Abstract
The actin nucleation factor Arp2/3 complex is a main regulator of actin assembly and is involved in multiple processes like cell migration and adhesion, endocytosis, and the establishment of cell polarity in mitosis. Our previous work showed that the Arp2/3 complex was involved in the actin-mediated mammalian oocyte asymmetric division. However, the regulatory mechanisms and signaling pathway of Arp2/3 complex in meiosis is still unclear. In the present work, we identified that the nucleation promoting factors (NPFs) JMY and WAVE2 were necessary for the expression and localization of Arp2/3 complex in mouse oocytes. RNAi of both caused the degradation of actin cap intensity, indicating the roles of NPFs in the formation of actin cap. Moreover, JMY and WAVE2 RNAi decreased the expression of ARP2, a key component of Arp2/3 complex. However, knock down of Arp2/3 complex by Arpc2 and Arpc3 siRNA microinjection did not affect the expression and localization of JMY and WAVE2. Our results indicate that the NPFs, JMY and WAVE2, are upstream regulators of Arp2/3 complex in mammalian oocyte asymmetric division.
Introduction
During mammalian oocyte meiotic maturation, a process called oocyte polarization occurs, which leads to a unique asymmetric division. The primary oocyte generates two daughter cells, a highly polarized big metaphase II (MII)-arrested oocyte and a small polar body, which is essential for the retention of maternal components for early embryo development [1]. The oocyte polarization experiences several steps including spindle migration, spindle anchoring and cortical reorganization [2]. This series of oocyte polarization processes are controlled by microtubule and microfilament cytoskeletons [2], [3]. After germinal vesicle breakdown (GVBD), the centrally-formed spindle migrates to the cortex of the oocyte in an actin-dependent manner. In addition, microfilaments are enriched to form an actin cap, microvilli are lost in the region overlaying the spindle, and cortical granules (CGs) are redistributed to form a CG-free domain (CGFD) [4]–[6]. All these processes are called cortical reorganization and polarization. After cortical reorganization, a small polar body is extruded, leaving a highly polarized oocyte. Different from common ligand-mediated cell polarity, the formation of oocyte polarity and cortical reorganization is independent of any external ligand and the signal is intrinsic to the oocyte [7]. Meanwhile, meiotic spindles in oocytes have no centrosome, which indicates that specialized mechanisms may be responsible for the off-centre positioning of the spindles. Until now, the molecular details of oocyte polarization have not been completely understood.
Actin is shown to play key roles during this process [5]. Actin nucleation is driven by actin binding proteins that are regulated by at least three distinct biochemical mechanisms [8]. These nucleation factors include formin family proteins [9], proteins harboring tandem actin monomer (G-actin) binding surfaces in variable numbers [10] and the Arp2/3 complex with its activators [11]–[13]. Arp2/3 complex, the first factor shown to drive actin filament nucleation, consists of Arp2, Arp3 and five other subunits; Arpc1 to Arpc5 [12], [14]. Since its discovery, the Arp2/3 complex has been shown to be involved in a wide range of cellular processes, from migration and endocytosis to trafficking and cell–cell communication [14]. In many species, inhibition of the activity of the complex by RNAi or inhibitory antibodies induces the disruption of cell migration and adhesion [15]–[17], endocytosis [18], [19], and the establishment of cell polarity during mitosis [14], [20]. Recent works by others and us have shown that Arp2/3 complex is essential for the maintenance of meiotic spindle position in mouse oocytes [21], [22].
Arp2/3 complex is activated by a class of proteins termed nucleation-promoting factors (NPFs), including WASP [23], N-WASP [24], [25], WAVE1 [26], [27], WAVE2 [28], [29], WAVE3, WASH [30], WHAMM [31], Abp1 [32], Pan1, Cortactin [33]–[35] and JMY [36]. WAVE2, a critical actin nucleation regulator, is widely expressed in mammals and is a member of the WASP family [37], [38]. WAVE2 consists of three independent domains at the C-terminus: the verprolin homology (V), central (C) and acidic (A) regions. These domains can bind and activate the Arp2/3 complex, which is directly involved in actin nucleation [39], [40]. JMY is originally identified as a cofactor of the transcriptional regulator p300/CBP which augments the p53 response [41]. JMY contains a series of WH2 domains that facilitate actin nucleation, and the isolated C-terminus of JMY stimulates Arp2/3-dependent actin assembly as potently as neural-Wiskott-Aldrich syndrome protein-WH2, connector, acidic (N-WASP-WWCA) [42]. Our recent work shows that WAVE2 and JMY are involved in oocyte polarization during meiotic maturation [42], [43]. Meiotic spindle stability, peripheral positioning and polar body emission are disturbed after knock down of WAVE2 in mouse oocytes [43]. And JMY RNAi disrupts the spindle migration, asymmetric division and cytokinesis during mouse oocyte maturation [44].
Although roles of NPFs and ARP2/3 in mouse oocyte have been separately uncovered, the signaling pathway leading to actin nucleation during meiosis is still unclear. Since WAVE2, JMY and ARP2/3 are involved in oocyte asymmetric division, their similar roles make us to investigate the relationship between NPFs and ARP2/3 during meiosis. In the present study, we injected the siRNA of these molecules into mouse oocytes and showed that knock down of NPFs caused the disruption of ARP2/3 complex localization in oocyte meiosis. Our results indicated that NPFs showed conserved roles and were the upstream regulators of ARP2/3 in oocyte meiosis.
Results
JMY RNAi disrupts the localization of ARP2/3 complex in mouse oocyte
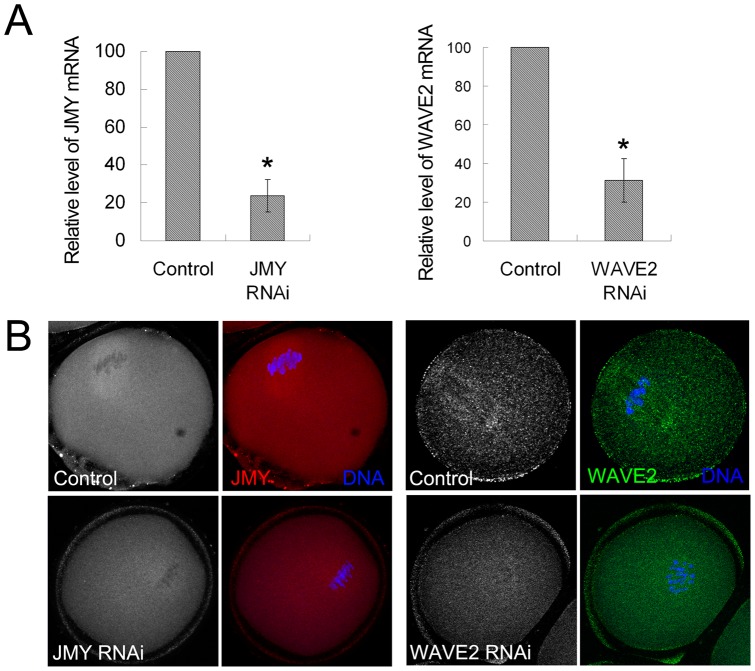
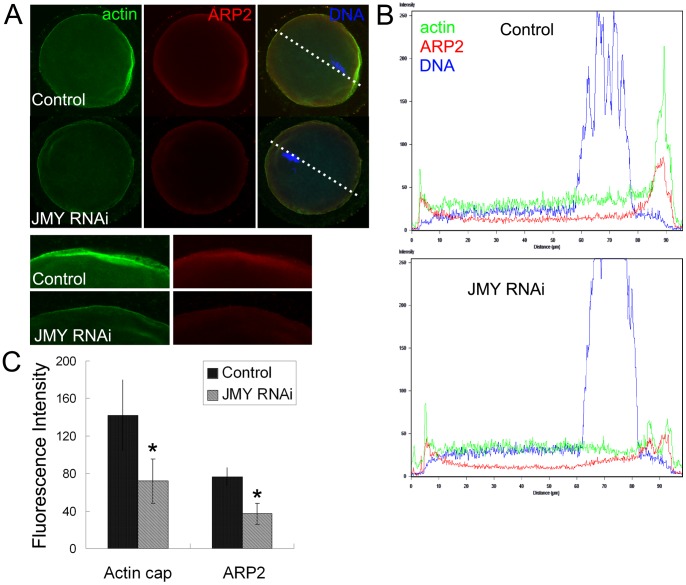
To investigate the effect of JMY on ARP2/3 in mouse oocytes, we used JMY siRNA injection to down-regulate the expression of JMY, and successfully depressed the mRNA level of JMY (23.7±8.6% versus 100%) (Fig. 1A). Immunofluorescent staining was adopted to examine the expression of JMY in oocytes. As shown in Fig. 1B, in the control group JMY accumulated at the spindle of the oocytes and in the cytoplasm. Compared to the control group, the protein expression of JMY in the RNAi group was significantly decreased. Then we examined the protein expression and localization of actin and ARP2 in mouse oocytes. As shown in Fig. 2A, an actin cap formed at the area where the chromosomes were closed to in the control group and ARP2 also accumulated at the actin cap area of the oocytes and the cytoplasm, whilst no actin cap formed and no ARP2 accumulated at this area in the JMY RNAi group. To confirm this, we examined the fluorescence intensity curve of oocytes. As shown in Fig. 2B, the intensity of actin and ARP2 at the dotted line area was significantly weakened in the JMY RNAi group compared to the control group. We also examined the average fluorescence intensity of the oocytes. As shown in Fig. 2C, the fluorescence intensity of actin in the control group (142.2±37.5) was significantly higher compared to that in the JMY RNAi group (71.9±23.5), while the fluorescence intensity of ARP2 in the control group (76.3±9.4) was also significantly higher compared to that of the JMY RNAi group (37.1±11.3), which further confirmed that the inhibition of JMY disrupted the localization of ARP2/3 complex in oocytes.
Figure 1. Efficiency of JMY and WAVE2 RNAi in mouse oocyte.
(A) JMY and WAVE2 mRNA level after JMY and WAVE2 siRNA injection. *, significantly different (p<0.05). (B) Immunofluorescence staining of JMY and WAVE2 in the oocytes after JMY and WAVE2 RNAi. In the control group, JMY localized at the spindle and WAVE2 accumulated around the spindle, whilst in the RNAi group, JMY and WAVE2 were barely expressed.
Figure 2. Expression and localization of actin and ARP2 after the injection of JMY siRNA.
(A) Localization of actin and ARP2 in oocytes by immunofluorescence staining after JMY RNAi. The expression of actin and ARP2 was significantly weaker in the JMY RNAi group. Green, actin; red, ARP2; blue, chromatin. (B) Fluorescence intensity curve of dotted line area in oocytes. The fluorescence intensity of actin and ARP2 was significantly weakened in the JMY RNAi group. (C) The average fluorescence intensity of actin and ARP2 in mouse oocytes. *, significantly different (p<0.05).
WAVE2 RNAi causes disruption of ARP2/3 complex localization in mouse oocyte
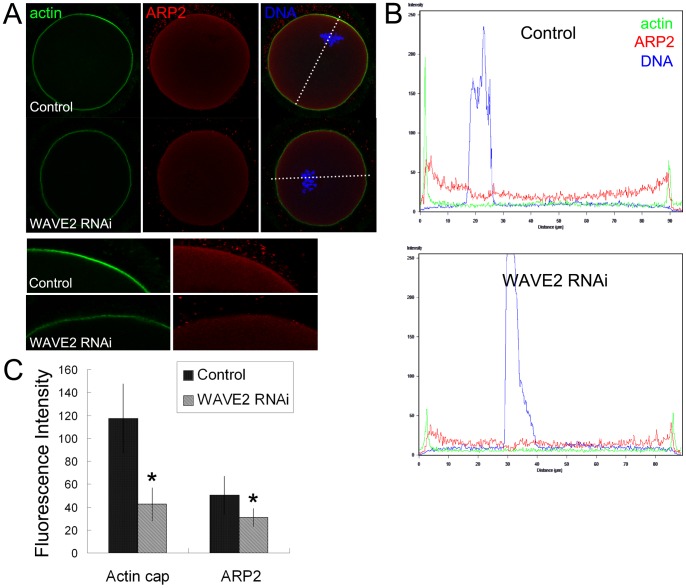
Analogously, to explore the effects of WAVE2 on ARP2/3 complex in oocytes, WAVE2 siRNA injection was used to down-regulate the expression of WAVE2, which successfully depressed the mRNA level of WAVE2 (31.3±11.2% versus 100%) (Fig. 1A). We also used immunofluorescent staining to examine the protein localization in the oocytes, and the results showed that WAVE2 accumulated around the spindle during spindle migration of late MI stage in the control group (Fig. 1B). However, WAVE2 was barely expressed in the WAVE2 RNAi group. Then we examined the protein expression and localization of actin and ARP2 in mouse oocytes. As shown in Fig. 3A, an actin cap formed and ARP2 accumulated at the cortex of the oocytes in the control group, but after WAVE2 siRNA injection, no actin cap was observed and no ARP2 accumulated. Furthermore, the fluorescence intensity curve of dotted line area also indicated that the fluorescence intensity of actin and ARP2 in the control group was significant higher than that in the WAVE2 RNAi group (Fig. 3B). Measurement of actin cap-fluorescence intensity revealed that actin expression was decreased in the WAVE2 RNAi group compared to the control group (42.5±14.4 vs. 117.4±30.2). Similarly, the expression of ARP2 was also decreased in the WAVE2 RNAi group (30.9±7.8 vs. 50.2±16.7) (Fig. 3C). These results indicated that WAVE2 is essential for the localization of ARP2/3 complex in oocytes.
Figure 3. Expression and localization of actin and ARP2 after the injection of WAVE2 siRNA.
(A) Localization of actin and ARP2 in oocytes by immunofluorescence staining after WAVE2 RNAi. The expression of actin and ARP2 was significantly weaker in the WAVE2 RNAi group. Green, actin; red, ARP2; blue, chromatin. (B) Fluorescence intensity curve of dotted line area. The fluorescence intensity of actin and ARP2 was weakened in the WAVE2 RNAi group. (C) The average fluorescence intensity of actin and ARP2 in oocytes. *, significantly different (p<0.05).
Arp2/3 complex is not required for the localization of JMY and WAVE2 in mouse oocyte
To further confirm the relationship between NPFs and ARP2/3 complex, we knocked down ARP2/3 complex by Arpc2 and Arpc3 siRNA injection, and the mRNA levels were significantly decreased [22]. After the expression of ARP2/3 complex was decreased, we examined the expression and localization of JMY and WAVE2 in oocytes by immunofluorescent staining. As shown in Fig. 4A, JMY accumulated at the spindle in the control group, whilst there was no change for the localization of JMY in the Arpc2 and Arpc3 RNAi group, indicating that the inhibition of ARP2/3 complex had no effect on the localization of JMY in oocytes. Similarly, in both control and Arpc2 and Arpc3 RNAi group, WAVE2 expressed normally and accumulated around the spindle in the oocytes, which also revealed that ARP2/3 complex was not a regulator of WAVE2 (Fig. 4B).
Figure 4. Expression and localization of JMY and WAVE2 after Arpc2 and Arpc3 RNAi.
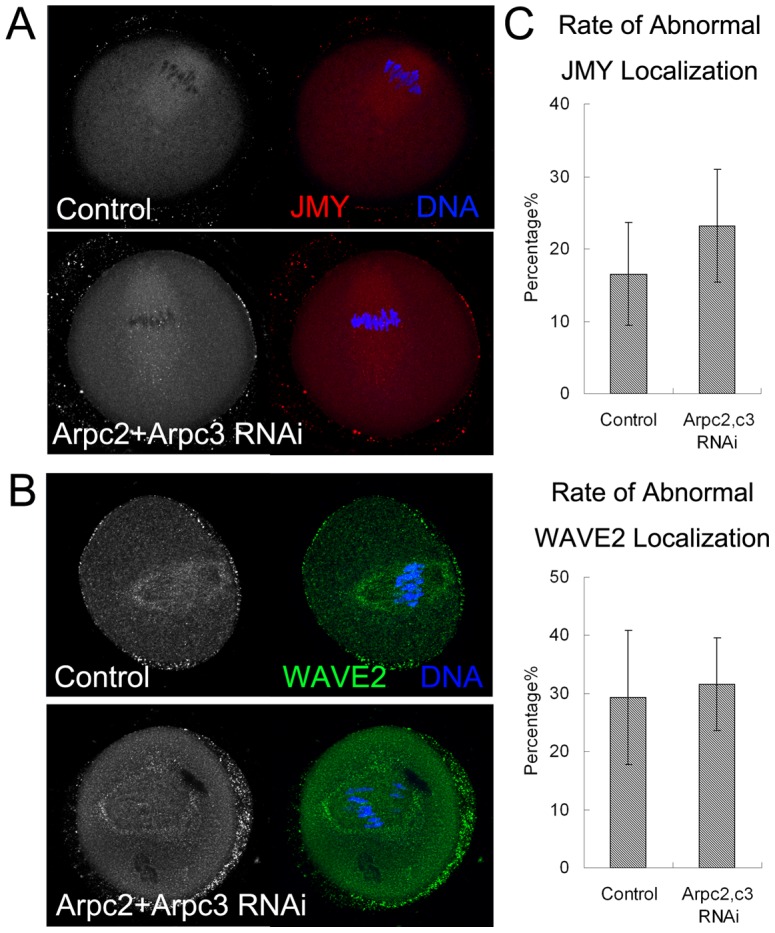
(A) Localization of JMY in oocytes by immunofluorescence staining after Arpc2 and Arpc3 RNAi. JMY expressed normally in both control and Arpc2 and Arpc3 RNAi group. (B) Localization of WAVE2 in oocytes by immunofluorescence staining after Arpc2 and Arpc3 RNAi. WAVE2 expressed normally in both control and Arpc2 and Arpc3 RNAi group. (C) Abnormal rate of JMY and WAVE2 localization in mouse oocytes after Arpc2 and Arpc3 RNAi.
To confirm this, the rate of abnormal JMY and WAVE2 localization was analyzed. The rate of abnormal JMY localization was no different for the Arpc2 and Arpc3 RNAi group compared with the control group (23.2%±7.8% vs. 16.5%±7.1%, n = 26, P>0.1) (Fig. 4C). Likewise, the rate of abnormal WAVE2 localization was also not different between the Arpc2 and Arpc3 RNAi group and the control group (31.6%±8% vs. 29.3%±11.6%, n = 32, P>0.1) (Fig. 4C). These results further demonstrated that ARP2/3 complex was not the upstream regulator of JMY and WAVE2.
Discussion
In the present study, we investigated the relationship between NPFs and ARP2/3 complex during mouse oocyte meiotic maturation. By the knock down of JMY, WAVE2 and Arp2/3 complex, our results revealed that the NPFs JMY and WAVE2 were the upstream regulators of Arp2/3 complex in asymmetric mouse oocyte division.
JMY is a newly identified nucleation promoting factor. In our previous study, JMY has been demonstrated to be involved in spindle migration, asymmetric cell division, and cytokinesis in mouse oocytes [44], but the mechanisms how JMY affects these series of processes are unknown. Previous work showed that Arp2/3 complex might be the target of JMY for the actin nucleation. The isolated C-terminus of JMY (WWLWCA; L refers to a short linker sequence discussed below) stimulated Arp2/3-dependent actin assembly as potently as N-WASP-WWCA [8]. In vitro, JMY-WWWCA promotes actin-based motility by an Arp2/3 complex–dependent mechanism, and full-length JMY nucleates actin filaments and activates the Arp2/3 complex [45]. In the current study, we attempted to find whether JMY was an upstream regulator of ARP2/3 complex in the unique mammalian meiotic oocyte model. Since ARP2/3 complex was proved to be involved into asymmetric division and cytokinesis in mouse oocytes [22]. The results showed that Arp2/3 complex failed to accumulate to the cortex area of oocyte after JMY RNAi, indicating that the regulatory roles of JMY on Arp2/3 complex, and that the proper localization of ARP2/3 complex in mouse oocyte requires the expression of JMY. Therefore, a JMY-Arp2/3 complex-actin signaling pathway was set up for the regulation of mammalian oocyte asymmetric division.
WAVE2, a WASP family member, is a traditional NPF binds to Arp2/3 complex and active it in mitosis [12], [14]. It also stimulates localized actin polymerization through its interaction with the Arp2/3 complex [46], [47]. It was shown in previous works that C. trachomatis activated Rac and promoted its interaction with WAVE2 to activate the Arp2/3 complex, resulting in the induction of actin cytoskeletal rearrangements required for chlamydial invasion [48]. Recently, the roles of WAVE2 in mouse oocyte was also revealed by us, and it was shown that WAVE2 might regulate p42/44 MAPK for the meiotic spindle formation [43]. However, it is still unclear how WAVE2 works for the meiotic spindle migration and asymmetric division in mouse oocyte. Our results showed that Arp2/3 complex might be the downstream molecule of WAVE2, since the expression of ARP2 decreased after WAVE2 RNAi. Therefore, in mammalian oocytes WAVE2 may regulate Arp2/3 complex to active actin assembly for the meiotic spindle migration and the asymmetry. Our data suggest that WAVE2 is an upstream regulator of ARP2/3 complex, and a WAVE2-Arp2/3-actin signaling pathway was set up for the regulation of mammalian oocyte asymmetric division.
To examine how Arp2/3 complex interacts with NPFs, we also examined the expression of JMY and WAVE2 after the depletion of Arp2/3 complex, and the results showed that there was no effect on the localization of these NPFs. Therefore, the regulatory mechanism for NPFs on Arp2/3 complex was not mutual in mouse oocytes; instead, NPFs functioned as the upstream molecules of Arp2/3 complex. Our results provide direct evidence of the regulation of NPFs on ARP2/3 complex during mouse oocytes maturation.
In conclusion, our results suggest that the inhibition of JMY and WAVE2 causes disruption of ARP2/3 complex localization in meiosis, indicating that NPFs JMY and WAVE2 are the upstream regulators of ARP2/3 complex in mouse oocytes.
Materials and Methods
Ethic statement
Animal care and use were conducted in accordance with the Animal Research Institute Committee guidelines of Nanjing Agricultural University, China. Mice were housed in a temperature-controlled room with proper darkness-light cycles, fed with a regular diet, and maintained under the care of the Laboratory Animal Unit, Nanjing Agricultural University, China. The mice were killed by cervical dislocation. This study was specifically approved by the Committee of Animal Research Institute, Nanjing Agricultural University, China.
Antibodies and chemicals
Goat polyclonal anti-JMY antibody and rabbit polyclonal anti-WAVE2 antibody were purchased from Santa Cruz (Santa Cruz, CA), whilst mouse monoclonal anti-ARP2 antibody was obtained from Abcam (Cambridge, UK). Phalloidin-TRITC, Lectin-FITC and mouse monoclonal anti-α-tubulin-FITC antibody was purchased from Sigma (St Louis, MO). Alexa Fluor 488, 568 antibodies were obtained from Invitrogen (Carlsbad, CA).
Oocyte collection and culture
Germinal vesicle-intact oocytes were collected from ovaries of 6- to 8-week-old ICR mice and cultured in M16 medium (Sigma, MO) under paraffin oil at 37°C 5%CO2. After a range of times in culture, oocytes were collected for immunostaining, real time RT-PCR and microinjection.
Microinjection of siRNA
Approximately 5–10 pl JMY siRNA (Sigma) was microinjected into the cytoplasm of a fully grown GV oocyte using a Eppendorf FemtoJet (Eppendorf AG, Hamburg, Germany) with a Nikon Diaphot ECLIPSE TE300 inverted microscope (Nikon UK Ltd., Kingston upon Thames, Surrey, UK) equipped with a Narishige MM0-202N hydraulic three-dimensional micromanipulator (Narishige Inc., Sea Cliff, NY). The oocytes were then cultured in M16 medium contained 5 μM milrinone for 24 h after injection. After culture, the oocytes were washed five times in fresh M16 medium, for 2 min each time, and then transferred to fresh M16 medium and cultured under paraffin oil at 37°C in an atmosphere of 5% CO2 in air. For the control oocytes, 5–10 pl of negative control siRNA were microinjected. The spindle, actin cap phenotypes and chromosome localization were examined by confocal microscopy (Zeiss LSM 710 META, Germany). For WAVE2, Arpc2 and Arpc3 siRNA injection, the same method was adopted as above.
Real-time quantitative PCR analysis
Analysis of JMY and WAVE2 gene expression was measured by real-time quantitative PCR and the ΔΔCT method. We used a Dynabead mRNA DIRECT kit (Invitrogen Dynal AS, Norway) to extract the total RNA from 50 oocytes. The first strand cDNA was generated with a cDNA synthesis kit (Takara), using Oligo(dT)12–18 primers (Invitrogen). JMY cDNA fragment was amplified using the following primers: F, TTC AAA TTA CAA GCC GTG CAC CCG; R, AGC TGC CTT CTG GAC CTT TAC TGA, while WAVE2 cDNA fragments were amplified using the following primers: F, AAC TCC ATG CTG TGC ATG TTT CCC; R, TCT ATT TGG AAG GAC CAC TGC CCT.
The DyNAmo HS SYBR Green qPCR kit (FINNZYMES) was used with a DNA Engine OPTICON 2 Continuous Fluorescence Detector (MJ Research) under the following conditions: 95°C for 10 sec, and 38 cycles of 95°C for 5 sec and 59°C for 32 sec.
Confocal microscopy
The protocol was basically the same as described in our previous work [44]. For immunostaining, oocytes were fixed in 4% paraformaldehyde in PBS for 30 min at room temperature and then transferred to membrane permeabilization solution (0.5% Triton X-100) for 20 min. After 1 h in blocking buffer (1% BSA-supplemented PBS), oocytes were incubated overnight at 4°C or for 4 h at room temperature with 1∶100 goat anti-JMY (1∶50 rabbit anti-WAVE2, 1∶200 mouse anti-ARP2) antibody. After three washes in washing buffer (0.1% Tween 20 and 0.01% Triton X-100 in PBS), the oocytes were labeled with 1∶100 Alexa Fluor rabbit-anti-goat 488 IgG or donkey anti-goat 568 IgG for 1 h at room temperature. The samples were co-stained with Hoechst 33342 (10 μg/ml in PBS) for 10 min and then mounted on glass slides and examined with a confocal laser-scanning microscope (Zeiss LSM 710 META, Germany). At least 20 oocytes were selected for examination for each group.
Statistical analysis
Three replicates were performed for each experiment. Statistical analyses were conducted using an analysis of variance (ANOVA) and differences between treatment groups were evaluated with Duncan's multiple comparison tests. Data were expressed as mean ± SEM and p<0.05 was considered significant. Fluorescence intensity statistics were examined by Image J (NIH) software and 10 oocytes were analyzed for each experiment.
Funding Statement
This work was supported by the Fundamental Research Funds for the Central Universities (KYRC201202) and a research grant (804051) from Nanjing Agricultural University in 2012, China; and the Biogreen 21 Program (PJ0090802012, PJ009098), RDA, Republic of Korea. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Maro B, Verlhac MH (2002) Polar body formation: new rules for asymmetric divisions. Nat Cell Biol 4: E281–283. [DOI] [PubMed] [Google Scholar]
- 2. Brunet S, Verlhac MH (2011) Positioning to get out of meiosis: the asymmetry of division. Hum Reprod Update 17: 68–75. [DOI] [PubMed] [Google Scholar]
- 3. Sun QY, Schatten H (2006) Regulation of dynamic events by microfilaments during oocyte maturation and fertilization. Reproduction 131: 193–205. [DOI] [PubMed] [Google Scholar]
- 4. Van Blerkom J, Bell H (1986) Regulation of development in the fully grown mouse oocyte: chromosome-mediated temporal and spatial differentiation of the cytoplasm and plasma membrane. J Embryol Exp Morphol 93: 213–238. [PubMed] [Google Scholar]
- 5. Longo FJ, Chen DY (1985) Development of cortical polarity in mouse eggs: involvement of the meiotic apparatus. Dev Biol 107: 382–394. [DOI] [PubMed] [Google Scholar]
- 6. Deng M, Kishikawa H, Yanagimachi R, Kopf GS, Schultz RM, et al. (2003) Chromatin-mediated cortical granule redistribution is responsible for the formation of the cortical granule-free domain in mouse eggs. Dev Biol 257: 166–176. [DOI] [PubMed] [Google Scholar]
- 7. Kutsuna H, Suzuki K, Kamata N, Kato T, Hato F, et al. (2004) Actin reorganization and morphological changes in human neutrophils stimulated by TNF, GM-CSF, and G-CSF: the role of MAP kinases. Am J Physiol Cell Physiol 286: C55–64. [DOI] [PubMed] [Google Scholar]
- 8. Rottner K, Hanisch J, Campellone KG (2010) WASH, WHAMM and JMY: regulation of Arp2/3 complex and beyond. Trends Cell Biol 20: 650–661. [DOI] [PubMed] [Google Scholar]
- 9. Chesarone MA, DuPage AG, Goode BL (2010) Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat Rev Mol Cell Biol 11: 62–74. [DOI] [PubMed] [Google Scholar]
- 10. Renault L, Bugyi B, Carlier MF (2008) Spire and Cordon-bleu: multifunctional regulators of actin dynamics. Trends Cell Biol 18: 494–504. [DOI] [PubMed] [Google Scholar]
- 11. Chesarone MA, Goode BL (2009) Actin nucleation and elongation factors: mechanisms and interplay. Curr Opin Cell Biol 21: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Campellone KG, Welch MD (2010) A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol 11: 237–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Veltman DM, Insall RH (2010) WASP family proteins: their evolution and its physiological implications. Mol Biol Cell 21: 2880–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goley ED, Welch MD (2006) The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol 7: 713–726. [DOI] [PubMed] [Google Scholar]
- 15. Bailly M, Ichetovkin I, Grant W, Zebda N, Machesky LM, et al. (2001) The F-actin side binding activity of the Arp2/3 complex is essential for actin nucleation and lamellipod extension. Curr Biol 11: 620–625. [DOI] [PubMed] [Google Scholar]
- 16. Rogers SL, Wiedemann U, Stuurman N, Vale RD (2003) Molecular requirements for actin-based lamella formation in Drosophila S2 cells. J Cell Biol 162: 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steffen A, Faix J, Resch GP, Linkner J, Wehland J, et al. (2006) Filopodia formation in the absence of functional WAVE- and Arp2/3-complexes. Mol Biol Cell 17: 2581–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moreau V, Galan JM, Devilliers G, HaguenauerTsapis R, Winsor B (1997) The yeast actin-related protein Arp2p is required for the internalization step of endocytosis. Mol Biol Cell 8: 1361–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schaerer-Brodbeck C, Riezman H (2000) Functional Interactions between the p35 Subunit of the Arp2/3 Complex and Calmodulin in Yeast. Mol Biol Cell 11: 1113–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rouiller I, Xu XP, Amann KJ, Egile C, Nickell S, et al. (2008) The structural basis of actin filament branching by the Arp2/3 complex. J Cell Biol 180: 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yi K, Unruh JR, Deng M, Slaughter BD, Rubinstein B, et al. (2011) Dynamic maintenance of asymmetric meiotic spindle position through Arp2/3-complex-driven cytoplasmic streaming in mouse oocytes. Nat Cell Biol 13: 1252–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun SC, Wang ZB, Xu YN, Lee SE, Cui XS, et al. (2011) Arp2/3 complex regulates asymmetric division and cytokinesis in mouse oocytes. PLoS One 6: e18392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bosticardo M, Marangoni F, Aiuti A, Villa A, Grazia Roncarolo M (2009) Recent advances in understanding the pathophysiology of Wiskott-Aldrich syndrome. Blood 113: 6288–6295. [DOI] [PubMed] [Google Scholar]
- 24. Lommel S, Benesch S, Rottner K, Franz T, Wehland J, et al. (2001) Actin pedestal formation by enteropathogenic Escherichia coli and intracellular motility of Shigella flexneri are abolished in N-WASP-defective cells. EMBO Rep 2: 850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Snapper SB, Takeshima F, Anton I, Liu CH, Thomas SM, et al. (2001) N-WASP deficiency reveals distinct pathways for cell surface projections and microbial actin-based motility. Nat Cell Biol 3: 897–904. [DOI] [PubMed] [Google Scholar]
- 26. Dahl JP, Wang-Dunlop J, Gonzalez C, Goad MEP, Mark RJ, et al. (2003) Characterization of the WAVE1 knock-out mouse: Implications for CNS development. Journal of Neuroscience 23: 3343–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soderling SH, Langeberg LK, Soderling JA, Davee SM, Simerly R, et al. (2003) Loss of WAVE-1 causes sensorimotor retardation and reduced learning and memory in mice. Proceedings of the National Academy of Sciences of the United States of America 100: 1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamazaki D, Suetsugu S, Miki H, Kataoka Y, Nishikawa SI, et al. (2003) WAVE2 is required for directed cell migration and cardiovascular development. Nature 424: 452–456. [DOI] [PubMed] [Google Scholar]
- 29. Yan C, Martinez-Quiles N, Eden S, Shibata T, Takeshima F, et al. (2003) WAVE2 deficiency reveals distinct roles in embryogenesis and Rac-mediated actin-based motility. Embo Journal 22: 3602–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Linardopoulou EV, Parghi SS, Friedman C, Osborn GE, Parkhurst SM, et al. (2007) Human subtelomeric WASH genes encode a new subclass of the WASP family. Plos Genetics 3: 2477–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Campellone KG, Webb NJ, Znameroski EA, Welch MD (2008) WHAMM is an Arp2/3 complex activator that binds microtubules and functions in ER to Golgi transport. Cell 134: 148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goode BL, Rodal AA, Barnes G, Drubin DG (2001) Activation of the Arp2/3 complex by the actin filament binding protein Abp1p. J Cell Biol 153: 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Uruno T, Liu JL, Zhang PJ, Fan YX, Egile C, et al. (2001) Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat Cell Biol 3: 259–266. [DOI] [PubMed] [Google Scholar]
- 34. Weaver AM, Karginov AV, Kinley AW, Weed SA, Li Y, et al. (2001) Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr Biol 11: 370–374. [DOI] [PubMed] [Google Scholar]
- 35. Weed SA, Karginov AV, Schafer DA, Weaver AM, Kinley AW, et al. (2000) Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. Journal of Cell Biology 151: 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zuchero JB, Coutts AS, Quinlan ME, La Thangue NB, Mullins RD (2009) p53-cofactor JMY is a multifunctional actin nucleation factor. Nat Cell Biol 11: 451–U198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suetsugu S, Miki H, Yamaguchi H, Obinata T, Takenawa T (2001) Enhancement of branching efficiency by the actin filament-binding activity of N-WASP/WAVE2. J Cell Sci 114: 4533–4542. [DOI] [PubMed] [Google Scholar]
- 38. Suetsugu S, Miki H, Yamaguchi H, Takenawa T (2001) Requirement of the basic region of N-WASP/WAVE2 for actin-based motility. Biochemical and Biophysical Research Communications 282: 739–744. [DOI] [PubMed] [Google Scholar]
- 39. Bompard G, Caron E (2004) Regulation of WASP/WAVE proteins: making a long story short. J Cell Biol 166: 957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takenawa T, Suetsugu S (2007) The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol 8: 37–48. [DOI] [PubMed] [Google Scholar]
- 41. Shikama N, Lee CW, France S, Delavaine L, Lyon J, et al. (1999) A novel cofactor for p300 that regulates the p53 response. Molecular Cell 4: 365–376. [DOI] [PubMed] [Google Scholar]
- 42. Zuchero JB, Coutts AS, Quinlan ME, La Thangue NB, Mullins RD (2009) p53-cofactor JMY is a multifunctional actin nucleation factor. Nature Cell Biology 11: 451–U198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sun SC, Xu YN, Li YH, Lee SE, Jin YX, et al. (2011) WAVE2 regulates meiotic spindle stability, peripheral positioning and polar body emission in mouse oocytes. Cell Cycle 10: 1853–1860. [DOI] [PubMed] [Google Scholar]
- 44. Sun SC, Sun QY, Kim NH (2011) JMY is required for asymmetric division and cytokinesis in mouse oocytes. Mol Hum Reprod 17: 296–304. [DOI] [PubMed] [Google Scholar]
- 45. Firat-Karalar EN, Hsiue PP, Welch MD (2011) The actin nucleation factor JMY is a negative regulator of neuritogenesis. Mol Biol Cell 22: 4563–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Machesky LM, Gould KL (1999) The Arp2/3 complex: a multifunctional actin organizer. Curr Opin Cell Biol 11: 117–121. [DOI] [PubMed] [Google Scholar]
- 47. Machesky LM, Insall RH (1999) Signaling to actin dynamics. J Cell Biol 146: 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carabeo RA, Dooley CA, Grieshaber SS, Hackstadt T (2007) Rac interacts with Abi-1 and WAVE2 to promote an Arp2/3-dependent actin recruitment during chlamydial invasion. Cell Microbiol 9: 2278–2288. [DOI] [PubMed] [Google Scholar]