Abstract
Objective
Although bipolar disorder has high heritability, the onset occurs during several decades of life, suggesting that social and environmental factors may have considerable influence on disease onset. This study examined the association between the age of onset and sunlight at the location of onset.
Method
Data were obtained from 2414 patients with a diagnosis of bipolar I disorder, according to DSM-IV criteria. Data were collected at 24 sites in 13 countries spanning latitudes 6.3 to 63.4 degrees from the equator, including data from both hemispheres. The age of onset and location of onset were obtained retrospectively, from patient records and/or direct interviews. Solar insolation data, or the amount of electromagnetic energy striking the surface of the earth, were obtained from the NASA Surface Meteorology and Solar Energy (SSE) database for each location of onset.
Results
The larger the maximum monthly increase in solar insolation at the location of onset, the younger the age of onset (coefficient= −4.724, 95% CI: −8.124 to −1.323, p = 0.006), controlling for each country’s median age. The maximum monthly increase in solar insolation occurred in springtime. No relationships were found between the age of onset and latitude, yearly total solar insolation, and the maximum monthly decrease in solar insolation. The largest maximum monthly increases in solar insolation occurred in diverse environments, including Norway, arid areas in California, and Chile.
Conclusion
The large maximum monthly increase in sunlight in springtime may have an important influence on the onset of bipolar disorder.
Keywords: age of onset, bipolar disorder, solar insolation, sunlight
Bipolar disorder may emerge during several decades of life. Studies from many countries have reported three peaks in the distribution of the age of onset, occurring at about ages 17, 26, and over 40 (1–5). Differences in the distribution of the age of onset were also reported among countries (6), although only limited data are available from some regions of the world. Patients living in North America generally have a younger age of onset than those living in European countries (6–8). In many studies conducted in the USA, about 60% of patients experience the onset of bipolar disorder before the age of 19 (6, 9–12), as compared to a third or less for many studies in European countries (1, 5, 6, 13–16).
Diverse factors may contribute to this variation in the age of onset. There is agreement among researchers that both a patient’s genetic makeup and methodological issues in defining onset contribute to the reported differences, and that the variability in the age of onset reflects the genetic heterogeneity underlying bipolar disorder (1–3, 17). A positive family history is one of the strongest predictors of early onset (18). However, in addition to the genetic component, both the broad variability in the age of onset, and the onset peaks occurring after puberty (19) suggest that non-genetic factors may have considerable influence (20). There is limited understanding of how non-genetic factors, such as socioeconomic status and the physical environment, may impact the expression of bipolar disorder (21).
In a recent study, a linear association was detected between the age of onset of schizophrenia and latitude, with a much younger onset occurring in individuals living closer to the equator (22). Latitude is often used as a proxy measure of sunlight, such as in studies of seasonal and geographical variation in affective disorders and suicide (23–28). However, more direct measures of the amount of electromagnetic energy striking the surface of the earth are now available, such as solar insolation (29). Sunlight is the primary synchronizer of circadian rhythmicity in most animals, including humans (30), and disruptions in circadian function may contribute to the pathophysiology of bipolar disorder (31). The purpose of this study was to investigate if the age of onset of bipolar I (BP-I) disorder was associated with sunlight at the location of onset, as measured by solar insolation.
Methods
Data collection
Data were collected at 24 sites in 13 countries: Melbourne and Geelong, Australia (n = 161); Wiener Neustadt, Austria (n = 253); São Paulo (n = 222) and Porto Alegre, Brazil (n = 205); Halifax, Canada (n = 102); Santiago, Chile (n = 59); Medellín, Colombia (n = 130); Dresden (n = 35) and Würzburg, Germany (n = 246); Poznan, Poland (n = 102); Sardinia (n = 206) and Siena, Italy (n = 60); Paris, France (n = 363); Oslo (n = 113) and Trondheim (n = 153); Norway; Barcelona (n = 200) and Vitoria, Spain (n = 251); and Kansas City, MO (n = 40), Los Angeles, CA (n = 159), Palo Alto, CA (n = 11), Rochester, MN (n = 74), San Diego, CA (n = 55), and Worcester, MA (n = 11) in the USA.
All data collected were from patients with a diagnosis of bipolar disorder according to DSM-IV criteria. For all patients, the diagnosis was made by a psychiatrist. Approval from the institutional review board was obtained locally as required. Data for this study were obtained retrospectively. Seven sites obtained data from direct questioning of patients by clinical staff and reviewing patient records (Dresden, Germany; Halifax, Canada; Kansas City, MO; Oslo, Norway; São Paulo, Brazil; Trondheim, Norway; Vitoria, Spain), six sites primarily from direct questioning (Barcelona, Spain; Los Angeles and Palo Alto, CA; Rochester, MN; Santiago, Chile; and Worcester, MA), and the remaining 11 sites primarily by extraction from patient records. The age of onset was defined as when a patient experienced the first episode of depression, mania, or hypomania according to DSM-IV criteria.
Only patients with a diagnosis of BP-I disorder were included in this analysis, for two reasons: there was an imbalance in the percent of BP-I patients at the collection sites ranging from 36% to 100%, and to eliminate potential bias associated with older patients who had their onsets before bipolar II (BP-II) was defined as a separate diagnostic category.
Solar insolation
The US National Aeronautics and Space Administration (NASA) has released the Surface Meteorology and Solar Energy (SSE) Version 6.0 database, which provides solar insolation values for the entire globe based on data collected from 1983–2005 (29). The monthly average insolation is available for every 1 × 1 degree grid of latitude and longitude. Solar insolation is a measure of the electromagnetic energy from the sun received for a given surface area on earth at a given time, expressed in kilowatt hours/square meter/day (kWh/m2/day). The solar insolation is determined by the earth–sun relationship (angle of the sun’s rays and the day length), absorption by clouds and atmospheric aerosols, and reflection into space by snow, ice, and desert sand (29).
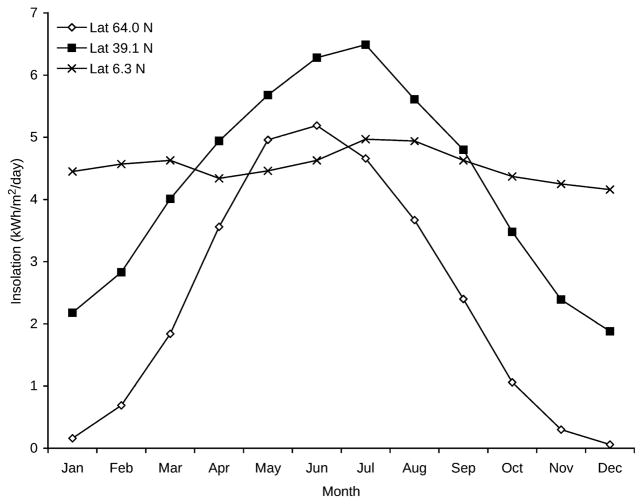
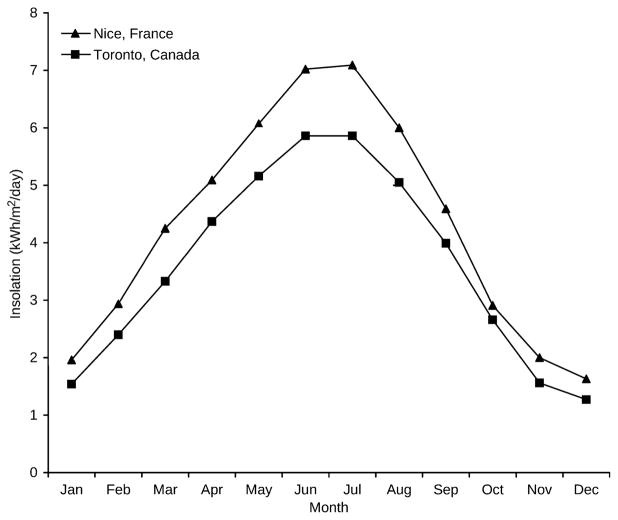
The pattern of monthly solar insolation varies dramatically by latitude. A location at the equator will show the least monthly variation throughout the year, while a location at 90 degrees from the equator (north or south pole) has the most variation. Figure 1 shows examples of different patterns of monthly solar insolation for sites in this study that are at equatorial, temperate, and northern latitudes. Additionally, monthly solar insolation values at the same latitude may differ significantly due to local conditions such as cloud cover, altitude, and proximity to large bodies of water, as shown in Figure 2.
Fig. 1.
Monthly solar insolation patterns at northern, temperate, and equatorial latitudes. The pattern of monthly solar insolation for three sites in this study: Trondheim, Norway (latitude 64.0 N), Kansas City, MO, USA (latitude 39.1 degrees N) and Medellin, Colombia (latitude 6.3 degrees N).
Fig. 2.
Monthly solar insolation values for cities at the same latitude. Locations at the same latitude may have very different solar insolation due to local conditions. The latitude of Nice, France, is 43.70 degrees N, with a maximum monthly increase in solar insolation of 1.31 kWh/m2/day between February and March. The latitude of Toronto, Canada is 43.67 degrees N, with a maximum monthly increase in solar insolation of 1.04 kWh/m2/day between March and April.
Solar insolation variables
The location of onset for each patient was grouped by reference cities, with each reference city representing all locations within the 1 × 1 degree grid of latitude and longitude. For example, Dresden, with latitude of 51.1 degrees north (N) and 13.8 degrees east (E), is the reference city for all locations between 51 and 52 degrees N, and 13 and 14 degrees E. The monthly average solar insolation values were obtained from the NASA SSE database for each reference city. Insolation data for reference cities in the southern hemisphere were shifted by six months to be comparable to those in the northern hemisphere.
Yearly, seasonal, and monthly solar insolation variables were calculated for each reference city. The monthly increase/decrease in solar insolation equals the current month minus the previous month. The maximum monthly increase/decrease equals the largest monthly increase/decrease over one year.
Demographic data
Gender and date of birth were collected for each patient. Country-specific parameters were obtained for each reference city including the lifetime prevalence of BP-I disorder, the country’s median age, the percent of the population aged 15–64, and the sex ratio for those aged 15–64 (32).
Statistics
The patient data within each reference city are correlated. To account for the correlated data, generalized estimating equation (GEE) models with an exchangeable correlation structure were used to analyze the age of onset, with each reference city treated as a cluster. This approach is suitable for studies containing a large number of clusters with variable cluster sizes, including many singleton clusters (clusters with a single observation) (33). A GEE model uses a population-averaged or marginal approach, estimating the effect across the entire population rather than within a cluster (34).
The country’s median age varied between 44.3 years for Germany and 26.7 years for Mexico. Since the age of onset of bipolar disorder spans childhood through late middle age, an older mean age of onset would be expected in a country with an older population as compared to one with a younger population (35, 36). Therefore, the country’s median age was included in all GEE models to adjust the age of onset for the country’s median age. Univariate GEE analyses were conducted to identify other demographic correlates of age of onset, and variables that were significant were included in the final models. All analyses were completed using SPSS version 18.0. All results were considered statistically significant if p < 0.05.
The sample size estimates for each collection center were not specified because there were no prior studies of solar insolation and age of onset from which to obtain variance values, and there was no way to predict how many patients at each collection site would have a location of onset somewhere else. The number of reference cities from each collection site varied considerably, reflecting differences in country size, culture, and migration patterns. For Melbourne (and Geelong), São Paulo, and Porto Alegre, the locations of onset were unavailable and the collection sites were used as proxies. These estimates were thought to adequately reflect the local population, and re-analysis of the data without these sites did not change the significance, direction, or magnitude of the results.
Results
Data for a total of 3211 patients were collected, of which 2414 had a diagnosis of BP-I disorder and were included in the analyses. Of the 797 excluded patients, 690 (86.6%) had a diagnosis of BP-II disorder and 107 (13.4%) had a diagnosis of bipolar disorder not otherwise specified or schizo-affective disorder. Considering the 2414 patients included in the analysis, 1452 (60.1%) were female and 962 (39.9%) male. The mean age of the 2414 patients was 47.0 ± 13.9 years and ranged from 16–99 years, with 269 (11.1%) older than age 64. The mean age of onset, unadjusted for the country’s median age, was calculated as 25.0 years for the 2414 patients to compare with prior studies that did not adjust for the country’s median age.
The onset locations for the 2414 patients were distributed over a wide range of latitudes, and included data from both the northern and southern hemispheres, as shown in Table 1. Although data were collected in 13 countries, 180 unique reference cities or clusters in 24 countries were obtained. The mean size of each cluster was 13.7, with 4.1% of the 2414 patients in singleton clusters.
Table 1.
Patient location of onset by latitude
| Degrees latitude (north and south)a | Number of patients |
|---|---|
| 0–9 | 130 |
| 10–19 | 3 |
| 20–29 | 210 |
| 30–39 | 652 |
| 40–49 | 1115 |
| 50–59 | 194 |
| 60–69 | 109 |
| 70–79 | 1 |
| Total | 2414 |
575 patients in the southern hemisphere.
In the univariate GEE analyses of age of onset, no patient demographic variables were significant, including sex (p = 0.315). Except for the country’s median age (p < 0.001), country-specific variables were not significant, including the prevalence of BP-I disorder (p = 0.704) and the sex ratio for those aged 15–64 (p = 0.769).
Table 2 presents the results of the models used to assess the relationship between solar insolation parameters and age of onset. The most striking result was the significant inverse relationship between the age of onset and the maximum monthly increase in solar insolation, with the coefficient estimated as −4.724 (p = 0.006). The larger the maximum monthly increase in solar insolation, the younger the age of onset, such that a 0.1 increase in maximum monthly solar insolation was associated with a nearly six-month decrease in age of onset. Table 3 presents the maximum monthly increase in solar insolation and mean age of onsets for representative reference onset locations in this study.
Table 2.
Estimated parameter coefficients explaining age of onset with 2414 patients in 180 clustersa
| Parameter | Coefficient estimate | 95% Wald confidence interval
|
Coefficient significance
|
||
|---|---|---|---|---|---|
| Lower | Upper | Wald Chi-square | p | ||
| Demographics | |||||
| Latitude | −0.040 | −0.170 | 0.090 | 0.367 | 0.545 |
| Gender (female) | −0.406 | −1.199 | 0.387 | 1.009 | 0.315 |
| Monthly insolation values | |||||
| Maximum monthly insolation | −1.268 | −2.304 | −0.231 | 5.743 | 0.017b |
| Minimum monthly insolation | −0.227 | −1.645 | 1.191 | 0.098 | 0.754 |
| Monthly insolation range | −1.168 | −2.117 | −0.218 | 5.811 | 0.016b |
| Cumulative insolation for year | −0.085 | −0.199 | 0.029 | 2.126 | 0.145 |
| Mean monthly isolation | −1.017 | −2.385 | 0.350 | 2.126 | 0.145 |
| Mean winter insolation | −0.067 | −1.405 | 1.270 | 0.010 | 0.921 |
| Mean spring insolation | −1.181 | −2.395 | 0.033 | 3.633 | 0.057 |
| Mean summer insolation | −1.125 | −2.265 | 0.016 | 3.737 | 0.053 |
| Mean fall insolation | −0.844 | −2.131 | 0.444 | 1.649 | 0.199 |
| Insolation range mean summer - mean winter | −1.265 | −2.333 | −0.197 | 5.393 | 0.020b |
| Monthly insolation changes | |||||
| Maximum monthly insolation increase | −4.724 | −8.124 | −1.323 | 7.413 | 0.006b |
| Maximum monthly insolation decrease | 0.412 | −4.360 | 5.183 | 0.029 | 0.866 |
| Percent maximum monthly insolation increase | −0.048 | −0.099 | 0.002 | 3.560 | 0.059 |
GEE model estimated age of onset using a constant, each country’s median age, and the listed parameter with an exchangeable correlation structure in each cluster. There was one degree of freedom for all models.
Significance < 0.05%.
Table 3.
Mean adjusted age of onset by maximum monthly increase in solar insolation (kWh/m2/day)
| Maximum monthly increase in solar insolation (kWh/m2/day) | Mean adjusted age of onset | Number of onset reference sites | Number of patients | Percent of patients | Example locations |
|---|---|---|---|---|---|
| < 1.0 | 23.43 | 19 | 355 | 15% | Medellín, Columbia Miami, FL, USA São Paulo, Brazil |
| ≥ 1.0 and < 1.1 | 22.37 | 36 | 428 | 17% | Halifax, Canada New York, NY, USA Porto Alegre, Brazil Rochester, MN, USA Würzburg, Germany |
| ≥ 1.1 and < 1.2 | 21.36 | 42 | 806 | 33% | Barcelona, Spain Kansas City, MO, USA Melbourne, Australia Paris, France Prince Edward Island, Canada Vienna, Austria |
| ≥ 1.2 and < 1.3 | 23.16 | 35 | 429 | 18% | Bordeaux, France Dresden, Germany Fargo, ND, USA San Diego, CA, USA Sardinia, Italy |
| ≥ 1.3 and < 1.4 | 22.08 | 24 | 142 | 6% | Mexicali, Mexico Nice, France San Francisco, CA, USA |
| ≥ 1.4 and < 1.5 | 19.64 | 10 | 12 | 1% | Las Vegas, NV, USA Lillehammer, Norway Valparaiso, Chile |
| ≥ 1.5 | 18.90 | 14 | 242 | 10% | Hermosillo, Mexico Los Angeles, CA, USA Oslo, Norway Sacramento, CA, USA Santiago, Chile Trondheim, Norway |
| Total | 180 | 2414 | 100% |
The inverse relationship between the maximum monthly increase in insolation and the age of onset was not solely due to the winter solar insolation value. Neither the minimum monthly insolation in winter (p = 0.754) nor the mean winter insolation (p = 0.921) were significantly associated with the age of onset. The percent change in maximum monthly increase in insolation, which magnifies the effect of a very low winter insolation in a northern latitude, was not significantly associated with the age of onset (p = 0.059). Additionally, many of the locations with the largest change in maximum monthly insolation were not at a northern latitude, as shown in Table 3. This suggests that a large maximum monthly increase in solar insolation was associated with a young age of onset, regardless of whether the change started from a very low level, as in Norway, or from a moderate level, as in California.
The parameters that indirectly assess the maximum monthly increase, such as the range in monthly insolation (p = .016), the range in mean summer–mean winter (p = 0.020), and maximum monthly insolation (p = 0.017), also supported the primary finding by having a significant inverse relationship to the age of onset. The cumulative yearly solar insolation (p = 0.145), maximum monthly decrease (p = 0.866), and mean seasonal insolation values were not associated with the age of onset. The maximum monthly increase occurred between February and March in 46% of locations, between March and April in 45% of locations, and between April and May in 6% of locations, excluding the 3% of the 180 reference cities near the equator which show little variation in monthly insolation, as shown in Figure 1. There was no significant relation between the month of the maximum monthly increase and the size of the maximum monthly increase in solar insolation.
In addition to the insolation effects, the country’s median age was significant in all GEE models. In the model to estimate the association with maximum monthly increase in solar insolation, the coefficient for the country’s median age was 0.444 (p < 0.001), where a one-year increase in the median age is associated with more than a five-month increase in age of onset.
Discussion
The primary finding of this study was that the larger the maximum monthly increase in solar insolation at the location of onset, the younger the age of onset of bipolar disorder. Comparing the extremes, the age of onset in the locations with the largest maximum monthly increase in solar insolation was about five years younger than in locations with the smallest increase. This inverse association was present regardless of the level of solar insolation in the starting month, with the largest increases occurring in locations that appeared to have little else in common geographically, including Norway, arid areas of the western USA, and Santiago, Chile. The maximum monthly increase in solar insolation occurred in the springtime, with the exact month varying by location.
In contrast to the inverse relationship between the maximum monthly increase in solar insolation and age of onset, neither the yearly total solar insolation nor the seasonal mean insolation values were associated with the age of onset. It is interesting that the magnitude of the monthly increase was significant, since prior studies with normal subjects have found a non-linear, dose–response relationship between light intensity and resetting the circadian clock (37), suppressing plasma melatonin concentration (38), and alertness (39).
No significant relationship was found between latitude at the location of onset and the age of onset. Since locations at the same latitude may have very different solar insolation, the limitations of latitude as a proxy for sunlight may have contributed to the unexpected results of prior research, such as inconsistencies in the seasonality of hospital admissions for bipolar disorder (40–44), and the weak correlation between the prevalence of seasonal affective disorder (SAD) and latitude in two review articles (24, 25). With the availability of solar insolation data from NASA, a new measure of sunlight is available for research.
Many lines of evidence converge to support the idea that a large monthly increase in solar insolation may be associated with the emergence of bipolar disorder. (i) Clinicians have long noted circadian abnormalities in patients with bipolar disorder, including sensitivity to light and sleep/wake cycle disturbances (31, 45). Even small circadian rhythm changes may be associated with adverse health outcomes such as suicide risk (46). (ii) Some patients with bipolar disorder experience seasonal variation (47). (iii) Light therapy for depression has induced a switch to mania in some patients (48, 49). (iv) Studies of seasonal variation in suicide have reported a spring peak in countries with large fluctuations in solar insolation, such as in northern Europe, and no peak near the equator (23, 50–52). (v) Violent suicide has been associated with an increase in sunlight duration (53), and this may involve seasonal variation in serotonergic, metabolic, and immune system variables (54–56). (vi) Within the brain, there is a seasonal variation in the concentration of serotonin and serotonin transporter binding (57, 58), and serotonin production is stimulated by light exposure (57). (vii) Patients with bipolar disorder may have abnormalities in melatonin secretion (59). (viii) There may be a genetic component underlying human rhythm disorders (30), and circadian gene polymorphisms may increase susceptibility to bipolar disorder (60). (ix) Light exerts effects through retinal ganglion cells, which are separate from rods and cones and have non-visual functions, including regulation of the biological clock (61). Overall, there is strong evidence that light affects human neurophysiology and behavior, making plausible the inverse association between the maximum monthly increase in solar insolation and the age of onset of bipolar disorder.
There are several limitations to this study. The total number of onset locations was relatively small. Although based on the DSM-IV criteria, the diagnostic assessment was not standardized across the collection sites. The age-of-onset data obtained by patient memory were subject to recall bias, although a retrospective approach has a precedent (3, 6, 10, 18). However, the unadjusted overall mean age of onset of 25.0 (n = 2414) in this study was similar to that for other international samples: 24.3 years for BP-I disorder (n = 1090) (15) and 25.6 years for any bipolar disorder (n = 1041) (13). Another limitation is that this study did not address individual activities. While most adults work indoors and have indoor hobbies, lifestyle choices such as outdoor work modify individual sun exposure (62). Finally, by shifting data from the southern hemisphere by six months, local cultural variations associated with the seasons were not accounted for.
The findings of this study suggest that variation in the size of the maximum monthly increase in solar insolation in springtime may have an important influence on the age of onset of bipolar disorder. Clinicians should be aware of the potential for a younger onset in locations that experience a large springtime increase in sunlight, and detailed questioning to identify symptoms of bipolar disorder, and more frequent monitoring may be indicated. Further research about the impact of sunlight on the onset and course of bipolar disorder is needed.
Acknowledgments
This research was funded in part by a grant entitled European Network of Bipolar Research Expert Centres (ENBREC) by the European Commission within the 7th Framework Programme (Ole A. Andreassen, Michael Bauer, Thomas D. Bjella, Letizia Bossini, Andrea Fagiolini, Ana Gonzalez-Pinto, Chantal Henry, Mónica Martinez-Cengotitabengoa, Ingrid Melle, Gunnar Morken, Andrea Pfennig, Carla Torrent, Eduard Vieta). Martin Alda received funds from the Canadian Institutes of Health Research (Grant 64410). Ole A. Andreassen received grants to the TOP study group from the University of Oslo, the Research Council of Norway (#167153/V50), and the South-Eastern Norway Health Authority (#2010-074). Frank Bellivier received grants from Assistance Publique-Hôpitaux de Paris (CRC 94232), the French Ministry of Research (PHRC, AOM98152), Institut National de la Santéet de la Recherche Médicale (INSERM), and the FondaMental foundation. Seetal Dodd collected data as part of a study that received financial support from Eli Lilly Australia. Ana González-Pinto was supported by Health Research Funds from the Spanish Government (CIBER Network, which is an initiative of ISCIII CB07/09/0024; P91A; P91E) and European Regional Development Funds (FEDER), and local grants (Kronikgune). Dr González Pinto is responsible for a specific collaborative agreement between the Spanish Government (SCIII) and the Basque Government to stabilize and intensify research in the National Health System (Boe nº 68 19, March 2010). The psychiatric research department in Santiago Apóstol Hospital is supported by the Stanley Research Foundation (03-RC-003). Chantal Henry received funds provided by EU Grant 223 102. Flavio Kapczinski received funding from CNPq, CAPES, Stanley Medical Research Institute, NARSAD, and FIPE-HCPA. Mauricio Kunz received support from CAPES, Brazil. Carlos Lopez-Jaramillo received support from Colciencias and CODI-UdeA. Mirko Manchia was supported by the Sobey Fellowship, Department of Psychiatry, Dalhousie University, Halifax, NS, Canada. Ingrid Melle received financial support from the Research Council of Norway (Grant # 181831, 147787/320, 167153/V50) and the South-Eastern Norway Health Authority (Grants # 2004123, #2006258). Gunnar Morken received support from St Olavs’ University Hospital, Trondheim, Norway.
Footnotes
Disclosures
Michael Bauer has been a consultant for AstraZeneca, Lilly, Servier, Lundbeck, and Bristol-Myers Squibb/Otsuka, received speaker honoraria from AstraZeneca, Lilly, Glaxo-SmithKline, Lundbeck, and Bristol-Myers Squibb/Otsuka, and grants/research support from The Stanley Medical Research Institute, NARSAD, and the European Commission (FP7).
Michael Berk has received grants/research support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, MBF, NHMRC, Beyond Blue, Geelong Medical Research Foundation, Bristol Myers Squibb, Eli Lilly & Co., GlaxoSmithKline, Organon, Novartis, Mayne Pharma, and Servier, has been a speaker for AstraZeneca, Bristol Myers Squibb, Eli Lilly & Co., GlaxoSmithKline, Janssen Cilag, Lundbeck, Merck, Pfizer, Sanofi Synthelabo, Servier, Solvay, and Wyeth, and served as a consultant to AstraZeneca, Bristol Myers Squibb, Eli Lilly & Co., GlaxoSmithKline, Janssen Cilag, Lundbeck, and Servier.
Seetal Dodd has received grants/research support from Eli Lilly & Co., GlaxoSmithKline, Organon, Mayne Pharma, and Servier, speaker’s fees from Eli Lilly & Co., and a travel grant from Servier.
Andrea Fagiolini has been a consultant and speaker for Angelini, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Pfizer, Eli Lilly & Co., Janssen, Lundbeck, Novartis, Otsuka, Sigma Tau, and Takeda.
Carlos Lopez-Jaramillo has been a consultant for AstraZeneca, Abbott, GSK, and Lundbeck.
Flavio Kapczinski has been a consultant for Lilly, AstraZeneca, Janssen, and Servier.
Andrea Pfennig has received a stipend/research support from GlaxoSmithKline and AstraZeneca, and speaker honoraria from AstraZeneca and Eli Lilly & Co.
Natalie Rasgon has received grant/research support from Bayer Pharmaceuticals, the American Diabetes Association, Abbot Laboratories, Inc., Forest Laboratories, GlaxoSmithKline, Pfizer, Inc., and Wyeth Pharmaceuticals, and has been a consultant or received lecture honoraria from Takeda Pharmaceuticals, Bristol-Myers Squibb Company, Forest Laboratories, Pfizer, Inc., and Wyeth Pharmaceuticals.
Eduard Vieta has received grants and been a consultant, advisor, or CME speaker for Almirall, AstraZeneca, Bristol-Myers Squibb, Eli Lilly & Co., Forest Research Institute, Gedeon Richter, GlaxoSmithKline, Janssen-Cilag, Jazz, Johnson & Johnson, Lundbeck, Merck, Novartis, Organon, Otsuka, Pfizer, Pierre-Fabre, Qualigen, Sanofi-Aventis, Servier, Shering-Plough, Solvay, Takeda, the Spanish Ministry of Science and Innovation (CIBERSAM), the Seventh European Framework Programme (ENBREC), the Stanley Medical Research Institute, United Biosource Corporation, and Wyeth.
Mark Zetin has received royalties from WW Norton for the book Challenging Depression and speaker’s fees from Sunovion, Bristol-Myers Squibb/Otsuka, Eli Lilly & Co., Pfizer, AstraZeneca, and Cephalon.
Tasha Glenn, Martin Alda, Ole A. Andreassen, Raffaella Ardau, Frank Bellivier, Thomas D. Bjella, Letizia Bossini, Maria Del Zompo, Mark A. Frye, Ana Gonzalez-Pinto, Chantal Henry, Sebastian Kliwicki, Barbara König, Mauricio Kunz, Beny Lafer, Mirko Manchia, Wendy Marsh, Mónica Martinez-Cengotitabengoa, Ingrid Melle, Gunnar Morken, Rodrigo Munoz, Fabiano G. Nery, Claire O’Donovan, Danilo Quiroz, Andreas Reif, Janusz Rybakowski, Kemal Sagduyu, Christian Simhandl, Carla Torrent, and Peter C. Whybrow do not have any conflicts of interest to report.
References
- 1.Bellivier F, Golmard JL, Rietschel M, et al. Age at onset in bipolar I affective disorder: further evidence for three subgroups. Am J Psychiatry. 2003;160:999–1001. doi: 10.1176/appi.ajp.160.5.999. [DOI] [PubMed] [Google Scholar]
- 2.Leboyer M, Henry C, Paillere-Martinot ML, Bellivier F. Age at onset in bipolar affective disorders: a review. Bipolar Disord. 2005;7:111–118. doi: 10.1111/j.1399-5618.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 3.Lin PI, McInnis MG, Potash JB, et al. Clinical correlates and familial aggregation of age at onset in bipolar disorder. Am J Psychiatry. 2006;163:240–246. doi: 10.1176/appi.ajp.163.2.240. [DOI] [PubMed] [Google Scholar]
- 4.Manchia M, Lampus S, Chillotti C, et al. Age at onset in Sardinian bipolar I patients: evidence for three subgroups. Bipolar Disord. 2008;10:443–446. doi: 10.1111/j.1399-5618.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 5.Hamshere ML, Gordon-Smith K, Forty L, et al. Age-at-onset in bipolar-I disorder: mixture analysis of 1369 cases identifies three distinct clinical sub-groups. J Affect Disord. 2009;116:23–29. doi: 10.1016/j.jad.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Post RM, Luckenbaugh DA, Leverich GS, et al. Incidence of childhood-onset bipolar illness in the USA and Europe. Br J Psychiatry. 2008;192:150–151. doi: 10.1192/bjp.bp.107.037820. [DOI] [PubMed] [Google Scholar]
- 7.Carter TD, Mundo E, Parikh SV, Kennedy JL. Early age at onset as a risk factor for poor outcome of bipolar disorder. J Psychiatr Res. 2003;37:297–303. doi: 10.1016/s0022-3956(03)00052-9. [DOI] [PubMed] [Google Scholar]
- 8.Soutullo CA, Chang KD, Díez-Suárez A, et al. Bipolar disorder in children and adolescents: international perspective on epidemiology and phenomenology. Bipolar Disord. 2005;7:497–506. doi: 10.1111/j.1399-5618.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- 9.Lish JD, Dime-Meenan S, Whybrow PC, Price RA, Hirschfeld RM. The National Depressive and Manic-depressive Association (DMDA) survey of bipolar members. J Affect Disord. 1994;31:281–294. doi: 10.1016/0165-0327(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 10.Perlis RH, Miyahara S, Marangell LB, et al. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2004;55:875–881. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 11.Leverich GS, Post RM, Keck PE, Jr, et al. The poor prognosis of childhood-onset bipolar disorder. J Pediatr. 2007;150:485–490. doi: 10.1016/j.jpeds.2006.10.070. [DOI] [PubMed] [Google Scholar]
- 12.Bauer M, Glenn T, Rasgon N, et al. Association between age of onset and mood in bipolar disorder: comparison of subgroups identified by cluster analysis and clinical observation. J Psychiatr Res. 2010;44:1170–1175. doi: 10.1016/j.jpsychires.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Morselli PL, Elgie R GAMIAN-Europe. GAMIAN-Europe/BEAM survey I–global analysis of a patient questionnaire circulated to 3450 members of 12 European advocacy groups operating in the field of mood disorders. Bipolar Disord. 2003;5:265–278. doi: 10.1034/j.1399-5618.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- 14.Suominen K, Mantere O, Valtonen H, et al. Early age at onset of bipolar disorder is associated with more severe clinical features but delayed treatment seeking. Bipolar Disord. 2007;9:698–705. doi: 10.1111/j.1399-5618.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- 15.Baldessarini RJ, Bolzani L, Cruz N, et al. Onset-age of bipolar disorders at six international sites. J Affect Disord. 2010;121:143–146. doi: 10.1016/j.jad.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 16.Larsson S, Lorentzen S, Mork E, et al. Age at onset of bipolar disorder in a Norwegian catchment area sample. J Affect Disord. 2010;124:174–177. doi: 10.1016/j.jad.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 17.Craddock N, Sklar P. Genetics of bipolar disorder: successful start to a long journey. Trends Genet. 2009;25:99–105. doi: 10.1016/j.tig.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Baldessarini RJ, Tondo L, Vazquez GH, et al. Age at onset versus family history and clinical outcomes in 1,665 international bipolar-I disorder patients. World Psychiatry. 2012;11:40–46. doi: 10.1016/j.wpsyc.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 20.Childs B, Valle D. Genetics, biology and disease. Annu Rev Genomics Hum Genet. 2000;1:1–19. doi: 10.1146/annurev.genom.1.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Tsuchiya KJ, Byrne M, Mortensen PB. Risk factors in relation to an emergence of bipolar disorder: a systematic review. Bipolar Disord. 2003;5:231–242. doi: 10.1034/j.1399-5618.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 22.Shaner A, Miller G, Mintz J. Evidence of a latitudinal gradient in the age at onset of schizophrenia. Schizophr Res. 2007;94:58–63. doi: 10.1016/j.schres.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Chew KS, McCleary R. The spring peak in suicides: a cross-national analysis. Soc Sci Med. 1995;40:223–230. doi: 10.1016/0277-9536(94)e0070-9. [DOI] [PubMed] [Google Scholar]
- 24.Mersch PP, Middendorp HM, Bouhuys AL, Beersma DG, van den Hoofdakker RH. Seasonal affective disorder and latitude: a review of the literature. J Affect Disord. 1999;53:35–48. doi: 10.1016/s0165-0327(98)00097-4. [DOI] [PubMed] [Google Scholar]
- 25.Haggarty JM, Cernovsky Z, Husni M. The limited influence of latitude on rates of seasonal affective disorder. J Nerv Ment Dis. 2001;189:482–484. doi: 10.1097/00005053-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Lambert G, Reid C, Kaye D, Jennings G, Esler M. Increased suicide rate in the middle-aged and its association with hours of sunlight. Am J Psychiatry. 2003;160:793–795. doi: 10.1176/appi.ajp.160.4.793. [DOI] [PubMed] [Google Scholar]
- 27.Friedman E, Gyulai L, Bhargava M, et al. Seasonal changes in clinical status in bipolar disorder: a prospective study in 1000 STEP-BD patients. Acta Psychiatr Scand. 2006;113:510–517. doi: 10.1111/j.1600-0447.2005.00701.x. [DOI] [PubMed] [Google Scholar]
- 28.Bauer M, Glenn T, Grof P, et al. Relationship among latitude, climate, season and self-reported mood in bipolar disorder. J Affect Disord. 2009;116:152–157. doi: 10.1016/j.jad.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 29. [accessed May 2011];NASA Surface meteorology and Solar Energy (SSE) Release 6.0 Methodology. 2011 Available from: http://eosweb.larc.nasa.gov/sse/
- 30.Cermakian N, Boivin DB. A molecular perspective of human circadian rhythm disorders. Brain Res Brain Res Rev. 2003;42:204–220. doi: 10.1016/s0165-0173(03)00171-1. [DOI] [PubMed] [Google Scholar]
- 31.Wehr TA, Sack D, Rosenthal N, Duncan W, Gillin JC. Circadian rhythm disturbances in manic-depressive illness. Fed Proc. 1983;42:2809–2814. [PubMed] [Google Scholar]
- 32. [accessed May 2011];CIA World Factbook. 2010 Available from: https://www.cia.gov/library/publications/the-world-factbook.
- 33.Stedman MR, Gagnon DR, Lew RA, Solomon DH, Brookhart MA. An evaluation of statistical approaches for analyzing physician-randomized quality improvement interventions. Contemp Clin Trials. 2008;29:687–695. doi: 10.1016/j.cct.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Zeger SL, Liang KY. An overview of methods for the analysis of longitudinal data. Stat Med. 1992;11:1825–1839. doi: 10.1002/sim.4780111406. [DOI] [PubMed] [Google Scholar]
- 35.Heimbuch RC, Matthysse S, Kidd KK. Estimating age-of-onset distributions for disorders with variable onset. Am J Hum Genet. 1980;32:564–574. [PMC free article] [PubMed] [Google Scholar]
- 36.Chen WJ, Faraone SV, Orav EJ, Tsuang MT. Estimating age at onset distributions: the bias from prevalent cases and its impact on risk estimation. Genet Epidemiol. 1993;10:43–59. doi: 10.1002/gepi.1370100106. [DOI] [PubMed] [Google Scholar]
- 37.Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Dose-response relationships for resetting of human circadian clock by light. Nature. 1996;379:540–542. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- 38.Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 40.Carney PA, Fitzgerald CT, Monaghan CE. Influence of climate on the prevalence of mania. Br J Psychiatry. 1988;152:820–823. doi: 10.1192/bjp.152.6.820. [DOI] [PubMed] [Google Scholar]
- 41.Silverstone T, Romans S, Hunt N, McPherson H. Is there a seasonal pattern of relapse in bipolar affective disorders? A dual northern and southern hemisphere cohort study. Br J Psychiatry. 1995;167:58–60. doi: 10.1192/bjp.167.1.58. [DOI] [PubMed] [Google Scholar]
- 42.Suhail K, Cochrane R. Seasonal variations in hospital admissions for affective disorders by gender and ethnicity. Soc Psychiatry Psychiatr Epidemiol. 1998;33:211–217. doi: 10.1007/s001270050045. [DOI] [PubMed] [Google Scholar]
- 43.Whitney DK, Sharma V, Kueneman K. Seasonality of manic depressive illness in Canada. J Affect Disord. 1999;55:99–105. doi: 10.1016/s0165-0327(98)00197-9. [DOI] [PubMed] [Google Scholar]
- 44.Lee HC, Tsai SY, Lin HC. Seasonal variations in bipolar disorder admissions and the association with climate: a population-based study. J Affect Disord. 2007;97:61–69. doi: 10.1016/j.jad.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 45.Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Rosenthal NE. Manic-depressive patients may be super-sensitive to light. Lancet. 1981;1:383–384. doi: 10.1016/s0140-6736(81)91697-4. [DOI] [PubMed] [Google Scholar]
- 46.Berk M, Dodd S, Hallam K, Berk L, Gleeson J, Henry M. Small shifts in diurnal rhythms are associated with an increase in suicide: the effect of daylight saving. Sleep and Biological Rhythms. 2008;6:22–25. [Google Scholar]
- 47.Goikolea JM, Colom F, Martínez-Arán A, et al. Clinical and prognostic implications of seasonal pattern in bipolar disorder: a 10-year follow-up of 302 patients. Psychol Med. 2007;37:1595–1599. doi: 10.1017/S0033291707000864. [DOI] [PubMed] [Google Scholar]
- 48.Schwitzer J, Neudorfer C, Blecha HG, Fleischhacker WW. Mania as a side effect of phototherapy. Biol Psychiatry. 1990;28:532–534. doi: 10.1016/0006-3223(90)90489-o. [DOI] [PubMed] [Google Scholar]
- 49.Sit D, Wisner KL, Hanusa BH, Stull S, Terman M. Light therapy for bipolar disorder: a case series in women. Bipolar Disord. 2007;9:918–27. doi: 10.1111/j.1399-5618.2007.00451.x. [DOI] [PubMed] [Google Scholar]
- 50.Parker G, Gao F, Machin D. Seasonality of suicide in Singapore: data from the equator. Psychol Med. 2001;31:549–553. doi: 10.1017/s0033291701003294. [DOI] [PubMed] [Google Scholar]
- 51.Petridou E, Papadopoulos FC, Frangakis CE, Skalkidou A, Trichopoulos D. A role of sunshine in the triggering of suicide. Epidemiology. 2002;13:106–109. doi: 10.1097/00001648-200201000-00017. [DOI] [PubMed] [Google Scholar]
- 52.Nejar KA, Benseñor IM, Lotufo PA. Sunshine and suicide at the tropic of Capricorn, São Paulo, Brazil, 1996–2004. Rev Saude Publica. 2007;41:1062–1064. doi: 10.1590/s0034-89102006005000046. [DOI] [PubMed] [Google Scholar]
- 53.Maes M, De Meyer F, Thompson P, Peeters D, Cosyns P. Synchronized annual rhythms in violent suicide rate, ambient temperature and the light-dark span. Acta Psychiatr Scand. 1994;90:391–396. doi: 10.1111/j.1600-0447.1994.tb01612.x. [DOI] [PubMed] [Google Scholar]
- 54.D’Hondt P, Maes M, Leysen JE, Gommeren W, Scharpé S, Cosyns P. Binding of [3H] paroxetine to platelets of depressed patients: seasonal differences and effects of diagnostic classification. J Affect Disord. 1994;32:27–35. doi: 10.1016/0165-0327(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 55.Maes M, Scharpé S, Verkerk R, et al. Seasonal variation in plasma L-tryptophan availability in healthy volunteers. Relationships to violent suicide occurrence. Arch Gen Psychiatry. 1995;52:937–946. doi: 10.1001/archpsyc.1995.03950230051008. [DOI] [PubMed] [Google Scholar]
- 56.Maes M, Scharpé S, D’Hondt P, et al. Biochemical, metabolic and immune correlates of seasonal variation in violent suicide: a chronoepidemiologic study. Eur Psychiatry. 1996;11:21–33. doi: 10.1016/0924-9338(96)80455-X. [DOI] [PubMed] [Google Scholar]
- 57.Lambert GW, Reid C, Kaye DM, Jennings GL, Esler MD. Effect of sunlight and season on serotonin turnover in the brain. Lancet. 2002;360:1840–1842. doi: 10.1016/s0140-6736(02)11737-5. [DOI] [PubMed] [Google Scholar]
- 58.Praschak-Rieder N, Willeit M, Wilson AA, Houle S, Meyer JH. Seasonal variation in human brain serotonin transporter binding. Arch Gen Psychiatry. 2008;65:1072–1078. doi: 10.1001/archpsyc.65.9.1072. [DOI] [PubMed] [Google Scholar]
- 59.Srinivasan V, Smits M, Spence W, et al. Melatonin in mood disorders. World J Biol Psychiatry. 2006;7:138–151. doi: 10.1080/15622970600571822. [DOI] [PubMed] [Google Scholar]
- 60.Mansour HA, Monk TH, Nimgaonkar VL. Circadian genes and bipolar disorder. Ann Med. 2005;37:196–205. doi: 10.1080/07853890510007377. [DOI] [PubMed] [Google Scholar]
- 61.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 62.Godar DE. UV doses worldwide. Photochem Photobiol. 2005;281:736–749. doi: 10.1562/2004-09-07-ir-308r.1. [DOI] [PubMed] [Google Scholar]