Abstract
Trogocytosis, which results in the acquisition of myeloma cell-derived membrane proteins by T cells, and hence generates novel regulatory T cells, adds to the growing list of immune defects of multiple myeloma patients. The increasing complexity of the cancer-associated immune defects must be attentively considered for attempting to improve the so-far unsatisfactory rates of clinical responses to immunotherapy in patients affected by multiple myeloma and other malignancies.
Keywords: dendritic cell, immunosurveillance, immunotherapy, multiple myeloma, T cell, T cell clones, Th17, Treg, trogocytosis
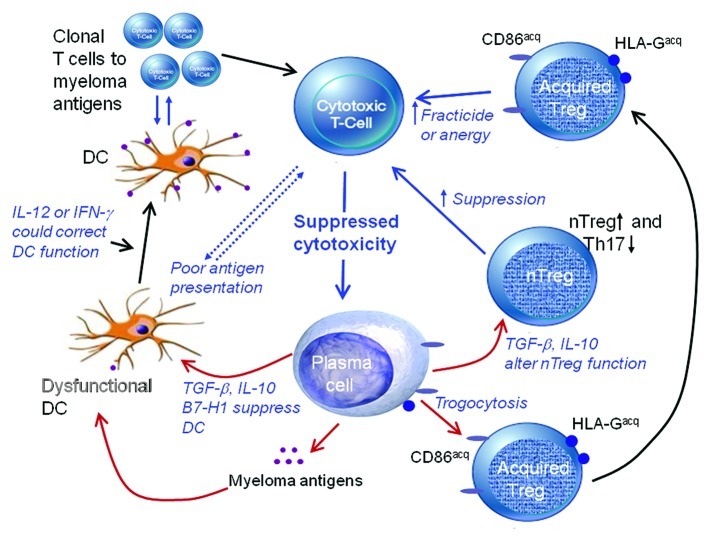
It is now clear that multiple myeloma (MM), a neoplasm of the most differentiated cells of the B lineage, is associated with a complex range of numerical, phenotypic and functional abnormalities within the dendritic cell (DC) and T-cell compartments. Malignant plasma cells use a broad range of subversive tactics to avoid recognition by the immune system, induce tolerance and prevent tumor rejection. Thus, MM cells can mimic “self” by downregulating MHC molecules (hence causing T cells to be “blind” to neo-antigens and differentiation antigens), can interfere with the upregulation of co-stimulatory molecules and antigen presentation, can produce soluble factors that impair DC function and hence directly paralyze the response of T cells to antigens. In addition, we have recently demonstrated that malignant plasma cells can generate acquired regulatory T cells (Tregs) by transferring membrane proteins to T cells by trogocytosis.1 Furthermore, the very same mechanisms designed to prevent harmful autoimmune responses can be hijacked by tumors, for instance upon tumor infiltration by natural Tregs (nTregs). Thus, tumor-specific cytotoxic T cells either never expand or, if they do so, are rendered ineffective.2 Figure 1 illustrates some of the mechanisms that promote tumor escape in MM and other malignancies.
Figure 1. Mechanisms associated with tumor-induced suppression of cytotoxic T cells in multiple myeloma include dysfunctional dendritic cells (DCs) due to plasma cell-derived transforming growth factor β (TGFβ) or interleukin-10 (IL-10), an imbalance between regulatory T cells (Tregs) and T helper 17 (Th17) cells, suppressing T-cell proliferation as well as fracticide or anergy induction as caused by novel Tregs generated by trogocytosis.
CD8+ T-cell expansion is common in the peripheral blood of patients with a variety of malignancies including chronic myeloid leukemia, myelodysplastic syndrome, melanoma, thymoma, MM and other monoclonal gammopathies, including Waldenstrom’s macroglobulinaemia (WM).3 In MM patients, such an expansion is associated with improved prognosis, are increased after IMiD therapy and involve T cells with a cytotoxic phenotype, i.e., CD8+CD45RA+CD57+CD28- cells that express perforin.3-6 Length analysis of complementarity determining region 3 (CDR3) fragments of the T-cell receptor (TCR) and nucleotide sequencing have confirmed that these cells are truly clonal.6 However, these clones fail to respond to proliferative stimuli, suggesting that they exist in a state of (near-to-) anergy.3 Genetic analyses have demonstrated that these cells upregulate RAS, CSK and TOB, while exhibiting an inactive ERK signaling pathway, which altogether contribute to the anergic state.3 Interestingly, we recently demonstrated that anergy is not present in clonal T-cell expansions from MM long-term (10 y) survivors, who were about 5% of the total MM cohort under investigation. Overcoming T-cell anergy should become an important component of any immunotherapeutic approach and the factors that control and suppress cytotoxic T cells in cancer patients need to be better understood.
Regulation by nTregs and Dysfunctional DCs
We have previously reported a failure of DCs isolated from MM patients to upregulate the B7 co-stimulatory molecules necessary for an effective immune response.7 This defect is primarily due to tumor cell-derived immunosuppressive factors including transforming growth factor β (TGFβ) and can be circumvented by the administration of recombinant interleukin-12 (rIL-12).8
There are conflicting reports of the number and function of nTregs and T helper 17 (Th17) cells in the blood of MM patients. This is due to both technical issues and the influence of recent therapeutic approaches.9 However, it is clear that the balance between nTreg and Th17 cells is abnormal in patients with MM.9 nTregs play an important role in limiting the host response to tumors. They appear to be increased in the course of many malignancies, tend to be more common as tumor-infiltrating cells than in the peripheral circulation and their rate of infiltration correlates with tumor progression.9
Novel Tregs Created by Trogocytosis
The term trogocytosis is used to describe the transfer of cell surface proteins and membrane patches from one cell to another upon physical cell-to-cell contact.10 We recently reported that T cells can acquire antigens from malignant cells by trogocytosis, resulting in T cells with novel antigen expression and altered function.1 T cells are more likely to be recipients of novel antigens and membrane patches than B or natural killer (NK) cells and trogocytosis is more common in MM than in other malignancies involving mature B cells. Trogocytosis is predominantly uni-directional and occurs independently of either the TCR or HLA status. While a wide range of different molecules are involved in trogocytosis, resulting in a novel T-cell surface immunophenotype, the acquisition of these neo-antigens will be random and usually serve no function. Thus, it is unlikely that the function of the acceptor cell will change upon trogocytosis, unless acquired antigens act as ligands for functional receptors on other cells or can be internalized and activate novel signaling pathways.
We have recently identified HLA-G and the B7 molecule CD86 as two antigens that, when acquired via trogocytosis, create novel acquired Tregs.1 Both HLA-G and CD86 are markers of poor prognosis when present on malignant plasma cells. Although both CD4+ and CD8+ T cells expressing HLA-G or CD86 are present in low numbers in the peripheral blood in physiological conditions, these cells are largely increased in patients affected by MM. Such HLA-G+ (and less so CD86+) cells act as potent inhibitors of T-cell proliferation - similar to nTregs.1,9,10
While novel chemotherapeutic regimens result in high remission rates among MM patients, a definitive cure for this neoplasm remains elusive. We suggest that a cure will only be achieved by restoring the normal immune state. In patients affected by MM and other cancers, the restoration of immune functions will involve overcoming the factors that induce immunosuppression. Our recent studies confirm that the acquisition of ectopic antigens by trogocytosis provides yet another mechanism for tumors to avoid immunosurveillance.
Glossary
Abbreviations:
- CDR3
complementarity determining region 3
- DC
dendritic cell
- MM
multiple myeloma
- NK
natural killer
- nTregs
natural regulatory T cells
- rIL-12
recombinant interleukin-12
- TCR
T-cell receptor
- TGFβ
transforming growth factor β
- Th
T helper
- WM
Waldenstrom’s macroglobuminaemia
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22032
References
- 1.Brown R, Kabani K, Favaloro J, Yang S, Ho PJ, Gibson J, et al. CD86+ or HLA-G+ can be transferred via trogocytosis from myeloma cells to T cells and are associated with poor prognosis. Blood. 2012;120:2055–63. doi: 10.1182/blood-2012-03-416792. [DOI] [PubMed] [Google Scholar]
- 2.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–74. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Sze DM, Brown RD, Cowley MJ, Kaplan W, Mo SL, et al. Clonal expansions of cytotoxic T cells exist in the blood of patients with Waldenstrom macroglobulinemia but exhibit anergic properties and are eliminated by nucleoside analogue therapy. Blood. 2010;115:3580–8. doi: 10.1182/blood-2009-10-246991. [DOI] [PubMed] [Google Scholar]
- 4.Raitakari M, Brown RD, Sze D, Yuen E, Barrow L, Nelson M, et al. T-cell expansions in patients with multiple myeloma have a phenotype of cytotoxic T cells. Br J Haematol. 2000;110:203–9. doi: 10.1046/j.1365-2141.2000.02131.x. [DOI] [PubMed] [Google Scholar]
- 5.Brown RD, Spencer A, Ho PJ, Kennedy N, Kabani K, Yang S, et al. Prognostically significant cytotoxic T cell clones are stimulated after thalidomide therapy in patients with multiple myeloma. Leuk Lymphoma. 2009;50:1860–4. doi: 10.3109/10428190903216804. [DOI] [PubMed] [Google Scholar]
- 6.Sze DM-Y, Giesajtis G, Brown RD, Raitakari M, Gibson J, Ho J, et al. Clonal cytotoxic T cells are expanded in myeloma and reside in the CD8(+)CD57(+)CD28(-) compartment. Blood. 2001;98:2817–27. doi: 10.1182/blood.V98.9.2817. [DOI] [PubMed] [Google Scholar]
- 7.Brown RD, Pope B, Murray A, Esdale W, Hart D, Gibson J, et al. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7-1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-beta1 and interleukin-10. Blood. 2001;98:2992–8. doi: 10.1182/blood.V98.10.2992. [DOI] [PubMed] [Google Scholar]
- 8.Brown R, Murray A, Pope B, Sze DM, Gibson J, Ho PJ, et al. Either interleukin-12 or interferon-gamma can correct the dendritic cell defect induced by transforming growth factor beta in patients with myeloma. Br J Haematol. 2004;125:743–8. doi: 10.1111/j.1365-2141.2004.04984.x. [DOI] [PubMed] [Google Scholar]
- 9.Joshua DE, Brown RD, Ho PJ, Gibson J. Regulatory T cells and multiple myeloma. Clin Lymphoma Myeloma. 2008;8:283–6. doi: 10.3816/CLM.2008.n.039. [DOI] [PubMed] [Google Scholar]
- 10.LeMaoult J, Caumartin J, Daouya M, Favier B, Le Rond S, Gonzalez A, et al. Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood. 2007;109:2040–8. doi: 10.1182/blood-2006-05-024547. [DOI] [PubMed] [Google Scholar]