimulating nucleic acid-mediated innate immunity.9 TIM-3 negatively regulates the HMGB1-mediated recruitment of nucleic acids to the endosomal compartment of DCs, thus shutting down the downstream signaling cascades mediated by TLRs and cytosolic sensors.8 TIM-3 on DCs thereby enables tumors to evade immunosurveillance by attenuating the sensing of nucleic acids that is potentially triggered by tumor-associated inflammation (Fig. 1).
Figure 1. This scheme illustrates the molecular machineries whereby TIM-3 on dendritic cells (DCs) negatively regulates innate immune signals that would be activated by endogenous danger signals under normal conditions. Tumor microenvironments frequently generate endogenous danger signals including HMGB1 due to smoldering inflammation. HMGB1 binds nucleic acids and facilitates the interaction of nucleic acids with pattern recognition receptors including Toll-like receptors (TLRs) and cytosolic sensors, activating innate immune signals. However, tumors counteract innate immunity by upregulating TIM-3 on tumor-infiltrating DCs. The interaction between TIM-3 and HMGB1 inhibits the recruitment of nucleic acids into the endosomal compartment of DCs. Thus, the interaction between TIM-3 and HMGB1 serves as an evasion strategy used by tumors to escape immunosurveillance.
Why TIM-3 on DCs is preferentially recognized by HMGB1 rather than galectin 9 remains largely obscure, but multiple tumor-derived mediators may regulate the repertoires of endogenous danger signals that contribute to the creation of inflammatory tumor microenvironments. Interestingly, a recent report revealed that pattern recognition receptor ligands including DAMPs may serve as driving forces that trigger the release of exogenous HMGB1 from the nucleus of host cells.10 This suggests that DNA vaccination or endogenous nucleic acids released from dying cells upon cytotoxic chemotherapy may increase the presence of HMGB1 in the tumor microenvironment, thus contributing to the generation of HMGB1-DNA complexes and the activation of innate immunity. With regard to this, tumors appear to utilize TIM-3 on tumor-infiltrating DCs as a means to evade innate immune responses elicited by anticancer therapeutic regimens.
A deeper understanding of the mechanisms linking innate immunity to tumor-associated inflammation and of the impact of negative regulatory pathways established by the tumor microenvironment will provide new strategies to promote endogenous antitumor immune responses and improve the efficacy of anticancer therapy.
Glossary
Abbreviations:
- DAMPs
danger-associated molecular patterns
- DC
dendritic cells
- HMGB1
high mobility group box 1
- TIM-3
T cell-immunoglobulin mucin protein 3
- TLRs
Toll-like receptors
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21681
References
- 1.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–15. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Desmet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat Rev Immunol. 2012;12:479–91. doi: 10.1038/nri3247. [DOI] [PubMed] [Google Scholar]
- 4.Marichal T, Ohata K, Bedoret D, Mesnil C, Sabatel C, Kobiyama K, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17:996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 5.Bernard JJ, Cowing-Zitron C, Nakatsuji T, Muehleisen B, Muto J, Borkowski AW, et al. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat Med. 2012 doi: 10.1038/nm.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuraishy A, Karin M, Grivennikov SI. Tumor promotion via injury- and death-induced inflammation. Immunity. 2011;35:467–77. doi: 10.1016/j.immuni.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–52. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 8.Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012 doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, et al. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 10.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–62. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]