Abstract
Regulatory T cells are essential to maintain immune homeostasis and prevent autoimmunity. Therapy with in vitro expanded human nTRegs is being tested to prevent graft versus host disease, which is a major cause for morbidity and mortality associated with hematopoietic stem cell transplantation. Their usefulness in therapy will depend on their capacity to survive, migrate appropriately and retain suppressive activity when introduced into a transplant recipient. The lack of a suitable animal model for studying the in vivo reconstitutive capability of human nTRegs is a major impediment for investigating the behavior of adoptively transferred nTRegs in vivo. We show that injection of a plasmid encoding human IL-2 is necessary and sufficient for long term engraftment of in vitro expanded nTRegs in NOD-SCID IL2rγcnull mice. We also demonstrate that these in vivo reconstituted TRegs traffic to different organs of the body and retain suppressive function. Finally, in an IL-2 accelerated GVHD model, we show that these in vivo reconstituted TRegs are capable of preventing severe xenogenic response of human PBMCs. Thus, this novel ‘hu-TReg mouse’ model offers a pre-clinical platform to study the in vivo function and stability of human nTRegs and their ability to modulate autoimmune diseases and GVHD.
Introduction
Naturally arising T regulatory cells (nTRegs) which originate in the thymus are a subset of CD4+ T cells, which are critical both for suppressing autoreactive lymphocyte responses and for preventing exaggerated antigen-specific immune responses. Their importance is clearly illustrated by lethal systemic autoimmunity and lymphoproliferative disease observed in humans with mutated forkhead box P3 transcription factor (Foxp3) gene and in Foxp3-deficient mice [1], [2], [3]. nTRegs are characterized by the co-expression of Foxp3 and interleukin-2Rα chain CD25. Another distinguishing feature is their dependence on exogenous interleukin-2 (IL-2) for growth and function [4]. With increased understanding of TReg biology and function, there has been a surge of interest in developing TReg-based cellular therapy for a variety of immunological diseases in humans, most notably to prevent graft rejection and reduce the severity of graft versus host disease (GVHD), a frequent and often severe complication following allogenic hematopoietic cell transplantation. The major limitation for TReg-based immunotherapy is their low number in peripheral blood, which makes it necessary to develop a robust method for large-scale expansion in vitro. The other major challenge is to ensure that in vitro expanded TRegs are not contaminated with conventional T cells that could potentially exacerbate inflammatory response in the transplantation setting. We and others have shown earlier that purified nTRegs can be expanded in vitro to clinically relevant numbers without loss of the signature CD25+Foxp3+ expression. Using anti-CD3/CD28 expander dynabeads and IL-2 in presence of rapamycin, we were able to achieve hundred-fold expansion of nTRegs that retained their phenotype and suppressive function with no evidence of conversion to inflammatory effector or Th17 T cells [5].
Successful use of human TRegs to suppress GVHD and graft rejection has recently been reported in humanized mouse models. Infusion of in vitro expanded human TRegs together with PBMCs could significantly reduce GVHD in NOD/SCID and NOD-SCID IL2rγcnull mice [6], [7]. Further, it was shown recently that expanded TRegs are effective in abrogating the development of transplant arteriosclerosis (TA) in a humanized mouse model [8]. Another study demonstrated the utility of cultured TRegs in preventing allograft rejection in a human skin graft model in BALB/c Rag IL2rγcnull mice [9]. Despite these encouraging results, translation to efficacy in humans still remains uncertain. Success in using in vitro expanded TRegs for immunosuppressive therapy in humans will depend on their capacity to survive, retain their phenotype, migrate appropriately and exert stable suppressive activity when introduced into the transplant recipient. Currently, there is no suitable model system to investigate the fate and function of human nTRegs in vivo. A preclinical model in which expanded human nTReg reconstitution, trafficking, stability and function can be studied systematically will allow in depth studies of their in vivo behavior and set the stage for testing novel approaches to manipulate the cells for more optimal therapeutic results. In this report, we show that in vitro expanded human nTRegs can be reconstituted in NOD-SCID IL2rγcnull mice by inducing the expression of IL-2 in vivo via hydrodynamic injection of hIL-2 expressing plasmid. Moreover, the reconstituted TRegs retained their characteristic phenotype as well as suppressive function and were able to traffic to various organs including liver, spleen and lungs. Finally, these in vivo reconstituted TRegs were capable of preventing severe xenogenic response of human PBMCs in an IL-2 accelerated GVHD model.
Results
Human IL-2 Expression by Hydrodynamic Injection of IL-2 Encoding Plasmid DNA Allows in vivo Expansion of Infused TRegs in NOD-SCID IL2rγcnull Mice
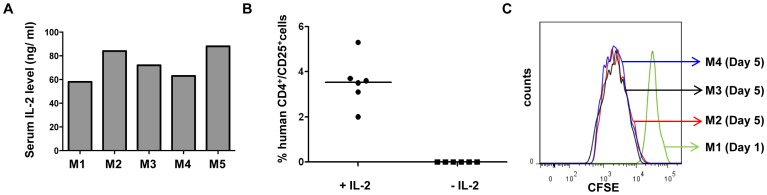
IL-2 signaling is required for both thymic development and peripheral expansion/maintenance of TRegs [4]. TRegs themselves do not produce this cytokine, so they have an obligatory requirement for IL-2 produced by other cells [4]. Large-scale in vitro expansion of polyclonal nTRegs after stimulation with CD3/CD28 antibodies is also dependent on provision of exogenous IL-2. Thus, although the lymphopenic environment in NOD-SCID IL2rγcnull mice is considered conducive to homeostatic expansion of transferred human T cells, unlike conventional T cells, TRegs are unlikely to engraft in the absence of an exogenous source of IL-2. The cytokine could be provided by administration of recombinant IL-2. However, the in vivo half life of the cytokine is in the range of minutes which would necessitate repeated injections for sustained action [10]. On the other hand, hydrodynamic delivery of plasmids expressing cytokine genes has been recently shown to be a simple, efficient and inexpensive method to express human cytokines in mice [11]. We injected hIL-2 expressing plasmid into these mice by hydrodynamic injection one day prior to nTRegs infusion. In vivo expression of IL-2 was confirmed by measuring cytokine levels in the serum by ELISA after 24 hr ( Figure 1A ).
Figure 1. hIL-2 is required for in vivo reconstitution of nTRegs in NOD-scid IL2rγcnull mice.
(A) NOD-scid IL2rγcnull mice were hydrodymanically injected with human IL2 encoding plasmid and after 24h, their sera were tested for hIL2 by ELISA. (B) hIL-2 expressing or control NOD-scid IL2rγcnull mice were iv injected with 5×106 in vitro expanded nTRegs. On day 12 MNCs from spleen were analyzed for presence of human CD4+ CD25+ cells on human CD45 gated population. Each symbol represents an individual mouse and horizontal bars indicate the mean values. (C) 5×106 CFSE labeled TRegs were iv injected into hIL-2 expressing NOD-scid IL2rγcnull mice and spleen cells obtained 1 (M1) or 5 days (M2–M4) later were analyzed for CFSE dilution on human CD4 gated population.
As shown in our previous study, nTRegs expanded in culture with CD3/CD28 stimulation in presence of IL-2 and rapamycin maintained their phenotypic and functional integrity (Figure S1A, B). To test reconstitution of the cells in vivo, we injected 5×106 expanded nTRegs into NOD-SCID IL2rγcnull mice in the presence or absence of hIL-2. The animals were sacrificed on day 12 and analyzed for reconstituted human cells in the spleen. MNCs isolated from spleen were stained with anti-human CD45, CD4 and CD25 antibodies. Our results show that human cells identified by expression of the three markers were present only in animals expressing human IL-2 (Figure 1B). The results demonstrate that provision of the cytokine from an exogenous source is essential for TReg reconstitution in NOD-SCID IL2rγcnull mice.
Next, we asked whether repopulation reflected just IL-2-dependent survival of the infused TRegs or their further expansion in vivo. For this, we labeled in vitro expanded nTRegs with CFSE dye just before injecting them into IL-2 expressing animals. The animals were sacrificed at day 1 or day 5 and cells isolated from spleen were assessed for CFSE dilution by flow cytometry. As shown in the Figure 1C, there was a substantial dilution of the dye on day 5 as compared to day 1, suggesting that the parent cells underwent cell division in vivo.
TRegs Maintain Stable Phenotype and Trafficking Capabilities after in vivo Reconstitution
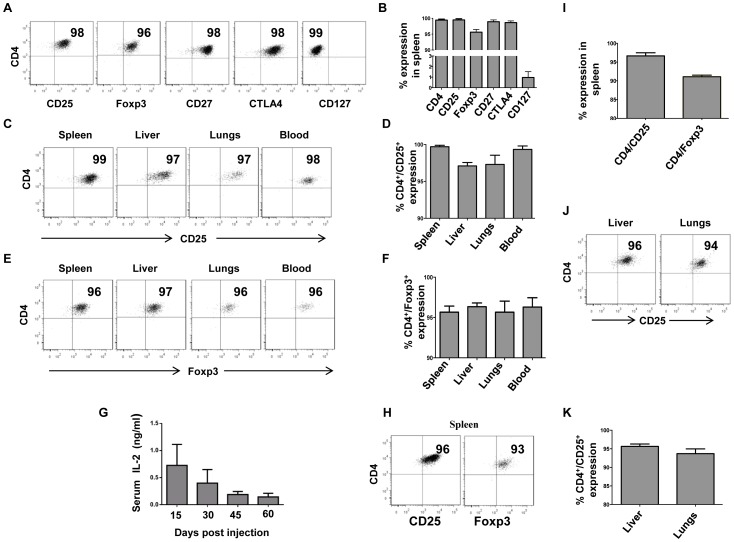
To determine whether expanded nTRegs maintain their phenotypic characteristics after in vivo reconstitution, we assessed the expression of multiple TReg-associated markers, including CD4, CD25, Foxp3, CD127, CTLA4 and CD27 on human cells isolated from the spleens12 days after reconstitution. We found the expression pattern of these markers to be identical to the parent nTReg culture that was infused into the animals (Figure 2A, B and Figure S1A respectively).
Figure 2. Reconstituted TRegs retain their phenotypic characteristics and traffic to different organs of NOD-scid IL2rγcnull mice.
(A, B) 5×106 expanded nTRegs were iv injected into NOD-scid IL2rγcnull mice in the presence hIL-2. On Day 12, MNCs were isolated from the spleen and surface stained for CD4, CD25, CD27, CD45 and CD127 and then intracellularly stained for CTLA4 and Foxp3. Representative flow cytometric analysis of human CD45 gated population from one mouse (A) and composite data (B) from all the three animals tested are shown. (C, D, E, F) MNCs from indicated organs were tested for TReg reconstitution by examining for CD4 and CD25 (C, D) and Foxp3 (E, F) expression. Representative results from one mouse (C, E) and composite data (D, F) from all the three animals analyzed are shown. (G) Serum IL-2 levels at different time points after a single hydrodynamic injection of the plasmid were tested by ELISA. (H, I, J, K) 33 days after TReg reconstitution, MNCs purified from spleen, liver and lungs were analyzed for CD4, CD25 and Foxp3 expression on human CD45+ gated cells. Representative results from one mouse (H, J) and composite data (I, K) from all animals tested are shown. The numbers in the panel depict percentage of positive cells within human CD45 gated populations and error bars indicate standard deviation of three different animals tested.
The usefulness of TReg therapy would also rely on their ability to efficiently traffic to different organs where their suppressive activity may be required. Thus in addition to spleen, we also evaluated liver, lungs and blood for the presence of transferred human TRegs 12 days after reconstitution. TRegs reconstitution was analyzed as before, by staining the cells with anti-human CD45, CD4 and CD25 antibodies. Our results show that, the reconstituted TRegs were present in spleen, liver, lungs and blood (Figure 2C, D). We also confirmed that these cells continue to express Foxp3 in different organs (Figure 2E, F). These data demonstrate that the reconstituted TRegs have the capability to traffic to different organs of the body to exert their regulatory activity.
To determine how long IL-2 from a single hydrodynamic plasmid injection could sustain TReg reconstitution, we first measured IL-2 levels at various time points after plasmid injection. As shown in the Figure 2G, IL-2 could be detected in the serum for up to 60 days, although the levels declined somewhat at later time points. Correspondingly, human TReg reconstitution was sustained in the spleen, liver and lungs for up to 33 days when the experiment was terminated and these cells continued to express Foxp3 (Figure 2H–K).
In vivo Reconstituted TRegs do not Convert to Th17 Cells and Retain their Characteristic Suppressive Function
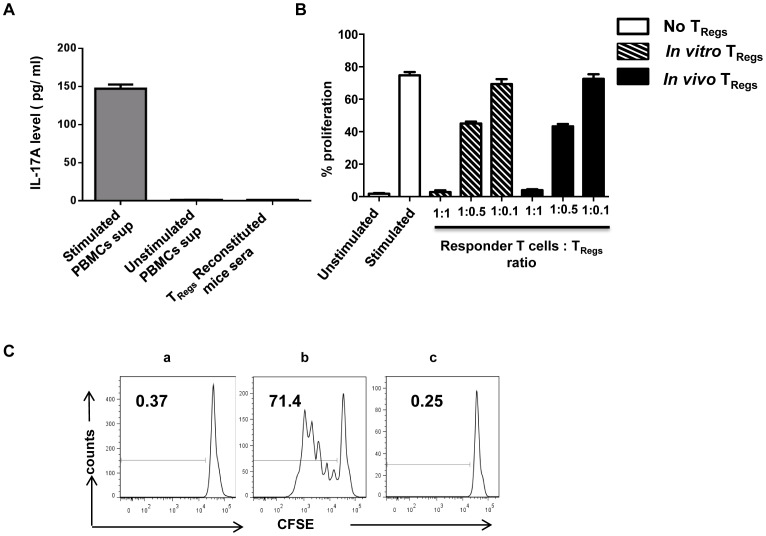
In vitro studies have shown that human TRegs can be reprogrammed to IL-17A secreting Th17 cells in the presence of IL-2 and Th17 polarizing conditions [12]. It was also shown that culture of ovarian cancer associated TRegs in presence of IL-2 resulted in their conversion to Th17 cells [13]. Based on these results, we also evaluated the possibility of in vitro expanded TRegs converting to Th17 cells after in vivo reconstitution in the presence of IL-2. As shown in the Figure 3A, we did not find detectable levels of IL-17A in the serum obtained 12 days after nTReg reconstitution, which rules out their possible conversion to inflammatory Th17 cells under the influence of IL-2 in vivo. Moreover, there was no detectable level of IL-17A even in serum obtained 33 days post nTReg reconstitution (data not shown).
Figure 3. In vivo expanded TRegs do not convert to Th17 cells and retain their characteristic suppressive function.
(A) IL-17A levels in the sera of nTReg reconstituted animals were analyzed by ELISA. Error bars indicate standard deviation for six different animals tested. Supernatants from normal human PBMCs stimulated with PMA (50 ng/ml) and ionomycin (1 µg/ml) served as positive control. (B) TRegs obtained from reconstituted mice and in vitro expanded TRegs were tested for their ability to suppress proliferation of autologous CD4+CD25− T cells at different TReg to CD4+CD25− responder T cell ratios. Responder cells were stimulated with anti-CD3/CD28 beads and CFSE dilution was analyzed after 5 days of culture under the following conditions: autologous T cells cultured in medium without stimulation (unstimulated), stimulated with anti-CD3/anti-CD28 coated micro beads in the absence of TRegs (stimulated), in the presence of cultured TRegs (in vitro TRegs) or in the presence of TRegs isolated from reconstituted mice 12 days after reconstitution (In vivo TRegs). Composite data from all the three animals tested are shown. (C) The functional stability of TRegs obtained 33 days after in vivo reconstitution was also analyzed by CFSE dilution of CD4+CD25− responder cells. Representative data from one of two animals tested is shown. Data depicts CD4+CD25− responder cells cultured with medium alone (a), stimulated in the absence of in vivo TRegs (b) and stimulated in the presence of in vivo TRegs (c).
The ability of transfused TRegs to retain their suppressive function in vivo would be the ultimate determinant for their usefulness as cellular therapy. To evaluate suppressive function of in vivo reconstituted TRegs, human T cells were purified from spleen harvested from animals on day 12 after reconstitution of human nTRegs. The isolated cells were tested for their ability to inhibit the proliferative response of autologous CD4+CD25− responder cells to CD3/CD28 stimulation, as this is used as the classical assay to assess suppression mediated by TRegs [14]. Autologous CD4+CD25− responder T cells were labeled with CFSE dye and mixed with TRegs isolated from in vivo reconstituted mice or in vitro cultured TRegs at a 1∶1 ratio, stimulated with CD3/CD28 and cultured for 5 days. Proliferation as determined by CFSE dilution with cell division was analyzed on day 5. As shown in Figure 3B, in vivo expanded TRegs retained potent suppressive activity as they were able to inhibit the proliferation of the responder CD4+CD25− T cells as efficiently as the parental nTRegs cells used for infusion. Furthermore, the suppressive capability of TRegs remained intact up to 33 days post transfer (Figure 3C).
In vivo Expression of IL-2 Accelerates Xenogenic GVHD
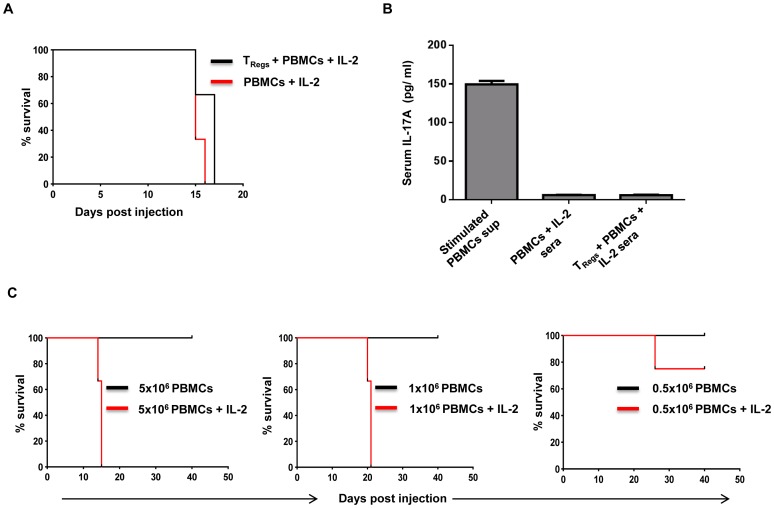
IL-2 therapy in vivo has been suggested to favor immunosuppression by TRegs rather than immune enhancement of effector T cells. Based on this premise, we evaluated the ability of NOD-SCID IL2rγcnull mice reconstituted with nTRegs in the presence of IL-2 to resist GVHD induced by injected human PBMCs. After hydrodynamic injection with plasmid expressing hIL-2, animals were either reconstituted with 5×106 nTRegs as described earlier or not reconstituted to be used as controls. After 10 days, both groups were injected with 5×106 allogenic human PBMCs by the iv route and observed for symptoms of GVHD over time. Contrary to expectations, animals with reconstituted TRegs were not protected. In fact, compared to what has been reported for xenogenic GVHD by human cells in the absence of exogenous IL-2, here we observed an accelerated course of GVHD in both groups. Previous studies have reported that NOD-SCID IL2rγcnull mice reconstituted with human PBMCs without IL-2 remain free of GVHD symptoms for nearly 30 days after engraftment even with the transfer of up to 20×106 PBMCs [15] whereas, here with just 5×106 cells, all of the animals succumbed to the disease by 15–17 days ( Figure 4A). No IL-17A was detected in the sera of animals injected with PBMCs with or without prior TRegs engraftment (Figure 4B), ruling out the possibility that the lack of protection was because of conversion of the in vivo expanded n TRegs to Th17 cells in the GVHD setting.
Figure 4. In vivo expression of hIL-2 accelerates the induction of xenogenic GVHD in the presence or absence of Treg reconstitution.
(A) Kaplan-Meir survival curve comparing IL-2 conditioned NOD-scid IL2rγcnull mice that received 5×106 hPBMCs with or without prior reconstitution with 5×106 TRegs 12 days before injection with PBMCs. (B) IL-17A levels in the sera collected from the animals at the time of euthanasia. Error bars indicate standard deviation for three different animals tested. Supernatants from normal human PBMCs stimulated with PMA (50 ng/ml) and ionomycin (1 µg/ml) was used as positive control. (C) Kaplan-Meir analysis for survival of NOD-scid IL2rγcnull mice injected with graded doses of human PBMCs in presence of IL-2. Control and IL-2 conditioned animals were injected with different doses of hPBMCs (5×106, 1×106 and 0.5×106) and observed for GVHD symptoms. Each group consisted of 3 mice.
We then determined the effect of human IL-2 in the initiation and progression of GVHD in NOD-SCID IL2rγcnull mice at different doses of PBMCs. Animals were injected with 5×106, 1×106 and 0.5×106 PBMCs in the presence or absence of human IL-2. As shown in Figure 4C, the presence of IL-2 dramatically altered the course of the disease, with all the animals developing GVHD with 5×106 PBMCs and 1×106 PBMCs by 15–16 and 21–23 days respectively. Even with 0.5×106 PBMCs 25% of the animals developed GVHD by day 30. None of the animals reconstituted in the absence of IL-2 developed signs and symptoms of GVHD until the experiment was terminated at day 40.
Co-injection of in vivo Expanded TRegs with Human PBMCs at 1∶1 Ratio Protects NOD-SCID IL2rγcnull Mice from IL-2 Accelerated GVHD
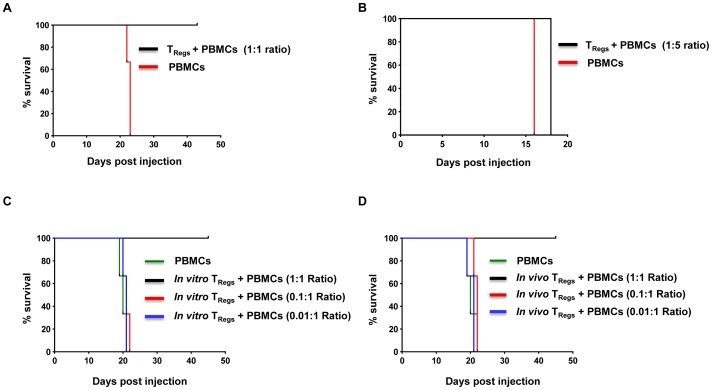
The inability of circulating TRegs in reconstituted animals to prevent IL-2 accelerated GVHD symptoms could be due to insufficient TReg numbers as TRegs are known to reliably silence GVHD in murine models only when used at the optimal dose [16]. To test the possibility that the lack of protection we observed in the IL-2 accelerated GVHD model was due to insufficient number of TRegs and not due to dysfunction of the in vivo expanded cells, we first tested protection from GVHD induced by transfer of 1×106 PBMCs in animals reconstituted with 5×106 TRegs 10 days earlier. However, here again, the control as well as the test group of animals succumbed to the disease (data not shown). To understand whether this implied TRegs dysfunction in vivo, we tested the efficacy of reconstituted TRegs in a co-transfer experiment, in which 1×106 PBMCs obtained from a normal donor were adoptively transferred iv into new IL-2 conditioned animals along with TRegs isolated from the spleens of mice 12 days after reconstitution at a 1∶1 or 1∶5 (TRegs: PBMCs) ratio. As only 0.2–0.4 million TRegs could be recovered from the spleen of each reconstituted animal, the cells were pooled from multiple animals to achieve the requisite numbers for co-transfer. As shown in Figure 5A and 5B, all the control animals receiving PBMCs only or those co-injected with TRegs at a 1∶5 (TReg: PBMCs) ratio developed severe symptoms and had to be euthanized by around 16–21 days, whereas animals co-injected with TRegs at a 1∶1 ratio were protected until day 45, when the experiment was terminated. The spleen and liver of diseased animals showed gross enlargement and flow cytometric analysis of cells isolated from the organs showed large-scale expansion of human CD4+ and CD8+ T cells. On the other hand in protected animals, the organs appeared to be of normal size and showed very low numbers of human cells (data not shown).
Figure 5. In vivo expanded TRegs co-transplanted with hPBMCs are capable of preventing IL-2 accelerated GVHD in NOD-scid IL2rγcnull mice.
(A, B) Kaplan-Meir curve showing the survival of IL-2 conditioned NOD-scid IL2rγcnull mice injected with hPBMCs alone or hPBMCs along with in vivo expanded TRegs. TRegs purified from animals 12 days after reconstitution were used for co-transfer along with hPBMCs at 1∶1 (A) or 1∶5 (B) ratio of TRegs:PBMCs and the animals were observed for GVHD symptoms. (C, D) Kaplan-Meir curve comparing protection from GVHD conferred by in vitro and in vivo expanded TRegs. IL-2 conditioned NOD-scid IL2rγcnull mice were injected with hPBMCs only or co-transferred with different ratios of in vitro (C) or in vivo (D) expanded TRegs and hPBMCs. In all experiments, each group consisted of 3 mice.
We also directly compared the in vivo potency of reconstituted TRegs with the cultured parent n TRegs in a separate experiment. Human PBMCs were transferred into IL-2 conditioned animals in the presence of various ratios of in vitro or in vivo expanded TReg (1∶0.01, 1∶0.1 and 1∶1 PBMC: TReg ratio). As shown in Figures 5C and 5D, in both groups, animals that received lower numbers of TRegs succumbed to GVHD, whereas animals co-injected with TRegs at 1∶1 ratio were protected until day 45, when the experiment was terminated. The equivalent protection observed with both populations also makes it unlikely that there was any significant conversion of TRegs to proinflammatory cells in presence of IL-2 in vivo either at the first reconstitution or after adoptive transfer into fresh animals. Further, the lack of protection at the lower TReg:PBMC ratio suggests that optimal numbers of TRegs are required for effective protection. As the number of in vivo expanded TRegs required for protection could only be achieved by pooling cells from multiple animals, the limited expansion of the cells in vivo may be a major factor for their inability to protect in the non co-transfer setting. The other possibility is that to exert their suppressive function, TRegs require cell: cell contact which is likely to be limiting when PBMCs are injected separately into TRegs reconstituted animals.
Protection from IL-2 accelerated GVHD at the higher 1∶1 (TReg: PBMC) ratio could also imply that the TRegs are preferentially using up all of the IL-2 produced in vivo. To rule out this possibility, in a separate experiment we also determined whether the same number of TRegs (1×106) could prevent GVHD induction when co-transferred at a 1∶5 ratio with 5×106 PBMCs. In this setting, all of the animals exhibited GVHD symptoms by around 16–18 days (data not shown), suggesting that there is enough of the cytokine to support the massive expansion of large numbers (5×106) of xenogenic PBMCs despite the presence of TRegs injected alongside.
Discussion
Induction of tolerance to allogeneic donor grafts is a clinically desirable goal in bone marrow and solid organ transplantation. As TRegs play a major role in tolerance induction, infusion of in vitro expanded TRegs is being developed as a novel therapeutic strategy to reduce the severity of GVHD and autoimmune diseases [17], [18]. Generating sufficient numbers of TRegs is no longer a constraint for their therapeutic use as methods have been developed for in vitro expansion of enriched TRegs without the loss of suppressive function [5], [7]. However, the lack of a preclinical animal model has been an impediment for gaining an improved understanding of their stability, trafficking capability and ability to expand in vivo without conversion to proinflammatory cells which are important issues for TReg therapy to gain acceptance in the clinic.
In this study we show that expanded human nTRegs can be reconstituted in immunodeficient NOD-scid IL2rγcnull mice by providing a source of IL-2 to sustain their survival and expansion. We were able to achieve long-term human TReg reconstitution with just a single hydrodynamic injection of hu-IL2 expressing plasmid. Human TRegs recovered from the animals retained the signature TReg-specific phenotypic markers. Further, repopulation of TRegs could be observed in multiple organs, including liver and lungs. More importantly, in vivo reconstituted TRegs remained functionally intact and they could potently inhibit GVHD symptoms and prevent lethality in a new IL-2 accelerated xenogenic GVHD model.
IL-2/IL-2R pathway is known to play a critical role in peripheral maintenance and fitness of TRegs [4]. Thus it is not surprising that in the absence of exogenous IL-2, we observed no TReg repopulation whatsoever, despite the lymphopenic milieu of NOD-scid IL2rγcnull mice, which should be conducive to homeostatic proliferation of lymphocytes. However, repopulation of human nTRegs in multiple organs was achieved after a single hydrodynamic injection of human IL-2 expressing plasmid. This brings up the question of whether TReg therapy in human transplant settings would also require supplemental IL-2 as the cytokine is likely to be deficient in the lymphopenic environment created by lymphoablation therapy preceding HSC or other transplants. It is possible that unlike the murine milieu, other cytokines like human IL-15 and IL-7 that are secreted by non-T cells may be sufficient to drive the homeostatic proliferation of TRegs to functionally effective numbers after infusions in humans. Our study also shows that although the IL-2 levels decline in the animals over time, the residual levels were sufficient for long-term reconstitution of TRegs that retained efficient suppressive activity.
IL-2 is also a critical cytokine for the amplification of immune effector cells in vivo [19]. Thus it is not surprising that human PBMC transfer into NOD-scid IL2rγcnull mice in the presence of IL-2 resulted in a dramatic acceleration of GVHD with comparatively fewer numbers of cells. In addition, this accelerated disease development was achieved without the need for irradiation of the animals prior to the PBMC transfer. Massive infiltration of lymphocytes was observed in the spleen, lung and liver, reminiscent of the pathophysiology of allogenic GVHD in HLA-mismatched human transplants [20]. Thus, PBMC transfer with provision of a source of IL-2 in vivo led to the development of a highly sensitive and reproducible xenogenic GVHD model, which would be useful for evaluating immunosuppressive therapeutic regimens, including TRegs therapy.
In this IL-2 stimulated accelerated GVHD model, it was clear that amelioration of the disease occurred only when the reconstituted TRegs were adoptively co-transferred with the PBMCs. Although the data shows that the TRegs proliferated in vivo in presence of IL-2 as assessed by CFSE dilution, it is possible that the level did not reach the critical threshold required for protection. The requirement of a threshold level of TRegs for suppressing GVHD was evident in the adoptive transfer setting in which, TRegs were able to suppress GVHD symptoms after co-transfer at a higher 1∶1 but not at the lower 1∶5 (TRegs: PBMCs) ratio. Transfer of equivalent numbers of TRegs and effector cells have also been shown to be necessary for silencing GVHD in murine models [17]. This underscores the requirement for sufficient numbers of TRegs to be infused for reliable therapeutic benefit in human transplant settings. Suboptimal numbers may be the reason for the fairly modest efficacy of TRegs in ameliorating GVHD symptoms in a recent Phase I clinical study that examined the safety and efficacy of ex vivo expanded umbilical cord blood-derived TRegs in 23 patients receiving allogenic cord blood HSC transplantation [21]. Low dose IL-2 supplementation could be considered as a strategy to enhance further TRegs expansion in vivo but this would require a balancing act as the cytokine could also result in the amplification of effector cell proliferation leading to exacerbation of GVHD symptoms and graft rejection. On the positive side, the Phase I/II clinical studies in HSC transplant recipients have shown that TRegs therapy is well tolerated with little or no infusion associated toxicity [21].
Another concern for using TRegs for therapy is the perceived plasticity of the cells, which poses potential risk of their conversion to effector T cells that can themselves play a pathogenic role in GVHD induction or severity. Studies in mouse models have yielded mixed reports on the vulnerability of TRegs to Th17 cell conversion under inflammatory conditions in vivo. Using Foxp3-reporter mouse strain, one group found that adoptively transferred TRegs downregulate their Foxp3 expression and become capable of IL-17 secretion under inflammatory conditions [22] whereas another group reported that adoptively transferred TRegs retained Foxp3 expression for several months under normal conditions [23]. In the presence of IL-2, human ovarian cancer-associated CD4+ regulatory T cells have also been shown to convert into proinflammatory IL17-producing helper T cells in vitro [13]. Hence we examined the possibility of in vitro expanded TRegs converting to Th17 after in vivo transfer. We did not find detectable levels of IL-17 in the serum of animals reconstituted with nTRegs in presence of IL-2. Although other cytokines that are upregulated under proinflammatory conditions may be required for reprogramming different subsets of TRegs to Th17 cells in vivo, this appears unlikely as both cultured and reconstituted population of TRegs could suppress GVHD lethality upon co-transfer with PBMCs at the optimal 1∶1 ratio. This is also borne out by studies showing that nTRegs isolated and expanded in vitro do not convert to Th17 phenotype [5]. Moreover, we did not detect IL-17A even in nTReg reconstituted animals that developed GVHD.
The data we presented here provide evidence for the in vivo engraftment capability of expanded human nTRegs. These findings hold important implications for studying the in vivo function of human nTRegs and allow researchers to develop new methods to improve the in vivo reconstitution of human nTRegs without affecting their suppressive function. Finally, we believe that, this novel hu-TReg mouse model offers a pre-clinical model to study the in vivo function and stability of human nTRegs and their ability to modulate autoimmune diseases and GVHD.
Materials and Methods
Isolation and Expansion of nTRegs
Human nTRegs were purified from buffy coat in two steps as described previously [5]. In the first step CD4+ T cells were enriched by negative selection using a cocktail of nine monoclonal antibodies. In the second step CD25+ cells were isolated by positive selection from purified CD4+ cells using anti-CD25 antibody in a Robosep instrument (Stem cell Technologies, Vancouver, BC, Canada). Isolated nTRegs were expanded in the presence of CD3/CD28 T-cell expander dynabeads (3∶1 ratio), rhIL-2 (1000 U ml−1) and rapamycin (100 ng ml−1) for 19 ± 1 days and at the end of the culture period, the beads were removed and the cells were used for testing phenotype, suppressive function and reconstitution.
Animals
NOD-SCID IL2rγcnull mice were obtained from Jackson Laboratory (Bar Harbor, ME) and maintained in specific pathogen free conditions at the Paul L. Foster School of Medicine, TTUHSC animal facility. All the experiments were done with 5–6 week old mice using study protocols approved by the TTUHSC IRB and IACUC committees.
Hydrodynamic Injection of Plasmids Expressing hIL-2
Human IL-2 gene was PCR amplified from PHA stimulated human PBMCs and cloned into the Adeno associated virus vector (pAAV-IRES-hrGFP) obtained from Agilent Technologies (Santa Clara, CA, USA). Plasmid DNA was purified using the endotoxin free maxi prep-kit from Qiagen (Valencia,CA,USA). For expressing hIL-2 in vivo, NOD-scid IL2rγcnull mice were injected with 70 µg of plasmid using 27-gauge needle in a volume of saline equivalent to 8% of the body mass of the mouse [24]. The total volume was delivered within 5–8 seconds and animals were bled periodically through retro-orbital puncture to measure serum cytokine levels.
TRegs Reconstitution and Isolation of TRegs from Reconstituted Animals
5–6 week old IL-2 expressing and control NOD-scid IL2rγcnull were iv injected with 5–10×106 in vitro expanded nTRegs and animals euthanized on different days and single cell suspensions prepared from blood, spleen, liver and lungs by standard procedures. Human cells were enriched from TRegs reconstituted animals by negative selection with mouse/human chimera enrichment Kit (Stem Cell Technologies) for use in suppression assays and for adoptive transfer experiments.
Antibodies and Flow Cytometry
Various fluorescent conjugated antibodies to human CD4, CD25, CD27, CD45, CD127, CTLA4, PD1 and Foxp3 were from BD Biosciences. Cells were surface stained first using appropriate combinations of mAbs, and either used directly for flow cytometric analyses or further processed for intracellular staining with CTLA4 and Foxp3 antibody. All stained samples were subsequently analyzed on FACS Canto II instrument (BD) using FlowJo software.
TRegs Suppression Assay
1×106 CD4+CD25− (responder) cells were labeled with carboxyfluorescein succinimidyl ester (CFSE, Invitrogen, Carlsbad, CA, USA) at a concentration of 1 µM/1×106 cells for 10 min at 37°C. Labeling was terminated by adding 5 volumes of ice cold RPMI medium containing 10% FBS and incubating on ice for 5 minutes. After 3 washes in complete RPMI medium, cells were cultured alone or with unlabeled in vivo or in vitro expanded nTRegs at various (responder : TRegs) ratio in the presence of anti-CD3/CD28 coated micro beads at a ratio of 50∶1 (responder cells: beads). CD4+CD25− cells in medium alone served as control. After 5 days of culture at 37°C under 5% CO2, the micro beads were removed and cells washed twice before analyzing cell division in various culture conditions. Cells undergoing division were identified by the decrease in CFSE, resulting from dilution of dye with each division.
ELISA for Serum IL-2 and IL-17A
To determine the IL-2 and IL-17A levels in the serum, animals were bled periodically through retro-orbital puncture. IL-2 or IL-17A serum levels were determined using commercial ELISA kits according to manufactures instructions (Biolegend Inc, San Diego, CA, USA).
Induction of Xenogenic GVHD
Human PBMCs were injected through tail vein (0.5–5×106/mouse) into control and hIL-2 expressing animals. In some experiments, PBMCs were mixed with pooled TRegs isolated from spleens of animals reconstituted for 12 days. The animals were monitored daily for symptoms of GVHD, including hunched back, ruffling of hair, diarrhea and weight loss. Animals were sacrificed when they lost 20% body weight.
Supporting Information
Phenotypic and functional characterization of in vitro expanded human nTRegs. (A) CD4+CD25+ cells were expanded in vitro as per protocol and were harvested on day 19 and surface stained for CD4, CD25, CD27, CD127 expression and intracellular staining was carried out for CTLA4 and Foxp3 expression. Data shown are one representative of three independent experiments and numbers indicate the percentage of positive cells. (B) Functional stability of TRegs expanded in vitro as determined by CFSE dilution of CD4+CD25− responder cells cultured with medium alone (a), stimulated in the absence of TRegs (b) and stimulated in the presence of in vitro expanded TRegs (c). One representative result from three independent experiments is shown.
(TIF)
Funding Statement
This work was supported by grants from the National Institutes of Health RO1 AI071882 and R01 AI084795 to Premlata Shankar. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, et al. (2001) X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 27: 18–20. [DOI] [PubMed] [Google Scholar]
- 2. Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, et al. (2001) Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet 27: 68–73. [DOI] [PubMed] [Google Scholar]
- 3. Sakaguchi S, Yamaguchi T, Nomura T, Ono M (2008) Regulatory T cells and immune tolerance. Cell 133: 775–787. [DOI] [PubMed] [Google Scholar]
- 4. Scheffold A, Huhn J, Hofer T (2005) Regulation of CD4+CD25+ regulatory T cell activity: it takes (IL-)two to tango. Eur J Immunol 35: 1336–1341. [DOI] [PubMed] [Google Scholar]
- 5. Pahwa R, Jaggaiahgari S, Pahwa S, Inverardi L, Tzakis A, et al. (2010) Isolation and expansion of human natural T regulatory cells for cellular therapy. J Immunol Methods 363: 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao T, Soto A, Zhou W, Wang W, Eck S, et al. (2009) Ex vivo expanded human CD4+CD25+Foxp3+ regulatory T cells prevent lethal xenogenic graft versus host disease (GVHD). Cell Immunol 258: 65–71. [DOI] [PubMed] [Google Scholar]
- 7. Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, et al. (2011) Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med 3: 83ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nadig SN, Wieckiewicz J, Wu DC, Warnecke G, Zhang W, et al. (2010) In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med 16: 809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Issa F, Hester J, Goto R, Nadig SN, Goodacre TE, et al. (2010) Ex vivo-expanded human regulatory T cells prevent the rejection of skin allografts in a humanized mouse model. Transplantation 90: 1321–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donohue JH, Rosenberg SA (1983) The fate of interleukin-2 after in vivo administration. J Immunol 130: 2203–2208. [PubMed] [Google Scholar]
- 11. Chen Q, Khoury M, Chen J (2009) Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc Natl Acad Sci U S A 106: 21783–21788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, et al. (2008) Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 112: 2340–2352. [DOI] [PubMed] [Google Scholar]
- 13. Leveque L, Deknuydt F, Bioley G, Old LJ, Matsuzaki J, et al. (2009) Interleukin 2-mediated conversion of ovarian cancer-associated CD4+ regulatory T cells into proinflammatory interleukin 17-producing helper T cells. J Immunother 32: 101–108. [DOI] [PubMed] [Google Scholar]
- 14. Brusko TM, Hulme MA, Myhr CB, Haller MJ, Atkinson MA (2007) Assessing the in vitro suppressive capacity of regulatory T cells. Immunol Invest 36: 607–628. [DOI] [PubMed] [Google Scholar]
- 15. King M, Pearson T, Shultz LD, Leif J, Bottino R, et al. (2008) A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin Immunol 126: 303–314. [DOI] [PubMed] [Google Scholar]
- 16. Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, et al. (2003) CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med 9: 1144–1150. [DOI] [PubMed] [Google Scholar]
- 17. Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S (2002) Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med 196: 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bluestone JA, Tang Q, Sedwick CE (2008) T regulatory cells in autoimmune diabetes: past challenges, future prospects. J Clin Immunol 28: 677–684. [DOI] [PubMed] [Google Scholar]
- 19. Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, et al. (2010) Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity 32: 91–103. [DOI] [PubMed] [Google Scholar]
- 20. Ferrara JL, Cooke KR, Pan L, Krenger W (1996) The immunopathophysiology of acute graft-versus-host-disease. Stem Cells 14: 473–489. [DOI] [PubMed] [Google Scholar]
- 21. Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, et al. (2011) Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 117: 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, et al. (2009) Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol 10: 1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, et al. (2010) Stability of the regulatory T cell lineage in vivo. Science 329: 1667–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu F, Song Y, Liu D (1999) Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther 6: 1258–1266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phenotypic and functional characterization of in vitro expanded human nTRegs. (A) CD4+CD25+ cells were expanded in vitro as per protocol and were harvested on day 19 and surface stained for CD4, CD25, CD27, CD127 expression and intracellular staining was carried out for CTLA4 and Foxp3 expression. Data shown are one representative of three independent experiments and numbers indicate the percentage of positive cells. (B) Functional stability of TRegs expanded in vitro as determined by CFSE dilution of CD4+CD25− responder cells cultured with medium alone (a), stimulated in the absence of TRegs (b) and stimulated in the presence of in vitro expanded TRegs (c). One representative result from three independent experiments is shown.
(TIF)