Abstract
Following activation, γδ T cells display many properties of lymphocytes from the innate immune system, yet how they mediate antigen presentation remains an open conundrum. In humans, circulating γδ T cells that express the Vγ9Vδ2 T-cell receptor become reversibly licensed for professional antigen presentation only upon interaction with a target cell opsonized with IgGs.
Keywords: APC, CD16, antibody opsonisation, antigen presentation, licensing, γδ T cells
γδ T cells in humans and mice share many properties with lymphocytes from the innate immune system, expressing, for instance, activatory natural killer (NK)-cell receptors such as NKG2D, inhibitory KIR receptors, as well as other cytotoxic surface molecules such as tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL). Like conventional αβ T lymphocytes, γδ T cells express rearranged T-cell receptor (TCRs) comprising two (one γ and one δ) chains, which - compared with rearranged and MHC-restricted αβ TCRs - have a limited range of MHC-unrestricted antigenic specificities. Of note, and again in common with NK cells, γδ T cells can express Fcγ receptors, most notably FcγRIII (CD16), which (at least in humans) is expressed to the highest levels by activated cells.1
A relatively new theme in the research field dealing with human γδ T lymphocytes has emerged following the seminal observation that, upon activation by γδ TCR ligation, γδ T cells become capable of taking up antigens and mediate professional antigen presentation to naïve αβ T cells.2,3 When compared with mature human dendritic cells, γδ T lymphocytes express equivalent levels of co-stimulatory molecules and CCR7, and are equally potent at promoting proliferative responses in αβ T cells.2
We initially hypothesized that the surface expression of CD16 by γδ T cells might be indicative of a phagocytic function, and we showed that human blood γδ T cells are indeed capable of taking up bacteria and beads, yet only upon target opsonisation by IgGs. Following the phagocytosis of beads coated with an influenza antigen, γδ T cells processed and presented the antigen to MHC Class II-restricted hybridoma T cells.4
We therefore wondered whether there might be a link between the recognition of antibody-coated target cells and the professional antigen presentation that had previously been reported by Moser and coworkers,2,3,5 and whether this might have implications for oncology, a field in which harnessing and regulating the function of professional antigen presenting cells (APCs) might be exploited therapeutically. A precedent for this type of regulation is provided by dendritic cells (DCs), for which licensing upon the interaction with CD40 ligand (CD40L)-expressing helper T lymphocytes in the T-cell areas of draining lymph nodes is required for the presentation of antigens taken up by immature DCs at a site of injury, infection or cancer.6-8 Interestingly, in the absence of a antibody-coated target cells, γδ T cells were capable of low levels of cross-presentation to MHC Class I-restricted αβ T cells. Conversely, the presence of opsonized target cells was sufficient to achieve a degree of cross-presentation by isoprenyl pyrophosphate (IPP)-activated human circulating γδ T cells that was equivalent to that of mature DCs.9 We have termed this phenomenon “licensing” for professional APC function by γδ T cells. Strikingly, neither antibodies alone, target cells alone, nor target cells in the presence of non-binding antibodies are capable of eliciting this “licensed” state. We demonstrated licensing using both rituximab and CH14.18, two humanized IgG1 antibodies targeting CD20 and the GD2 ganglioside, respectively, which are clinically used for the treatment of B-cell malignancies and neuroblastoma. Human IgG1 antibodies efficiently bind FcγRIII (CD16) and, accordingly, licensing was abrogated by CD16 blocking antibodies.9
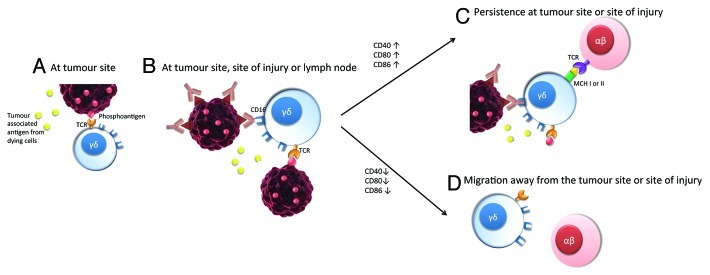
A model is therefore emerging suggesting that human γδ T cells are capable of operating as professional APCs similar to DCs and that - like DC - a specific licensing signal is required for them to acquire full-blown APC functions (Fig. 1). We have also observed that, for acting as professional APCs, γδ T cells require the engagement of their TCR as well as a correct cytokine milieu, as demonstrated by the fact that γδ T cells acquired APC functions only in media conditioned by B-cell lymphoblastoid lines (B-LCLs). Indeed, we observed the aggregation of the γδ TCR in an immune complex at the sites of interaction between γδ T cells and rituximab-opsonized Daudi cells.9 It will be interesting to determine the involvement of CD16-Fc interactions in this complex.
Figure 1. (A) At the tumor site, circulating γδ T cells bearing the Vγ9Vδ2 T-cell receptor (TCR) become activated and expand as a result of TCR ligation, for instance by the isoprenyl pyrophosphate (IPP) phosphoantigen. (B) In the context of an established or nascent immune response, target cells become opsonized with antibodies and activated γδ T cells become licensed through IgG/CD16 interactions. (C) This licensing is highly regulated: in the absence of continuative interactions with opsonized target cells, γδ T cells rapidly lose their ability to take up and present soluble antigens, such a loss being associated with the downregulation of co-stimulatory molecules. Hence the APC function of γδ T cells is localized and antigen presentation is restricted to tumor-associated antigens, de facto decreasing the risk of autoimmune phenomena.
Unlike DCs, the licensing of γδ T cells is a reversible process. On separating licensed circulating γδ T cells from opsonized target cells and culturing them in the presence of IPP, we found that their ability to cross-present antigen is lost over a 24 h period. . Strikingly, however, a licensed and then unlicensed γδ T cell can be “re-licensed” by the same antibody-opsonized target. We have recently observed that the “de-licensing” process is associated with the downregulation of the co-stimulatory molecules CD80 and CD86, while “re-licensing” is associated with their re-expression (unpublished observations).
Many aspects of the regulation of the APC function of γδ T cells remain to be investigated. For example, from a signal transduction perspective, it will be interesting to determine whether CD16 interacts with the γδ TCR at the synapse between γδ T cells and target cells and whether the rapid and reversible changes in co-stimulatory molecule expression are driven by cytokine secretion or reflect direct transcriptional effects. The APC function of γδ T cells from different anatomical sites and bearing different TCRs (that exhibit distinct ligand specificities) warrants detailed investigation. From an even more fundamental research-oriented perspective, it will be important to understand whether γδ T cells represent an evolutionarily primitive type of lymphocyte or rather one that has emerged after the evolution of adaptive immune responses, to perform two specialized functions (i.e., killing and antigen presentation) in a highly regulated manner. Our findings are perhaps most exciting from a therapeutic perspective, as they imply that monoclonal antibodies used in anticancer therapy might be combined with γδ T cells or γδ T-cell stimulating factors to modulate innate effector functions as well as the generation of secondary immune responses.
Glossary
Abbreviations:
- APC
antigen-presenting cell
- IPP
isoprenyl pyrophosphate
- NK
natural killer
- TCR
T-cell receptor
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21971
References
- 1.Braakman E, van de Winkel JG, van Krimpen BA, Jansze M, Bolhuis RL. CD16 on human gamma delta T lymphocytes: expression, function, and specificity for mouse IgG isotypes. Cell Immunol. 1992;143:97–107. doi: 10.1016/0008-8749(92)90008-D. [DOI] [PubMed] [Google Scholar]
- 2.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309:264–8. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 3.Brandes M, Willimann K, Bioley G, Lévy N, Eberl M, Luo M, et al. Cross-presenting human gammadelta T cells induce robust CD8+ alphabeta T cell responses. Proc Natl Acad Sci U S A. 2009;106:2307–12. doi: 10.1073/pnas.0810059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y, Wu W, Wong WM, Ward E, Thrasher AJ, Goldblatt D, et al. Human gamma delta T cells: a lymphoid lineage cell capable of professional phagocytosis. J Immunol. 2009;183:5622–9. doi: 10.4049/jimmunol.0901772. [DOI] [PubMed] [Google Scholar]
- 5.Meuter S, Eberl M, Moser B. Prolonged antigen survival and cytosolic export in cross-presenting human gammadelta T cells. Proc Natl Acad Sci U S A. 2010;107:8730–5. doi: 10.1073/pnas.1002769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 7.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 8.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–80. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 9.Himoudi N, Morgenstern DA, Yan M, Vernay B, Saraiva L, Wu Y, et al. Human γδ T lymphocytes are licensed for professional antigen presentation by interaction with opsonized target cells. J Immunol. 2012;188:1708–16. doi: 10.4049/jimmunol.1102654. [DOI] [PubMed] [Google Scholar]