Abstract
Objective
Hippocampal volume decrease associated with illness burden is among the most replicated findings in unipolar depression. The absence of hippocampal volume changes in most studies of individuals with bipolar disorder (BD) may reflect neuroprotective effects of lithium (Li).
Methods
We recruited 17 BD patients from specialized Li clinics, with at least two years of regularly monitored Li treatment (Li group), and compared them to 12 BD participants with < 3 months of lifetime Li exposure and no Li treatment within two years prior to the scanning (non-Li group) and 11 healthy controls. All BD patients had at least 10 years of illness and five episodes. We also recruited 13 Li-naïve, young BD participants (15–30 years of age) and 18 sex- and age-matched healthy controls. We compared hippocampal volumes obtained from 1.5-T magnetic resonance imaging (MRI) scans using optimized voxel-based morphometry with small volume correction.
Results
The non-Li group had smaller left hippocampal volumes than controls (corrected p < 0.05), with a trend for lower volumes than the Li group (corrected p < 0.1), which did not differ from controls. Young, Li-naïve BD patients close to the typical age of onset had comparable hippocampal volumes to controls.
Conclusions
Whereas patients with limited lifetime Li exposure had significantly lower hippocampal volumes than controls, patients with comparable illness burden, but with over two years of Li treatment, or young Li-naïve BD patients without Li exposure, showed hippocampal volumes comparable to controls. These results provide indirect support for neuroprotective effects of Li and negative effects of illness burden on hippocampal volumes in bipolar disorders.
Keywords: bipolar disorders, hippocampus, illness burden, lithium, neuroprotection
Hippocampal volume decrease secondary to illness burden is among the most replicated neuroimaging findings in unipolar depression, where it is thought to reflect hypothalamo-pituitary-adrenal axis (HPA) dysregulation during recurrences of illness (1–3). The phenotype as well as the HPA abnormalities overlap between unipolar and bipolar depression (4). In addition, patients with bipolar disorder (BD) typically spend more time depressed than manic (5), and bipolar depression is more recurrent (6) and may start earlier (7) than unipolar depression. It is thus surprising that the majority of studies showed comparable (8–13) or even larger hippocampal volumes (14–16) in BD patients relative to healthy controls. Hippocampal volume decreases have, however, been reported in groups of BD individuals with limited exposure to lithium (Li) (17, 18). Since Li treatment has been found to lead to increased hippocampal volume in prospective studies (19) and Li-exposed patients in retrospective studies have typically shown larger hippocampal volumes than unmedicated participants (17, 20, 21), it is possible that hippocampal volume changes in BD are masked by exposure to putative neuroprotective effects of Li (22, 23).
The intercorrelated nature of illness burden and medication exposure makes it difficult to study hippocampal volume changes in BD. Hippocampal volume decrease is associated with illness burden (24, 25) and unlikely to be present in the early stages of illness, even in the absence of Li treatment (12). Investigating the effects of Li on hippocampal volumes thus requires maximizing the burden of illness. On the other hand, since even a short exposure to Li may be associated with increases in hippocampal volumes (21), testing the negative effects of illness burden on hippocampal volumes requires controlling for exposure to Li. To complicate things further, medication-naïve patients are typically at the early stages of their illness, whereas patients with sufficient burden of illness are unlikely to remain well for long periods of time without medication. Available studies thus recruited participants with a wide range of illness burden and durations of Li treatment or with only short Li-free periods (weeks to months) (17, 20, 21, 26). An alternative would be to maximize the differences between the groups in illness burden and duration of exposure to Li by using strict inclusion and exclusion criteria. Such an approach has not yet been used.
We therefore designed this study to test the effects of illness burden and Li on hippocampal volumes by recruiting cohorts of patients selected for either low or high burden of illness as well as for either limited lifetime or chronic ongoing treatment with Li. Our a priori hypotheses were that hippocampal volumes would be smaller only among patients with substantial burden of illness and limited exposure to Li, whereas patients with substantial burden of illness and ongoing Li treatment or young BD patients with lower illness burden and no Li exposure would have comparable hippocampal volumes to controls.
Patients and methods
In order to vary the illness burden and exposure to Li, we recruited two separate cohorts of patients and matched controls. In the Halifax Lithium Study, we attempted to maximize the illness burden and vary the exposure to Li. In the Prague Study of Young BD Patients, we attempted to minimize the burden of illness and eliminate lifetime or current exposure to Li.
Halifax Lithium Study
Participants were recruited from patients followed up at a specialized Mood Disorders Program at Dalhousie University (Halifax, NS, Canada). The program is a tertiary care clinic providing consultation services to family physicians and community psychiatrists and following up patients with BD. All patients were diagnosed by psychiatrists using the Structured Clinical Interview for DSM-IV (SCID) and had regular follow ups at the clinic, including monitoring of Li levels at least twice per year. Recruitment of Li-treated BD patients from a specialized clinic ensured Li levels in the therapeutic range. This prevented subtherapeutic levels, which could be insufficient to elicit neuroprotective changes, or levels above the therapeutic range, which could lead to neurotoxicity. We also recruited control participants among hospital employees, matched to the BD patients by age and sex. Each control participant underwent a SCID interview and was included if found to have no lifetime history of Axis I psychiatric disorders.
Inclusion criteria
The BD patients (both Li and non-Li groups) had to have: (i) a diagnosis of bipolar I or II disorder made by a psychiatrist using the SCID; (ii) at least 10 years of illness; (iii) a history of at least five episodes of illness (including manic, depressive, or mixed episodes); (iv) current Hamilton Depression Rating Scale, 17-item version (HAM-D-17) score < 7; (v) current Young Mania Rating Scale (YMRS) score < 5; (vi) current Clinical Global Impressions Scale–Bipolar (CGI-BP) score < 3; and (vii) a period of euthymia for at least four months prior to scanning, as aside from state-related factors, patients in acute episodes may present with additional difficult to control confounding variables, including recent medication change or substance abuse.
The non-Li group had to have less than three months of lifetime Li exposure, more than 24 months prior to the scanning. The Li group had to have a current Li treatment lasting a minimum of 24 months.
Exclusion criteria
Individuals from any of the three groups were excluded if they met any of the magnetic resonance imaging (MRI) exclusion criteria or had any serious medical illness (e.g., brain injury, Cushing’s disease, or conditions treated with corticosteroids).
Individuals with BD were excluded if they had: (i) more than one lifetime course of electroconvulsive therapy (ECT) or ECT in the previous 12 months; (ii) comorbid psychiatric disorders, and/or personality disorder; (iii) active substance abuse in the previous 12 months; (iv) significant change in their medication in the previous three months; or (v) current psychotic features or acute suicidality. Individuals from the non-Li group were excluded if they had: (i) Li exposure < 2 years before the scanning; or (ii) lifetime Li exposure of more than three months.
The neuropsychiatrically healthy individuals were excluded if they had a personal history of psychiatric disorders.
After providing a complete description of the study to the participants, written informed consent was obtained. The study was approved by the Research Ethics Boards of IWK Health Center and Capital District Health Authority (Halifax, NS, Canada).
Prague Study of Young BD Patients
Thirteen BD patients close to the typical age of onset, with no lifetime history of Li treatment, were identified through hospital database and outpatient clinics at the Prague Psychiatric Centre according to methods described previously (27). Each identified participant underwent a Schedule for Affective Disorders and Schizophrenia–Lifetime version (SADS-L) interview conducted by an experienced research psychiatrist (TN or MK). Control participants matched by age and sex were recruited via advertisements from similar sociodemographic populations to the patients. Each participant underwent a SADS-L interview conducted by an experienced research psychiatrist (TN or MK) and was included if found to have no lifetime history of Axis I psychiatric disorders.
Inclusion/exclusion criteria
Participants in both groups were required to be between 15 and 30 years of age. This age range was selected in order to limit the burden of illness. Our prospective (28) as well as retrospective studies (29) have shown the most typical age of onset to be in this range. The BD patients had to have a diagnosis of bipolar I or II disorder made by a psychiatrist using the SADS-L. Participants were excluded if they had a current or lifetime history of Li treatment, met MRI exclusion criteria (pacemaker or metal implants), had any serious medical illness (e.g., Cushing’s disease, or conditions treated with corticosteroids) or neurological disorder (e.g., epilepsy, brain injury, or demyelinating disorders), or if they met criteria for substance abuse or dependence during the previous six months. Additional exclusion criteria for the control group included a personal or family history of psychiatric disorders. Participants were allowed to continue the use of psychotropic medications at the time of scanning. All participants were deemed to be euthymic during MRI assessment by the psychiatrist according to current symptoms description from the SADS-L interview, which was conducted within one week of the MRI scan date.
After a complete description of the study to the participants, written informed consent was obtained. The study was approved by the Prague Psychiatric Centre Institutional Review Board.
MRI methods
MRI acquisition parameters
The Halifax Lithium Study participants were scanned in Halifax, whereas the participants in the Prague Study of Young BD Patients were scanned in Prague. We used the same scanner type and the same scanning parameters at both sites. In particular, all magnetic resonance acquisitions were performed with a 1.5 Tesla General Electric Signa scanner (General Electric Medical Systems, Milwaukee, WI, USA) and a standard single-channel head coil. After a localizer scan, a T1-weighted spoiled gradient recalled (SPGR) scan was acquired with the following parameters: flip angle = 40 degrees, echo time (TE) = 5 msec, repetition time (TR) = 25 msec, field of view (FOV) = 24 cm × 18 cm, matrix = 256 × 160 pixels, NEX = 1, no inter-slice gap, 124 images with 1.5 mm slice thickness.
Voxel-based morphometry (VBM) data processing
Structural data were analyzed with an optimized VBM-style analysis [Oxford Centre for Functional MRI of the Brain (FMRIB)’ s Software Library (FSL)–VBM], carried out with FSL Tools (30). First, structural images were brain-extracted using the Brain Extraction Tool (31). Next, tissue-type segmentation was carried out using FMRIB’s Automated Segmentation Tool, version 4 (32). Overall gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) volumes in native space were obtained at this step. The resulting GM partial volume images were then aligned to Montreal Neurological Institute (MNI) 152 standard space using the affine registration tool FLIRT (33), followed by nonlinear registration using FNIRT, which uses a b-spline representation of the registration warp field (34). The resulting images were averaged to create a study-specific template, to which the native GM images were then nonlinearly re-registered. Creating a study-specific template further reduces any potential bias for spatial normalization. As a result of nonlinear spatial normalization, the volumes of certain brain regions may grow, whereas others may shrink. In order to preserve the volume of a particular tissue (GM, WM, or CSF) within a voxel, the registered partial volume images were then modulated by dividing by the Jacobian of the warp field to correct for local expansion or contraction. In effect, an analysis of modulated data tests for regional differences in the absolute amount (volume) of GM (35). The modulated segmented images were then smoothed with an isotropic Gaussian kernel with a sigma of 3 mm. The intensity in each voxel of the smoothed data is a locally weighted average of GM density from a region of surrounding voxels, the size of the region being defined by the size of the smoothing kernel. This conditions the data to conform more closely to the Gaussian field model underlying the statistical procedures used for making inferences about regionally specific effects (36).
Statistical analyses
For comparisons of clinical and demographic variables, we used one-way ANOVA or t-test for continuous and χ2 test for categorical variables.
Voxel-wise paired GM differences for data preprocessed in FSL were determined using ‘FSL randomize’, a permutation based, nonparametric program, which enables modeling and inferences on statistical parametric maps with an unknown null distribution using a general linear model design (37), thus requiring no assumptions about the underlying distributions. We selected the hippocampus as the a priori region of interest (ROI), using Wake Forest University (WFU) PickAtlas version 2.5 (http://fmri.wfubmc.edu/software/PickAtlas). We applied a threshold of p < 0.05 corrected for multiple comparison using the threshold free cluster estimation (TFCE) analysis (38). This technique does not use a specific threshold but takes into account both the spatial extent and height of any between-group differences.
All results are presented using MNI coordinates. Four contrasts were conducted: (i) non-Li group versus matched controls; (ii) Li group versus matched controls; (iii) Li versus non-Li groups; and (iv) young BD participants versus matched controls. All participants for contrasts (i) to (iii) were scanned in Halifax, whereas those in contrast (iv) were scanned in Prague. Data from different sites were not combined in any analysis. We did not directly compare the young BD individuals (Prague Study) with the Halifax non-Li group, as these two groups were scanned in different sites and differed not only in the duration of illness, but also in age. Since both age and duration of illness are inter-correlated and negatively associated with hippocampal volumes (25), comparisons between these two groups would be confounded. Instead we contrasted each of these groups with age-matched controls.
Results
Description of the participants
We recruited 17 Li, 12 non-Li, and 11 control participants for the Halifax Lithium Study. The three groups did not differ in age, sex or education levels (see Table 1). Both patient groups (i.e., Li and non-Li) had a marked burden of illness, but without statistically significant differences between the Li and the non-Li groups in cumulative time spent ill, numbers of episodes, duration of illness, number of currently used psychotropic medications, duration of follow up, HAM-D-17, YMRS, or CGI-BP, or in the proportion of participants with a chronic course of illness, a history of psychiatric hospitalization or a diagnosis of bipolar I versus bipolar II disorder (see Table 2). More non-Li than Li participants were treated with anticonvulsants. Two of the BD patients in the non-Li group had lifetime exposure to Li, 9 and 13 years prior to the scanning; the rest were Li naïve. The duration of Li treatment in the Li group was mean ± standard deviation (SD) 10.6 ± 6.3 years. The Li levels at the time of scanning in the Li-treated group were mean 0.73 ± 0.16 mmol/l, range: 0.50–0.97 mmol/l. There were no differences between the groups in the volumes of the whole brain, GM, WM, or CSF (see Table 1).
Table 1.
Comparison between the non-lithium (non-Li), lithium (Li), and control participants in the Halifax Lithium Study
| Variable | Non-Li (n = 12) | Li (n = 17) | Controls (n = 11) | df | Fa or (χ2)b | p-value |
|---|---|---|---|---|---|---|
| Sex, female, n (%) | 6 (50.0) | 12 (70.6) | 8 (72.7) | 2 | 1.71 | 0.43 |
| Age, years, mean (SD) | 45.6 (8.9) | 47.8 (10.1) | 46.0 (8.6) | 2,37 | 0.22 | 0.80 |
| Education level, n (%) | 8 | 9.09 | 0.33 | |||
| Elementary school | 0 (0) | 2 (12) | 1 (9) | |||
| High school | 3 (25) | 6 (35) | 2 (18) | |||
| Apprenticeship | 1 (8) | 0 (0) | 0 (0) | |||
| College | 3 (25) | 0 (0) | 3 (27) | |||
| University | 5 (42) | 9 (53) | 5 (46) | |||
| CSF volume, mm3, mean (SD) | 327247.0 (56072.9) | 305693.9 (49463.1) | 289078.7 (52890.1) | 2,37 | 1.54 | 0.23 |
| GM volume, mm3, mean (SD) | 587298.0 (76492.1) | 605841.1 (60912.0) | 594566.0 (67134.3) | 2,37 | 0.28 | 0.76 |
| WM volume, mm3, mean (SD) | 431889.5 (74003.1) | 403444.5 (65279.7) | 381516.2 (69802.5) | 2,37 | 1.54 | 0.23 |
| Total brain volume, mm3, mean (SD) | 1346400.0 (168330.5) | 1315000.0 (146355.0) | 1265200.0 (134251.6) | 2,37 | 0.85 | 0.43 |
CSF = cerebrospinal fluid; df = degrees of freedom; GM = gray matter; SD = standard deviation; WM = white matter.
For continuous variables.
For categorical variables.
Table 2.
Comparison of clinical, illness burden, and treatment-related variables between the non-lithium (non-Li) and lithium (Li) bipolar disorder patients in the Halifax Lithium Study
| Variable | Non-Li (n = 12) | Li (n = 17) | df | t-testa or (χ2)b | p-value |
|---|---|---|---|---|---|
| Duration of follow-up, years, mean (SD) | 4.4 (4.3) | 6.7 (2.8) | 25 | −1.71 | 0.10 |
| Years since diagnosis of depression, mean (SD) | 25.3 (8.9) | 26.3 (8.9) | 25 | −0.29 | 0.77 |
| Lifetime episodes of depression, n (%) | 8.3 (5.8) | 7.1 (5.0) | 27 | 0.59 | 0.56 |
| Cumulative duration of depressions, months, mean (SD) | 21.0 (11.9) | 19.6 (15.5) | 26 | 0.25 | 0.80 |
| Years since diagnosis of mania, mean (SD) | 20.9 (11.4) | 19.9 (7.3) | 27 | 0.28 | 0.78 |
| Lifetime episodes of mania, n (%) | 2.3 (1.4) | 3.9 (3.3) | 26 | −1.66 | 0.11 |
| Cumulative duration of manias, months, mean (SD) | 6.2 (8.0) | 7.8 (6.6) | 27 | −0.61 | 0.54 |
| YMRS total score, mean (SD) | 1.1 (1.4) | 1.1 (1.4) | 26 | −0.05 | 0.96 |
| HAM-D-17 total score, mean (SD) | 2.6 (2.9) | 2.3 (1.6) | 26 | 0.40 | 0.70 |
| Clinical course chronic, n (%) | 2 (16.7) | 4 (23.5) | 1 | 0.20 | 0.65 |
| Total duration of illness, years, mean (SD) | 25.6 (9.8) | 27.1 (8.2) | 27 | −0.45 | 0.65 |
| Total duration of illness, years, median [range] | 26.0 [10.0–38.5] | 26.5 [12.3–42.0] | N/A | N/A | N/A |
| Total no. of episodes, mean (SD) | 10.5 (5.1) | 10.8 (5.5) | 27 | −0.13 | 0.89 |
| Total no. of episodes, median [range] | 8.0 [5.0–20.0] | 9.0 [5.0–22.0] | N/A | N/A | N/A |
| Duration of untreated illness, years, mean (SD) | 9.6 (6.8) | 9.4 (10.0) | 26 | 0.07 | 0.94 |
| No. of psychotropic medications at the time of scan, mean (SD)c | 1.8 (1.3) | 1.8 (0.7) | 27 | 0.19 | 0.85 |
| Li duration at the time of scan, years, mean (SD) | N/A | 10.6 (6.3) | N/A | N/A | N/A |
| Anticonvulsants at the time of scan, n (%) | 9 (75.0) | 4 (23.5) | 1 | 7.35 | 0.01 |
| Anticonvulsants: duration at the time of scan, years, mean (SD) | 7.1 (4.2) | 8.3 (6.2) | 10 | −0.38 | 0.71 |
| Antidepressants at time of scan, n (%) | 6 (50.0) | 7 (41.2) | 1 | 0.22 | 0.64 |
| Antidepressants: duration at the time of scan, years, mean (SD) | 7.2 (6.0) | 4.8 (6.3) | 10 | 0.66 | 0.52 |
| Antipsychotics at the time of scan, n (%) | 4 (33.3) | 2 (11.8) | 1 | 1.99 | 0.16 |
| Antipsychotics: duration at the time of scan, years, mean (SD) | 7.0 (1.5) | 4.3 (1.3) | 3 | 2.01 | 0.14 |
| History of psychiatric hospitalizations, n (%) | 7 (58.3) | 12 (70.6) | 1 | 0.47 | 0.49 |
| Bipolar disorder diagnosis, n (%) | 1 | 0.35 | 0.56 | ||
| Bipolar I disorder | 9 (75.0) | 11 (64.7) | |||
| Bipolar II disorder | 3 (25.0) | 6 (35.3) |
df = degrees of freedom; HAM-D-17 = Hamilton Depression Rating Scale, 17-item version; N/A = not applicable; SD = standard deviation; YMRS = Young Mania Rating Scale.
For continuous variables.
For categorical variables.
Excluding benzodiazepines.
For the Prague Study of Young BD Patients, we recruited 13 BD patients with no lifetime history of Li treatment and 18 controls. The two groups did not differ in age, sex or education levels (see Table 3). The BD patients in this study had an average of 5.3 ± 4.1 years of illness (range: 0.008–11.5 years, median 5.25 years), and 3.1 ± 2.3 episodes (range: 1–9, median 2.0 episodes). Ten of the 13 young BD patients had a history of psychiatric hospitalizations. The BD patients were currently being treated with anticonvulsants (n = 4), antipsychotics (n = 6), and antidepressants (n = 3). The average duration of treatment was 4.1 ± 4.05 years. None of the participants had any current or lifetime exposure to Li. There were no differences between the groups in the volumes of the whole brain, GM, WM, or CSF (see Table 3).
Table 3.
Comparison between bipolar disorder and control participants in the Prague Study of Young Bipolar Disorders Patients
| Variable | Bipolar disorders (n = 13) | Controls (n = 18) | df | t-testa or (χ2)b | p-value |
|---|---|---|---|---|---|
| Sex, female, n (%) | 9 (69.2) | 11 (61.1) | 1 | 0.22 | 0.64 |
| Age, years, mean (SD) | 25.7 (3.5) | 23.0 (4.0) | 29 | 1.97 | 0.06 |
| Education level, n (%) | 4 | 2.27 | 0.63 | ||
| Elementary school | 2 (15) | 2 (11) | |||
| High school | 3 (23) | 5 (28) | |||
| Apprenticeship | 1 (8) | 5 (28) | |||
| College | 3 (23) | 3 (17) | |||
| University | 4 (31) | 3 (17) | |||
| CSF volume, mm3, mean (SD) | 279316.4 (45679.9) | 275249.3 (47724.3) | 29 | 0.24 | 0.81 |
| GM volume, mm3, mean (SD) | 588102.3 (56045.4) | 584938.3 (77938.1) | 29 | 0.12 | 0.90 |
| WM volume, mm3, mean (SD) | 393207.8 (64305.8) | 387482.4 (67183.9) | 29 | 0.24 | 0.81 |
| Total brain volume, mm3, mean (SD) | 1247700.0 (174698.4) | 1260600.0 (139115.1) | 29 | 0.22 | 0.83 |
CSF = cerebrospinal fluid; df = degrees of freedom; GM = gray matter; SD = standard deviation; WM = white matter.
For continuous variables.
For categorical variables.
VBM results
In the Halifax Lithium Study, BD patients without current exposure to Li (non-Li group) showed significantly smaller GM volumes in the left hippocampus relative to healthy controls (corrected p < 0.05, voxel level; 141 contiguous voxels, maximum difference at x = −24, y = −28, z = −16; t = 4.68, corrected p = 0.015) and a trend for smaller GM volume in the left hippocampus relative to the Li group (corrected p < 0.1, voxel level; 11 contiguous voxels, maximum difference at x = −18, y = −22, z = −16; t = 3.97, corrected p = 0.087) (Fig. 1). Nine of the voxels showing decreased hippocampal GM volume among the non-Li relative to Li-treated participants directly overlapped with the decreased left hippocampal GM volume among the non-Li relative to control participants. Excluding the two individuals with previous Li exposure from the non-Li group and thus redoing the analyses in 10 Li-naïve participants did not change the results.
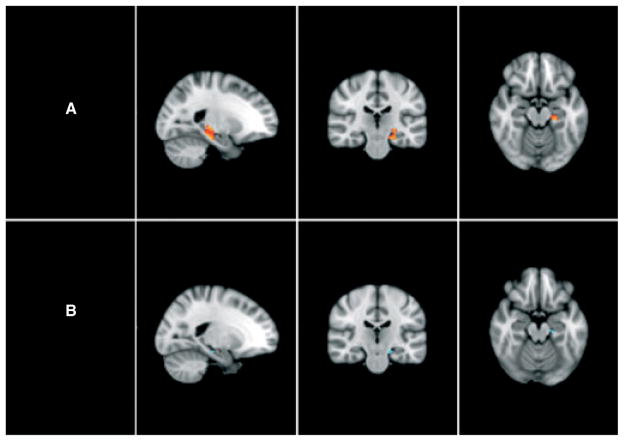
Fig. 1.
Differences between groups in hippocampal volumes. (A) Non-lithium (non-Li) participants with bipolar disorder (BD) versus controls; a red cluster denotes a significant hippocampal volume decrease in non-Li relative to control participants (corrected p < 0.05). (B) Non-Li BD participants versus lithium (Li)-treated BD patients; a blue cluster denotes a trend for hippocampal volume decrease in non-Li relative to Li-treated BD patients (corrected p < 0.1).
There were no significant voxelwise differences (increases or decreases) within the left or right hippocampal mask between the Li-treated BD patients and healthy controls in the Halifax study or between the young BD patients and matched controls in the Prague study (corrected p > 0.05 for all voxels), not even when we increased the p-values to corrected p = 0.30 and corrected p = 0.50, respectively.
Discussion
Among BD participants selected for substantial illness burden, only the group with no or limited lifetime exposure to Li had lower hippocampal volumes than controls. BD patients with similar illness burden and ongoing Li treatment, or young, Li-naïve BD individuals with lower illness burden had hippocampal volumes comparable to controls. Our findings are in keeping with our a priori hypotheses, as well as with other studies which reported lower hippocampal volumes among non-Li patients relative to controls (17, 18) or Li-treated patients (17, 20, 21, 26). The Li and non-Li groups in our study did not differ in the cumulative time spent ill, numbers of episodes of either polarity, duration of illness, proportion of participants with a history of psychiatric hospitalization or proportion of individuals with the diagnosis of bipolar I versus bipolar II disorder. Therefore, patient-related factors were unlikely to underlie the results. It is more parsimonious to assume that the observed differences were related to differential exposure to Li.
Whereas the comparison between the non-Li and control participants (n = 23) showed marked differences with a stringent statistical threshold (corrected p < 0.05), there were no differences between young BD patients and controls or between the Li-treated BD and control participants even at a much higher p level (0.50 and 0.30, respectively) and with a larger sample size (n = 31 and n = 28, respectively). This suggests that the absence of hippocampal volume changes in these contrasts was not a false negative finding. These results are also in keeping with previous investigations, which showed a lack of hippocampal volume differences among Li-treated BD patients (9, 21), or BD patients at the early stages of illness (12, 39) and controls.
The association between the duration of Li treatment and hippocampal volumes may be nonlinear, with initial steep increases, followed by a plateau or even decreases (19, 40). The maximum average duration of treatment in previous studies of hippocampal volumes in Li-treated patients was up to 2.4 ± 4.3 years (17). It is thus of interest that in our study BD patients with substantial illness burden did not show hippocampal volume loss relative to controls even after an average of 10.6 ± 6.3 years of Li treatment.
The non-Li group in our study had decreased hippocampal volumes despite exposure to other mood stabilizers (valproate, or atypical antipsychotics) also considered neuroprotective in mostly preclinical studies (23). This is in keeping with a number of previous clinical neuroimaging studies which also showed greater effects of Li than of other mood stabilizers on hippocampal volumes (14, 21, 26) but also on magnetic resonance spectroscopy measures of N-acetyl aspartate, a putative marker of neuronal density (41–43). In addition, the increased risk of dementia in BD seems to be reduced with continuous Li, but not anticonvulsant or antipsychotic treatment (44). Focusing on unique biochemical effects of Li may help elucidate some of these unique clinical and neurobiological actions of this element.
The smaller hippocampal volumes relative to controls in BD patients selected for a substantial illness burden (mean 25.6 ± 9.8 years of illness and 10.5 ± 5.1 episodes) and minimal lifetime exposure to Li together with the absence of such changes in Li-naïve BD participants with a much lower duration of illness (mean 5.3 ± 4.1 years) and numbers of episodes (mean 3.1 ± 2.3) suggests that, similar to unipolar depression (24), hippocampal volume changes in BD may be secondary to burden of illness. This is a novel finding in BD. A single prospective study reported a larger decline in hippocampal GM density over four years in BD than control participants (25). In most studies of BD patients no association between illness burden and hippocampal volumes was detected (8, 13, 14, 20). Some studies even observed a trend for smaller hippocampal volumes in first episode relative to multiple episode or control participants (45). Other studies have shown a positive association between duration of illness (46) or age (17) and hippocampal/temporal lobe volumes, as well as larger hippocampal volumes in elderly BD patients relative to controls (14). Inclusion of patients with current as well as lifetime history of Li treatment likely underlies these contradictory results. Longer duration of illness increases the chance of Li exposure (14), and even a short-term Li exposure leads to increased hippocampal volumes (21). Controlling for exposure to Li is thus critical in studies investigating the effects of illness burden on brain structure.
A frequent concern in volumetric studies of Li treatment is whether the observed changes are related to shifts in water content. This explanation is unlikely, as there were no overall differences in GM or WM volumes between the groups. Furthermore, instead of generalized hippocampal volume changes, as would be expected in case of shifts in water content, we only found small and circumscribed differences in hippocampal GM.
This study has several limitations. A prospective design would have better allowed us to establish a causal effect of Li exposure or illness burden on hippocampal volumes. The sample size in the non-Li group was not large, due to strict inclusion criteria. Previous studies have, however, used smaller sample sizes of patients with much less detailed clinical information. Exposure to other potentially neuroprotective medications was allowed in both treatment groups. This was motivated by the fact that requiring Li monotherapy would further decrease feasibility and would select for more Li-responsive participants, who may differ in their neurobiology from other BD patients. Contrasting the Li-treated participants with a medication-naïve group would not be optimal either, as medication-naïve BD patients are typically at the early stages of the illness where hippocampal volume changes are unlikely to be present. Exposure to other neuroprotective medications was unlikely to have confounded the results, as the groups were comparable with regard to exposure to antidepressants or antipsychotics. The only difference was in exposure to anticonvulsants, for which clinical evidence for neuroprotective effects is predominantly negative (14, 21, 26). This study was not designed to test for correlations between the duration of illness or duration of Li treatment and hippocampal volumes. The strict inclusion and exclusion criteria used in our study limit the generalizability of the findings. Previous studies have assessed less selected, more generalizable populations, which, however, may make the interpretation and replicability of findings difficult due to the presence of uncontrolled confounders. In this study our main goal was to test specific questions and to allow for a clean interpretation of the data, which required a more selected sample. All participants in this study were required to be euthymic at the time of scanning. This is critical for minimization of confounding factors. It is possible that younger patients who have poorer symptom control would demonstrate smaller hippocampal volumes. On the other hand, illness course is only a poor proxy of illness severity. The majority of the Prague BD participants had bipolar I disorder and a history of psychiatric hospitalizations, indicating a marked severity of illness. The age range selection criterion did not fully ensure low illness burden in all of the Prague young BD patients. However, it was sufficient to ensure a large and systematic separation in duration of illness and numbers of episodes between the clinical groups recruited in Prague and Halifax.
The current study provides several key benefits over prior investigations. With 71 participants this is one of the largest studies of hippocampal volumes in BD patients. The study was a priori designed to test neuroprotective effects of Li. Participants were carefully prospectively monitored for Li treatment at a specialized clinic, thus ensuring that neither too low (subtherapeutic), nor too high (potentially neurotoxic) levels could have confounded the results. We used more stringent criteria for exposure as well as lack of exposure to Li than any other previous study, as well as controlled for lifetime exposure to Li. Unlike previous investigations we used inclusion criteria to ensure a minimum required illness burden and a lack of differences in illness burden between the Li-exposed and Li non-exposed BD patients. We included young BD patients to allow for better interpretation of the relationship between Li, illness burden, and hippocampal volume.
To conclude, among patients selected for substantial illness burden, only the group with no or limited lifetime exposure to Li had lower hippocampal volumes than controls. BD patients with similar illness burden, but with an average of 10.6 ± 6.3 years of ongoing Li treatment or young, Li-naïve BD patients with lower illness burden showed hippocampal volumes comparable to controls. These results provide indirect support for neuroprotective effects of Li and negative effects of illness burden on hippocampal volumes in bipolar disorders.
Acknowledgments
This study was supported by funding from the Canadian Institutes of Health Research, Nova Scotia Health Research Foundation, Dalhousie Clinical Research Scholarship (to TH), grants from the Ministry of Health of the Czech Republic (grant NR8786), and the Ministry of Education of Czech Republic (MSMT 1M0517).
Footnotes
Disclosures
The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
References
- 1.Hoschl C, Hajek T. Hippocampal damage mediated by corticosteroids–a neuropsychiatric research challenge. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 2):II81–II88. doi: 10.1007/BF03035134. [DOI] [PubMed] [Google Scholar]
- 2.MacQueen GM, Campbell S, McEwen BS, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rush AJ, Giles DE, Schlesser MA, et al. Dexamethasone response, thyrotropin-releasing hormone stimulation, rapid eye movement latency, and subtypes of depression. Biol Psychiatry. 1997;41:915–928. doi: 10.1016/S0006-3223(97)00148-0. [DOI] [PubMed] [Google Scholar]
- 5.Post RM, Denicoff KD, Leverich GS, et al. Morbidity in 258 bipolar outpatients followed for 1 year with daily prospective ratings on the NIMH life chart method. J Clin Psychiatry. 2003;64:680–690. doi: 10.4088/jcp.v64n0610. [DOI] [PubMed] [Google Scholar]
- 6.Winokur G, Coryell W, Keller M, Endicott J, Akiskal HS. A prospective follow-up of patients with bipolar and primary unipolar affective disorder. Arch Gen Psychiatry. 1993;50:457–465. doi: 10.1001/archpsyc.1993.01820180059006. [DOI] [PubMed] [Google Scholar]
- 7.Winokur G, Coryell W, Endicott J, Akiskal H. Further distinctions between manic-depressive illness (bipolar disorder) and primary depressive disorder (unipolar depression) Am J Psychiatry. 1993;150:1176–1181. doi: 10.1176/ajp.150.8.1176. [DOI] [PubMed] [Google Scholar]
- 8.Altshuler LL, Bartzokis G, Grieder T, et al. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol Psychiatry. 2000;48:147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- 9.Brambilla P, Harenski K, Nicoletti M, et al. MRI investigation of temporal lobe structures in bipolar patients. J Psychiatr Res. 2003;37:287–295. doi: 10.1016/s0022-3956(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 10.Chen BK, Sassi R, Axelson D, et al. Cross-sectional study of abnormal amygdala development in adolescents and young adults with bipolar disorder. Biol Psychiatry. 2004;56:399–405. doi: 10.1016/j.biopsych.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 11.Delaloye C, de Bilbao F, Moy G, et al. Neuroanatomical and neuropsychological features of euthymic patients with bipolar disorder. Am J Geriatr Psychiatry. 2009;17:1012–1021. doi: 10.1097/JGP.0b013e3181b7f0e2. [DOI] [PubMed] [Google Scholar]
- 12.Hajek T, Gunde E, Slaney C, et al. Amygdala and hippocampal volumes in relatives of bipolar patients–high-risk study. Can J Psychiatry. 2009;54:726–733. doi: 10.1177/070674370905401102. [DOI] [PubMed] [Google Scholar]
- 13.Strakowski SM, DelBello MP, Sax KW, et al. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry. 1999;56:254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- 14.Beyer JL, Kuchibhatla M, Payne ME, et al. Hippocampal volume measurement in older adults with bipolar disorder. Am J Geriatr Psychiatry. 2004;12:613–620. doi: 10.1176/appi.ajgp.12.6.613. [DOI] [PubMed] [Google Scholar]
- 15.Blumberg HP, Kaufman J, Martin A, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- 16.Javadapour A, Malhi GS, Ivanovski B, Chen X, Wen W, Sachdev P. Hippocampal volumes in adults with bipolar disorder. J Neuropsychiatry Clin Neurosci. 2010;22:55–62. doi: 10.1176/jnp.2010.22.1.55. [DOI] [PubMed] [Google Scholar]
- 17.Bearden CE, Thompson PM, Dutton RA, et al. Three-dimensional mapping of hippocampal anatomy in unmedicated and lithium-treated patients with bipolar disorder. Neuropsychopharmacol. 2008;33:1229–1238. doi: 10.1038/sj.npp.1301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chepenik LG, Fredericks C, Papademetris X, et al. Effects of the brain-derived neurotrophic growth factor val66met variation on hippocampus morphology in bipolar disorder. Neuropsychopharmacol. 2009;34:944–951. doi: 10.1038/npp.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yucel K, McKinnon MC, Taylor VH, et al. Bilateral hippocampal volume increases after long-term lithium treatment in patients with bipolar disorder: a longitudinal MRI study. Psychopharmacol. 2007;195:357–367. doi: 10.1007/s00213-007-0906-9. [DOI] [PubMed] [Google Scholar]
- 20.Foland LC, Altshuler LL, Sugar CA, et al. Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. NeuroReport. 2008;19:221–224. doi: 10.1097/WNR.0b013e3282f48108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yucel K, Taylor VH, McKinnon MC, et al. Bilateral hippocampal volume increase in patients with bipolar disorder and short-term lithium treatment. Neuropsychopharmacol. 2008;33:361–367. doi: 10.1038/sj.npp.1301405. [DOI] [PubMed] [Google Scholar]
- 22.Gould TD, Picchini AM, Einat H, Manji HK. Targeting glycogen synthase kinase-3 in the CNS: implications for the development of new treatments for mood disorders. Curr Drug Targets. 2006;7:1399–1409. doi: 10.2174/1389450110607011399. [DOI] [PubMed] [Google Scholar]
- 23.Zarate CA, Jr, Singh J, Manji HK. Cellular plasticity cascades: targets for the development of novel therapeutics for bipolar disorder. Biol Psychiatry. 2006;59:1006–1020. doi: 10.1016/j.biopsych.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 24.McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- 25.Moorhead TW, McKirdy J, Sussmann JE, et al. Progressive gray matter loss in patients with bipolar disorder. Biol Psychiatry. 2007;62:894–900. doi: 10.1016/j.biopsych.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Germana C, Kempton MJ, Sarnicola A, et al. The effects of lithium and anticonvulsants on brain structure in bipolar disorder. Acta Psychiatr Scand. 2010;122:481–487. doi: 10.1111/j.1600-0447.2010.01582.x. [DOI] [PubMed] [Google Scholar]
- 27.Hajek T, Novak T, Kopecek M, Gunde E, Alda M, Hoschl C. Subgenual cingulate volumes in offspring of bipolar parents and in sporadic bipolar patients. Eur Arch Psychiatry Clin Neurosci. 2010;260:297–304. doi: 10.1007/s00406-009-0077-2. [DOI] [PubMed] [Google Scholar]
- 28.Duffy A, Alda M, Hajek T, Grof P. The early course of bipolar disorder as captured in a prospective study of high-risk children. Br J Psychiatry. 2009;195:457–458. doi: 10.1192/bjp.bp.108.062810. [DOI] [PubMed] [Google Scholar]
- 29.Ortiz A, Bradler K, Slaney C, et al. An admixture analysis of the age at index episodes in bipolar disorder. Psychiatry Res. 2011;188:34–39. doi: 10.1016/j.psychres.2010.10.033. [DOI] [PubMed] [Google Scholar]
- 30.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 31.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 33.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 34.Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- 35.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 36.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 37.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 39.McDonald C, Marshall N, Sham PC, et al. Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am J Psychiatry. 2006;163:478–487. doi: 10.1176/appi.ajp.163.3.478. [DOI] [PubMed] [Google Scholar]
- 40.Lyoo IK, Dager SR, Kim JE, et al. Lithium-induced gray matter volume increase as a neural correlate of treatment response in bipolar disorder: a longitudinal brain imaging study. Neuropsychopharmacol. 2010;35:1743–1750. doi: 10.1038/npp.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallelli KA, Wagner CM, Karchemskiy A, et al. N-acetyl-aspartate levels in bipolar offspring with and at high-risk for bipolar disorder. Bipolar Disord. 2005;7:589–597. doi: 10.1111/j.1399-5618.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 42.Garcia M, Huppertz HJ, Ziyeh S, Buechert M, Schumacher M, Mader I. Valproate-induced metabolic changes in patients with epilepsy: assessment with H-MRS. Epilepsia. 2009;50:486–492. doi: 10.1111/j.1528-1167.2008.01801.x. [DOI] [PubMed] [Google Scholar]
- 43.Silverstone PH, Wu RH, O’Donnell T, Ulrich M, Asghar SJ, Hanstock CC. Chronic treatment with lithium, but not sodium valproate, increases cortical N-acetyl-aspartate concentrations in euthymic bipolar patients. Int Clin Psychopharmacol. 2003;18:73–79. doi: 10.1097/00004850-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Kessing LV, Forman JL, Andersen PK. Does lithium protect against dementia? Bipolar Disord. 2010;12:87–94. doi: 10.1111/j.1399-5618.2009.00788.x. [DOI] [PubMed] [Google Scholar]
- 45.Strakowski SM, DelBello MP, Zimmerman ME, et al. Ventricular and periventricular structural volumes in first-versus multiple-episode bipolar disorder. Am J Psychiatry. 2002;159:1841–1847. doi: 10.1176/appi.ajp.159.11.1841. [DOI] [PubMed] [Google Scholar]
- 46.Ali SO, Denicoff KD, Altshuler LL, et al. Relationship between prior course of illness and neuroanatomic structures in bipolar disorder: a preliminary study. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:227–232. [PubMed] [Google Scholar]