Abstract
Retrospective clinical data indicate that cardiac glycosides (CGs), notably digoxin, prolong the survival of carcinoma patients treated with conventional chemotherapy. CGs are known to influence the immune response at multiple levels. In addition, recent results suggest that CGs trigger the immunogenic demise of cancer cells, an effect that most likely contributes to their clinical anticancer activity.
Keywords: calreticulin, digitoxin, digoxin, HMGB1, immunogenic cell death, Na+/K+ ATPase
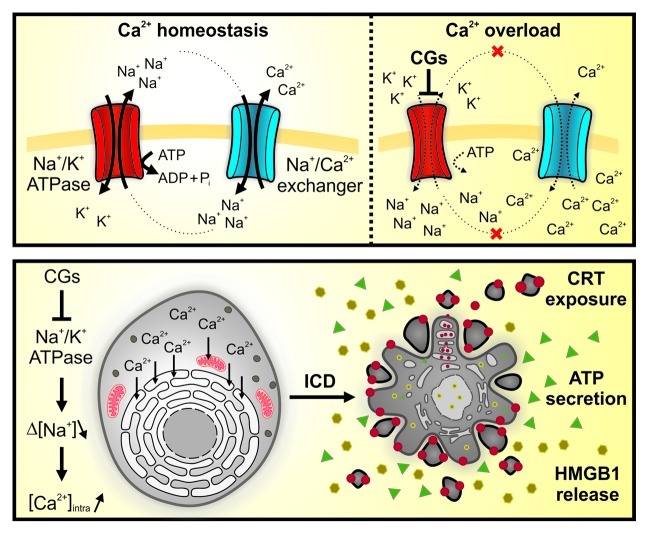
Digoxin and digitoxin, two digitalis derivatives also known as cardiac glycosides (CGs), are among the most ancient and effective therapies for congestive heart failure and arrhythmia.1 The major pharmacological effect of these compounds, which are still largely used in the clinic, derives from the inhibition of the plasma membrane Na+/K+ ATPase (Fig. 1).1 Thus, upon binding to this essential cationic pump, CGs reduce the intracellular concentration of K+ while augmenting that of Na+. In turn, high intracellular levels of Na+ block the antiporter activity of the Na+/Ca2+ exchanger, favoring the accumulation of Ca2+ within the endoplasmic reticulum and mitochondria. Eventually, this allows for an increased release of Ca2+ from the endoplasmic reticulum (via inositol 1,4,5-trisphosphate-gated channels) in response to contraction stimuli, which de facto improves the performance of the heart muscle.1 Since human cancer cells tend to express particular isoforms of the subunits that build up the Na+/K+ ATPase, they may be especially sensitive to the cytotoxic effect of CGs.2 Multiple studies have previously explored this hypothesis, testing the cytotoxic potential of CGs on human carcinoma or leukemia cells.2,3 Of note, the human Na+/K+ ATPase has a much higher affinity for digoxin than its mouse equivalent. Hence, it is possible to transfect human cells with vectors expressing murine Na+/K+ ATPase subunits, rendering them resistant to CG-mediated cytotoxicity. This methodological approach allows for the discrimination between the bona fide on-target activity of CGs and possible off-target effects.4
Figure 1. Proposed mode of action of cardiac glycosides. Cardiac glycosides (CGs) bind to (and hence inhibit) the plasma membrane Na+/K+ ATPase, resulting in the accumulation of intracellular Na+ ions. As the Na+ gradient [Δ(Na+)] normally drives Ca2+ extrusion via the Na+/Ca2+ exchanger, CGs increase the intracellular concentration of Ca2+ [(Ca2+)intra], which is readily taken up by the endoplasmic reticulum and by mitochondria (A). In cardiomyocytes, this allows for an increased release of Ca2+ from the endoplasmic reticulum (via inositol 1,4,5-trisphosphate-gated channels) in response to contraction stimuli, de facto improving the cardiac performance. Conversely, cancer cells express particular Na+/K+ ATPase subunits and hence respond to CGs with an endoplasmic reticulum stress that eventually is lethal. Thus, CGs can induce immunogenic cell death (ICD), featuring the exposure of calreticulin (CRT) at the cell surface, the secretion of ATP as well as the release of the nuclear protein HMGB1 into the extracellular space (B). Pi, inorganic phosphate.
Interestingly, CGs can mediate anti-inflammatory effects by an on-target mechanism. Indeed, the increase in intracellular Na+ induced by digoxin can inhibit the ATPase activity of the RNA sensor RIG-I, an essential and early component in the signal transduction pathway leading to interferon β secretion.5 As a result, CGs interfere with the transactivation of IFNB by viruses, double-stranded RNA or double-stranded DNA, an effect that can be overcome by the expression of a CG-resistant variant of the Na+/K+ ATPase. CGs also inhibit tumor necrosis factor α signaling, at least in part by interfering with the nuclear translocation of NFκB.5
Recently, we performed a chemical screen to identify components that would induce immunogenic cell death (ICD) in vitro.6 To this aim, we generated a panel of human osteosarcoma U2OS cells stably expressing a series of biosensors that measure the hallmarks of ICD, namely, (1) the pre-apoptotic exposure of calreticulin at the cell surface (which depends on the establishment of an endoplasmic reticulum stress), (2) the secretion of ATP during the blebbing phase of apoptosis (which depends on the autophagic machinery) and (3) the post-apoptotic release of the non-histone chromatin protein HMGB1.7 Among a panel of FDA-approved drugs, we identified several CGs (i.e., digoxin, digitoxin, ouabain and lanatoside C) as particularly efficient inducers of the three hallmarks of ICD in vitro. Having validated the capacity of digoxin to stimulate calreticulin exposure, ATP secretion and HMGB1 release in vitro, on a broad panel of human and mouse cancer cell lines, we characterized the mechanisms underlying these effects. We found that digoxin and digitoxin lose their cytotoxicity, as well as their potential to stimulate the hallmarks of ICD, on human cells that are transfected with mouse Na+/K+ ATPase subunits.6 Hence, CG-induced ICD originates from an on-target mechanism.
Subsequent functional experiments confirmed the capacity of CGs to stimulate anticancer immune responses in vivo. Thus, murine colon cancer CT26 cells or fibrosarcoma MCA205 cells succumbing to a combination of chemotherapy plus digoxin were able to efficiently vaccinate syngenic mice against a subsequent challenge with living cells of the same type.6 Moreover, CGs exacerbated the antineoplastic effects of DNA-damaging agents such as mitomycin C and cisplatin in immunocompetent, but not in immunodeficient, mice. In this model, the combination of digoxin and mitomycin C induced a more robust infiltration of tumors by interferon γ-producing α/β CD4+ or CD8+ T lymphocytes than did either of these two agents alone.6 These results suggest that digoxin can indeed mediate antineoplastic effects by stimulating a tumor-specific immune response.
Encouraged by these observations, we decided to engage in an extensive retrospective clinical study. To this aim, we identified within the clinical files of the Institut Gustave Roussy (Villejuif, France) all carcinoma patients that received digoxin—owing to an underlying cardiac disorder—along with conventional anticancer therapies. In addition, for each of these carcinoma patients, we selected two control patients that were perfectly matched according to demographic criteria (age, sex, department of treatment), clinical parameters (tumor type, TNM stage), biological characteristics of the tumor (histological type, hormone receptor status, human papilloma virus infection, etc…) and type of treatment (chemotherapy, radiotherapy, hormonotherapy). Surprisingly, the overall survival of carcinoma patients receiving digoxin was superior as compared with that of control patients, in spite of the presence of an underlying cardiac pathology. Subgroup analyses revealed that digoxin ameliorated the overall survival of breast, head and neck, hepatocellular and colorectal carcinoma patients, but not of subjects affected by non-small cell lung cancer or prostate carcinoma.6 Moreover, patients that received anthracyclines or oxaliplatin did not benefit from the presence of digoxin, possibly because anthracyclines and oxaliplatin constitute optimal ICD inducers per se.8,9 In contrast, patients receiving drugs other than anthracyclines and oxaliplatin did obtain a survival benefit if they were co-treated with digoxin.6 Altogether, these clinical data suggest that digoxin improves the life expectancy of cancer patients by virtue of its ICD-inducing capacity.
It must be noted that CGs can also have off-target effects. In particular, digitalis compounds are phytoestrogens and bind to the estrogen receptor (ER), albeit with a lower affinity than estrogen itself. A large study enrolling more than 100,000 women revealed that current (but not former) digoxin use increase the relative risk (RR) of developing breast cancer (current users vs. non-users: RR = 1.39; 95% CI = 1.32–1.46), with a trend in favor of ER-positive, rather than ER-negative, lesions.10 An even larger study, involving 2.1 million women, confirmed that the risk of developing uterus cancer is increased among current (but not former) digoxin users (current users vs. non-users: RR = 1.48, 95% CI = 1.32–1.65). Conversely, long-term (for ≥ 10 y) users of digoxin appear to have a significantly reduced risk to develop prostate cancer (long-term users vs. non-users: RR = 0.54, 95% CI = 0.37–0.79, p value < 0.001), as determined on a cohort of close to 50,000 men followed for over 20 years.11
Beyond these effects, which may be attributed to an agonistic activity on ERs, digoxin may also act on other steroid receptors. This notion is supported by the results of a recent chemical screen, leading to the discovery that digoxin is a specific inhibitor of the retinoic acid receptor (RAR)-related nuclear orphan receptor RORγt.12 Subsequent optimization steps allowed for the generation of the non-toxic digoxin derivatives 20,22-dihydrodigoxin-21,23-diol and digoxin-21-salicylidene, which specifically inhibit RORγt but have lost the ability to bind the Na+/K+ ATPase. RORγt is required for the transactivation of the gene encoding interleukin-17 (IL-17) and for the manifestation of TH17-dependent autoimmune diseases in mice.12 In line with its capacity to inhibit RORγt, digoxin has been shown to prevent experimental autoimmune encephalitis in mice and to block the induction of IL-17 in human CD4+ T cells.12 Nonetheless, we believe that these steroid-like effects of digoxin cannot explain the results of our retrospective study, for multiple reasons. First, digoxin improved (rather than reduced) the life expectancy of breast cancer patients. Second, digoxin had neither beneficial nor detrimental effects on the survival of prostate cancer patients. Finally, in preclinical experiments, digoxin failed to affect the intratumoral secretion of IL-17 by γ/δ T cells in vivo.6
Based on our retrospective study, we are now designing a prospective clinical trial in which digoxin will be administered to patients bearing locally advanced, human papilloma virus-negative head and neck cancer, obviously upon the exclusion of subjects affected by coronary disease or other contraindications of CGs. Together with a team of clinical oncologists, we will then monitor whether the combination of digoxin with standard, cisplatin-based chemo/radiotherapy regimens will be tolerated, assess the infiltration of the tumor by immune effectors and determine the potential clinical benefits or this approach.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21684
References
- 1.Ehle M, Patel C, Giugliano RP. Digoxin: clinical highlights: a review of digoxin and its use in contemporary medicine. Crit Pathw Cardiol. 2011;10:93–8. doi: 10.1097/HPC.0b013e318221e7dd. [DOI] [PubMed] [Google Scholar]
- 2.Newman RA, Yang P, Pawlus AD, Block KI. Cardiac glycosides as novel cancer therapeutic agents. Mol Interv. 2008;8:36–49. doi: 10.1124/mi.8.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Tailler M, Senovilla L, Lainey E, Thépot S, Métivier D, Sébert M, et al. Antineoplastic activity of ouabain and pyrithione zinc in acute myeloid leukemia. Oncogene. 2012;31:3536–46. doi: 10.1038/onc.2011.521. [DOI] [PubMed] [Google Scholar]
- 4.Perne A, Muellner MK, Steinrueck M, Craig-Mueller N, Mayerhofer J, Schwarzinger I, et al. Cardiac glycosides induce cell death in human cells by inhibiting general protein synthesis. PLoS One. 2009;4:e8292. doi: 10.1371/journal.pone.0008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye J, Chen S, Maniatis T. Cardiac glycosides are potent inhibitors of interferon-β gene expression. Nat Chem Biol. 2011;7:25–33. doi: 10.1038/nchembio.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, Shen S, et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med. 2012;4:143ra99. doi: 10.1126/scitranslmed.3003807. [DOI] [PubMed] [Google Scholar]
- 7.Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140:798–804. doi: 10.1016/j.cell.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–33. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 9.Vacchelli E, Galluzzi L, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, et al. Trial watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2012;1:179–88. doi: 10.4161/onci.1.2.19026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biggar RJ, Wohlfahrt J, Oudin A, Hjuler T, Melbye M. Digoxin use and the risk of breast cancer in women. J Clin Oncol. 2011;29:2165–70. doi: 10.1200/JCO.2010.32.8146. [DOI] [PubMed] [Google Scholar]
- 11.Platz EA, Yegnasubramanian S, Liu JO, Chong CR, Shim JS, Kenfield SA, et al. A novel two-stage, transdisciplinary study identifies digoxin as a possible drug for prostate cancer treatment. Cancer Discov. 2011;1:68–77. doi: 10.1158/2159-8274.CD-10-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature. 2011;472:486–90. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]