Abstract
Combining electrochemotherapy with dendritic cell-based immunotherapy is a promising strategy against human metastatic melanoma that deserves to be clinically assessed. While electrochemotherapy induces a rapid regression of metastases, immunotherapy generates systemic anticancer immunity, contributes to eradicate the tumor and maintains an immunological memory to control relapse.
Keywords: cancer immunity, cancer immunotherapy, dendritic cells, electrochemotherapy, melanoma
In the last few years, there has been a renovated interest in cancer immunotherapy, and a specific field of this area, dendritic cell (DC)-based immunotherapy, is living a renaissance.
Given the ability of DCs to orchestrate the immune system and their essential role in generating antitumor immunity in mice, several efforts have been made to harness these properties for cancer therapy.1,2 Although sporadic regressions of metastases have been described, the laborious protocols for in vitro DC preparation have greatly limited the use of this appraoch.2 However, recent studies in animal models have shown the possibility to exploit endogenous DC populations.3 Targeting of tumor antigens to local DCs is feasible and represents an effective form of cancer immunotherapy, paving the way for other forms of in vivo DC-based immunotherapy.2,3
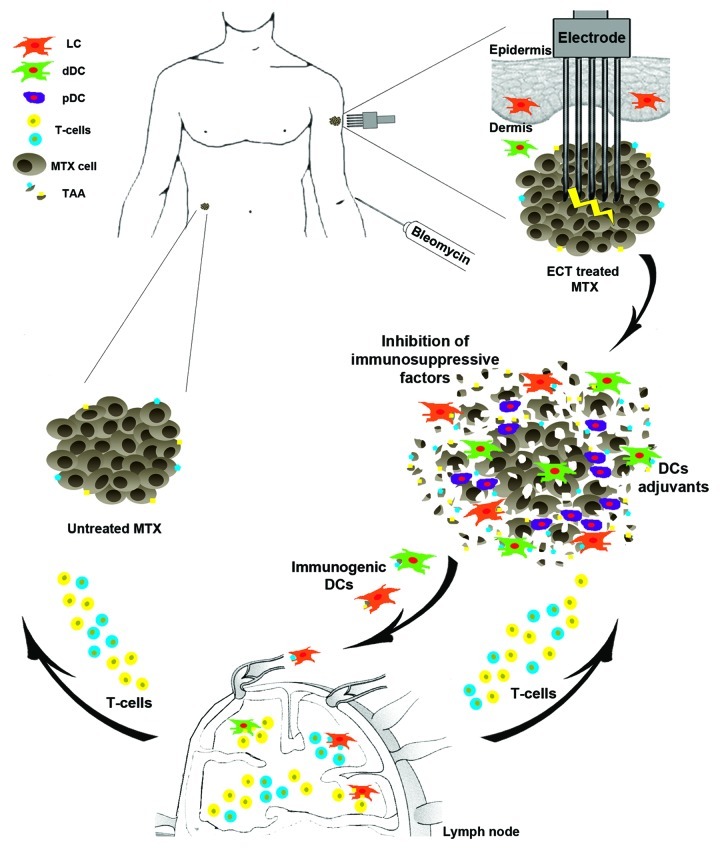
A particular type of chemotherapy, electrochemotherapy (ECT), is emerging as a highly effective treatment for human cancers and particularly for unresectable metastatic melanoma.4 ECT is based on the administration of antineoplastic drugs followed by the electroporation of tumor cells, which strongly increases therapeutic efficacy. Although ECT has only local effects, the innovative combination of ECT and CpG oligodeoxynucleotides (CpG-ODNs), a potent immune adjuvant, triggers a systemic anticancer immunity with regression of distant untreated tumors in mouse melanoma models.5 The underlying rationale is that ECT-induced cell death releases tumor-associated antigens (TAA), which can be captured by local DCs and presented to tumor-specific cytotoxic T cells in draining lymph nodes5 (Fig. 1). It is worthwhile to reason on the possibility to modulate this scenario in cancer patients to enhance the potential of ECT to induce a systemic response. Combining ECT with DC activation protocols would be a valid method to reach this goal also in humans.
Figure 1. The immunological scenario in electrochemotherapy treated melanoma metastases. A schematic representation of electrochemotherapy (ECT) treatment is shown: the electrode inserted in the subcutaneous melanoma metastasis (MTX) after bleomycin administration induces tumor cell electroporation. This results in massive tumor cell death with the release of tumor-associated antigens (TAA) and mitigation of the immunosuppressive microenvironment. These TAAs are captured by different dendritic cell (DC) subsets—Langerhans cells (LCs), dermal DCs (dDCs), plasmacytoid DCs (pDCs)—that migrate to draining lymph nodes and present them to tumor-specific T cells. The combination of ECT with DC-targeting adjuvants and/or with the inhibition of immunosuppressive factors might lead to an effective systemic anti-melanoma immunity, accompanied by the regression of untreated distant metastases.
A recent study by our group provides a contribute to rationally associate these two approaches in a clinical setting.6 Our results indicate that the inflammatory infiltrate of ECT-treated melanoma metastases contains a high number of plasmacytoid (pDCs) and dermal DCs (dDCs). It also suggests that ECT induces a rapid migration of tumor-associated epidermal Langerhans cells (LCs) to draining lymph nodes. The role played by these DC subsets in tumor regression is still unknown but certainly these cells represent key targets to strengthen the efficacy of the therapy.
DC recruitment and TAA availability at the tumor site are two essential factors for generating systemic anticancer immunity (Fig. 1). This is essential but not sufficient, as the regression of untreated distant metastases does not occur in the clinical practice. Probably, a heavily immunosuppressive microenvironment at metastatic sites may drive DC to induce tolerance.1 However, it is possible that the massive tumor regression triggered by ECT may be accompanied by a significant decrease in the levels of immunosuppressive factors. This is a crucial point that requires further investigation. A tumor milieu more permissive for immune responses could be further conditioned by adding DC activators (or immune adjuvants). Thus, DCs together with TAAs and immune adjuvants are the ingredients required for in vivo DC-based cancer immunotherapy.
Among various adjuvants, Toll-like receptor (TLR) agonists administered locally deserve a special consideration. The presence of pDCs, which express both TLR7 and TLR9, in the infiltrate makes the corresponding agonists (imiquimod and CpG-ODNs, respectively), two promising candidates.1-3,5 Imiquimod and CpG-ODNs have been shown to exert a strong anti-melanoma immunity either in combination with ECT5 or in the contest of a DC-based immunotherapy.3 Importantly, although pDCs may play a tolerogenic role in cancer immunology,1,2 the treatment of melanoma with imiquimod leads to pDC recruitment and their transformation into cytotoxic pDCs, which are able to directly kill tumor cells.7 Another crucial DC-targeting adjuvant is the granulocyte-macrophage colony-stimulating factor (GM-CSF), a cytokine that further increases DC recruitment at tumor site, enhances the ability of DCs to capture TAAs and promotes DC maturation. Most importantly, GM-CSF, in synergy with TLR agonists, promotes the secretion of pro-inflammatory cytokines such as interleukin (IL)-12 that stimulate Th1-cell and cytotoxic T-cell proliferation (Fig. 1).8 To enhance immunity, an alternative or complementary strategy to the use of adjuvants could be the blockade of immune system regulatory checkpoints. Inhibition of the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) molecule results in strong and durable antitumor responses.9 Notably, a combinatorial approach including radiotherapy and immunotherapy with anti-CTLA-4 antibodies has been shown to exert a synergic effect leading to the generation of systemic antitumor responses and complete regression of distant non irradiated lesions.10 Similarly, the blockade of immune checkpoints could strengthen and extend the beneficial effects of ECT. In analogy, removal of other tumor promoting mechanisms such as those centered around IL-10, transforming growth factor β and regulatory T cells (Tregs) may be instrumental to promote anticancer immune responses.
Combination of ECT with DC-based immunotherapy might have further advantages. First, the efficacy of DC-based immunotherapy would be higher in ECT-treated patients, who have reduced tumor burden thanks to the potent clinical response, than in late stage cancer patients, who are the current candidates of this immunotherapeutic intervention. Second, the development of antitumor responses and the consequent clinical effects usually take time with immunotherapeutic approaches, while ECT has more rapidly visible effects. This latter aspect is of primary importance for the quality of life of cancer patients.
Currently, there is great interest in combinatorial approaches to cancer therapy. Here, we have proposed some hints for a novel combinatorial therapeutic strategy based on ECT and in vivo DC-based immunotherapy, two approaches that might have synergistic effects.
Glossary
Abbreviations:
- CpG-ODN
CpG oligodeoxynucleotide
- CTLA-4
cytotoxic T-lymphocyte-associated antigen 4
- DC
dendritic cell
- dDC
dermal DC
- ECT
electrochemotherapy
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- LC
Langerhans cell
- pDC
plasmacytoid dendritic cell
- TAA
tumor associated antigen
- TLR
Toll-like receptor
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21991
References
- 1.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 2.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–77. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoitzner P, Green LK, Jung JY, Price KM, Tripp CH, Malissen B, et al. Tumor immunotherapy by epicutaneous immunization requires langerhans cells. J Immunol. 2008;180:1991–8. doi: 10.4049/jimmunol.180.3.1991. [DOI] [PubMed] [Google Scholar]
- 4.Marty M, Sersa G, Garbay JR, Gehl J, Collins CG, Snoj M, et al. Electrochemotherapy – An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur J Cancer. 2006;4:3–13. [Google Scholar]
- 5.Roux S, Bernat C, Al-Sakere B, Ghiringhelli F, Opolon P, Carpentier AF, et al. Tumor destruction using electrochemotherapy followed by CpG oligodeoxynucleotide injection induces distant tumor responses. Cancer Immunol Immunother. 2008;57:1291–300. doi: 10.1007/s00262-008-0462-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerlini G, Sestini S, Di Gennaro P, Urso C, Pimpinelli N, Borgognoni L. Dendritic cells recruitment in melanoma metastasis treated by electrochemotherapy. Clin Exp Metastasis. 2012 doi: 10.1007/s10585-012-9505-1. In Press. [DOI] [PubMed] [Google Scholar]
- 7.Drobits B, Holcmann M, Amberg N, Swiecki M, Grundtner R, Hammer M, et al. Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. J Clin Invest. 2012;122:575–85. doi: 10.1172/JCI61034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min L, Mohammad Isa SA, Shuai W, Piang CB, Nih FW, Kotaka M, et al. Cutting edge: granulocyte-macrophage colony-stimulating factor is the major CD8+ T cell-derived licensing factor for dendritic cell activation. J Immunol. 2010;184:4625–9. doi: 10.4049/jimmunol.0903873. [DOI] [PubMed] [Google Scholar]
- 9.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]