Abstract
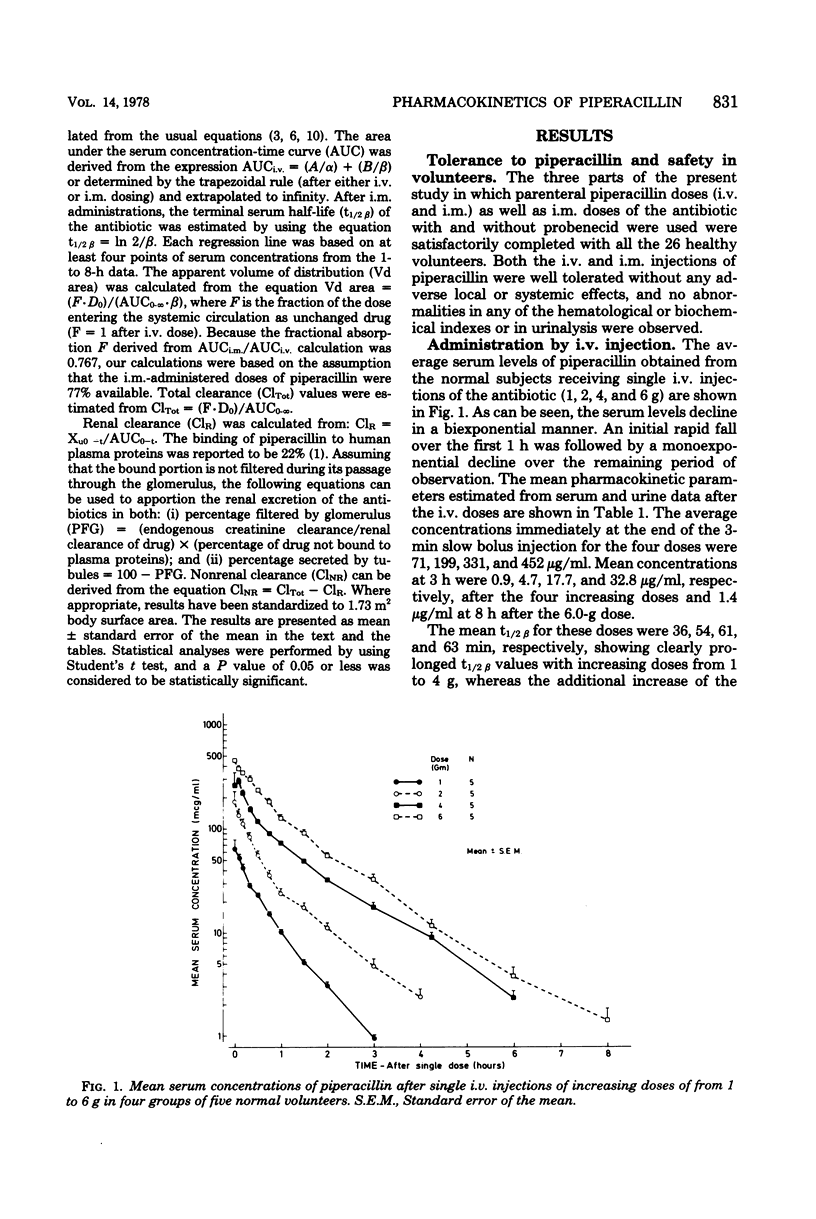
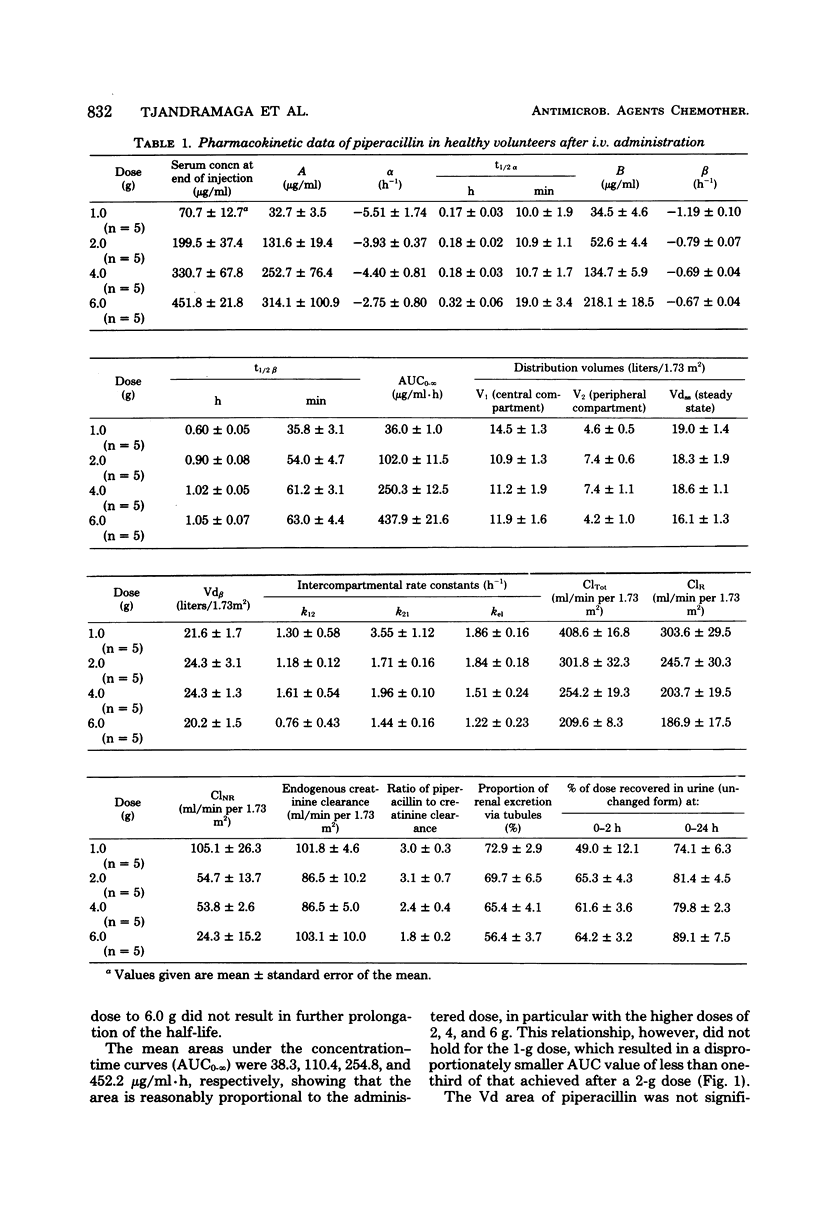
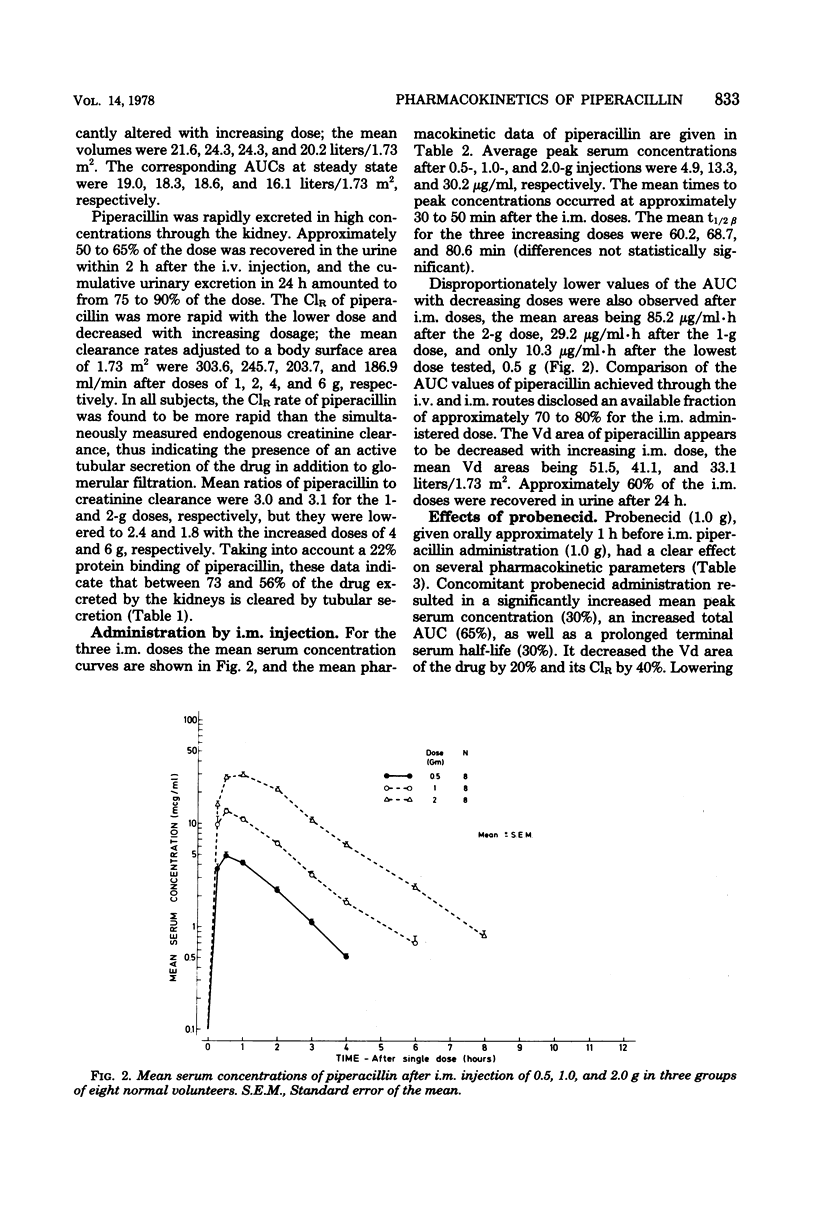
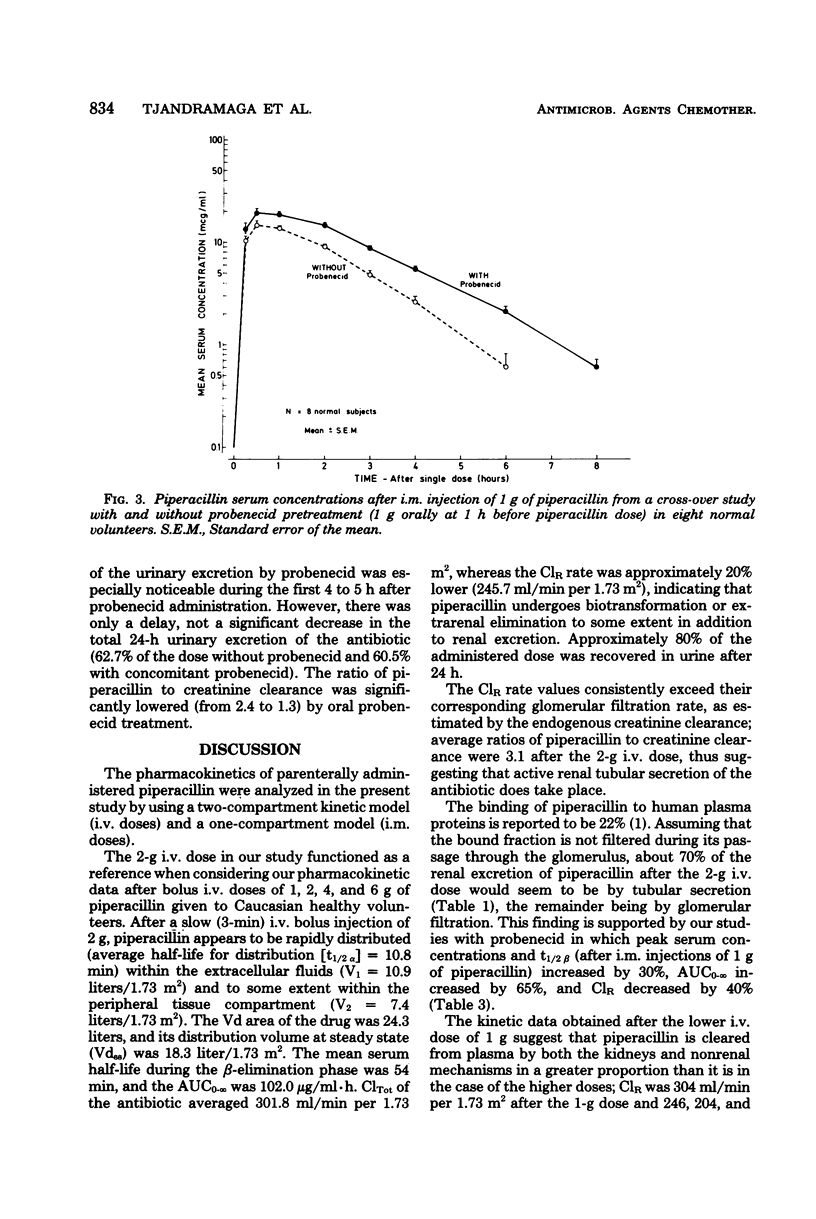
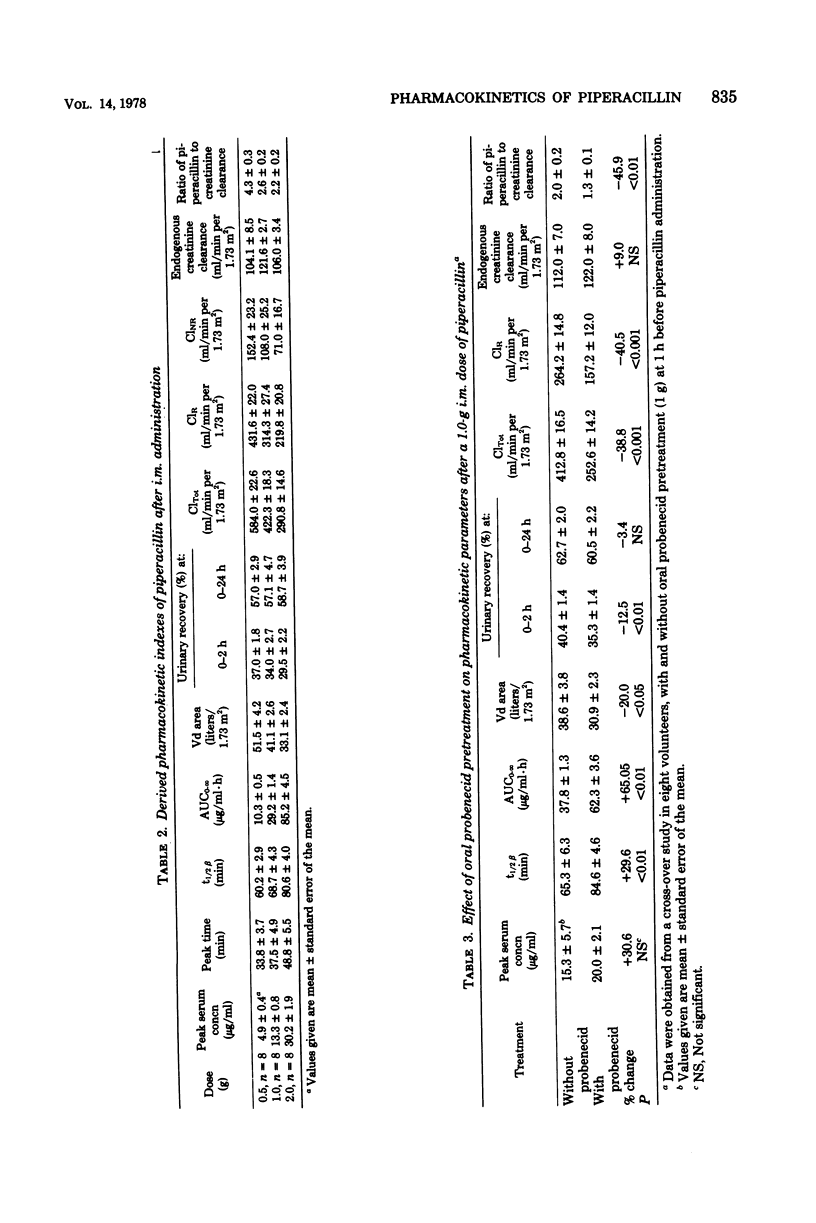
The pharmacokinetics of piperacillin were studied in a total of 26 Caucasian normal male volunteers. Single intramuscular doses of 0.5, 1.0, and 2.0 g were given to three groups, each containing eight volunteers. Mean peak serum concentrations of 4.9, 13.3, and 30.2 μg/ml were assayed at 30 to 50 min, and measurable levels were present up to 4, 6, and 8 h, respectively, after dosing. Single intravenous bolus doses of 1.0, 2.0, 4.0, and 6.0 g were given to four groups of five subjects, and mean serum concentrations of 70.7, 199.5, 330.7, and 451.8 μg/ml were measured at the end of the injections. The antibiotic had a mean terminal serum half-life of 60 to 80 min after the intramuscular doses and 36 to 63 min after intravenous administrations, depending on the dose. The apparent distribution volume was 20 to 24 liters/1.73 m2, and distribution volume at steady state was 16 to 19 liters/1.73 m2. Mean urinary recovery in 24 h was 74 to 89% for the intravenous doses and 57 to 59% for the intramuscular doses. The piperacillin-creatinine clearance ratios indicated that the proportion of renal excretion of piperacillin through tubular secretion was 56 to 73%, and this was confirmed by the renal clearance data from eight volunteers receiving probenecid treatment before the piperacillin dose. Probenecid (1 g given orally before administration of piperacillin) increased peak serum concentration by 30%, terminal serum half-life by 30%, and the area under the plasma concentration curve by 60%, and it decreased the apparent distribution volume by 20% and the renal clearance of the intramuscularly administered (1 g) antibiotic by 40%. Injections of piperacillin by both parenteral routes were well tolerated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fu K. P., Neu H. C. Piperacillin, a new penicillin active against many bacteria resistant to other penicillins. Antimicrob Agents Chemother. 1978 Mar;13(3):358–367. doi: 10.1128/aac.13.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibaldi M., Schwartz M. A. Apparent effect of probenecid on the distribution of penicillins in man. Clin Pharmacol Ther. 1968 May-Jun;9(3):345–349. doi: 10.1002/cpt196893345. [DOI] [PubMed] [Google Scholar]
- Jusko W. J., Gibaldi M. Effects of change in elimination on varous parameters of the two-compartment open model. J Pharm Sci. 1972 Aug;61(8):1270–1273. doi: 10.1002/jps.2600610820. [DOI] [PubMed] [Google Scholar]
- Riegelman S., Loo J. C., Rowland M. Shortcomings in pharmacokinetic analysis by conceiving the body to exhibit properties of a single compartment. J Pharm Sci. 1968 Jan;57(1):117–123. doi: 10.1002/jps.2600570123. [DOI] [PubMed] [Google Scholar]
- Ueo K., Fukuoka Y., Hayashi T., Yasuda T., Taki H., Tai M., Watanabe Y., Saikawa I., Mitsuhashi S. In vitro and in vivo antibacterial activity of T-1220, a new semisynthetic penicillin. Antimicrob Agents Chemother. 1977 Oct;12(4):455–460. doi: 10.1128/aac.12.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbist L. In vitro activity of piperacillin, a new semisynthetic penicillin with an unusually broad spectrum of activity. Antimicrob Agents Chemother. 1978 Mar;13(3):349–357. doi: 10.1128/aac.13.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbist L. Triple crossover study on absorption and excretion of ampicillin, pivampicillin, and amoxycillin. Antimicrob Agents Chemother. 1974 Nov;6(5):588–593. doi: 10.1128/aac.6.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. G., Northam J. I. Estimation of volume of distribution and half-life of a compound after rapid intravenous injection. J Pharm Sci. 1967 Apr;56(4):529–531. doi: 10.1002/jps.2600560424. [DOI] [PubMed] [Google Scholar]



