Abstract
Uterine fibroids are common reproductive-age benign tumors that contribute to severe morbidity and infertility. Cumulative incidence is 4 times higher in Africian-Americans compared to Caucasians and constitutes a major health disparity challenge. Fibroids are the leading indication for hysterectomy and their management averages $21 billion annually in the US. No long term minimally invasive therapies exist. Thus, promising drug therapies, their chemistry, pharmacology, and clinical efficacy, focusing first on innovative drug delivery approaches, are reviewed.
Introduction
Uterine fibroids are the most common benign pelvic tumor in women with a 70–80% cumulative incidence during childbearing years. African-Americans develop fibroids at younger ages than Caucasians and they tend to persist to menopause. [1] Fibroids tend to regress in size before menopause in Caucasian women. The etiology remains elusive although progress has been made. Previously reported similarities between fibroids and keloids [2; 3] corroborate with recent findings.[4] Fibroids cells secret high levels of collagen and resist apoptosis. Ranging in location and size, growth is influenced by female gonadal steroids by apocrine and paracrine mechanisms. Subserosal or intramural fibroids can negatively impact fertility,[5] and all fibroids are the leading cause of hysterectomy. Two Food and Drug Administration approved alternatives to hysterectomy are uterine artery embolization (UAE) and magnetic resonance–guided focused ultrasound surgery (MRgFUS).[6] UAE is an angiographic technique that treats the whole uterus by causing ischemic fibroid necrosis. This therapy, like hysterectomy, is considered a standard treatment for women with no desire for future fertility. Alternatively, MRgFUS provides noninvasive fibroid-specific therapy utilizing high-intensity ultrasonography through the abdominal wall to cause coagulative necrosis in specific fibroids. Guidance and thermal monitoring is provided by dynamic real-time magnetic resonance imaging. However, fibroid regrowth after treatment in some cases may necessitate follow up therapies for both UAE and MRgFUS. Current studies are evaluating the long term outcomes of these procedures. Thus, fibroids are a major public health challenge.[7] US treatment costs (~$21 billion annually) contribute more to healthcare expenditures than breast, colon or ovarian cancer.[8] As researchers develop next generation therapies, innovative approaches to drug delivery should be considered.
Drug Therapies for Uterine Fibroids
Treatment options for fibroids vary — severity of the symptoms, size and location of the fibroid lesions, the patient’s desire to maintain fertility— with the ultimate goal of therapy being relief of the symptoms. As we learn more about the impact of fibroids on fertility, it becomes important for patients and their physicians to have a toolbox of therapeutic options with viable drug therapies being one of those tools. To this end, promising drug therapies, their chemistry, mechanism of action, pharmacology, clinical efficacy and side effects, focusing first on innovative drug delivery approaches, will be highlighted.
Potential of Novel Therapies Enabled by Smart Nanocarriers
The development of nanocarriers designed to deliver and protect drug therapeutics (e.g. anti-fibrotic, aromatase inhibitors, progestins, etc) is an emerging field. Advances in guided-ultrasound technology (e.g. human in vitro fertilization where oocytes as small as 3–5 mm are manipulated)[9] make it feasible to envision utilizing nanocarriers to create a drug depot inside the fibroid by local injection. Thus, skilled physicians could inject the therapy into the uterine fibroid under guided-ultrasound in an outpatient setting. This approach would impede diffusion and distribution of the drug away from the injected fibroid, prolong release, delay inactivation, and therefore reduce the need for repeat injections. Examples of the most promising thermoresponsive delivery systems are given below.
Atrigel®
Atrigel® comprises a water-insoluble biodegradable polymer (e.g., poly(lactic-coglycolic acid, PLGA) dissolved in a biocompatible, water-miscible organic solvent (e.g., N-methyl-2-pyrrolidone, NMP). A drug is added, forming a solution or suspension. Both the PLGA molecular weight and lactide:glycolide molar ratio governs drug delivery. Leuprolide acetate was incorporated into Atrigel® and depending on the targeted duration of leuprolide delivery, the L:G ratio in the PLGA varied from 50:50 to 85:15 and the polymer concentration varied from 34 to 50%. Clinical studies demonstrated a 22.5 mg leuprolide depot maintained an effective suppression of serum testosterone (50 ng/dL) for more than 3 months.[10]
ReGel®
ReGel® is a ~4,000 Da triblock copolymer formed from PLGA and polyethylene glycol (PEG, 1000 Da or 1450 Da) in repetitions of PLGA-PEG-PLGA or PEG-PLGA-PEG. ReGel® is formulated as a 23 wt% copolymer solution in aqueous media. A drug is added to the solution and upon temperature elevation to 37 °C the whole system gels. Degradation of ReGel® to final products of lactic acid, glycolic acid and PEG occurs over 1–6 weeks depending on copolymer molar composition. Chemically distinct drugs like porcine growth hormone and glucagon-like peptide-1 (GLP-1) may be incorporated, one at a time, and released from ReGel®.[10]
LiquoGel™
We’ve engineered LiquoGel to work by mechanistically independent drug delivery routes: entrapment and covalent linkage.[11] This later feature distinguishes LiquoGel from other thermoresponsive injectables, as in theory, two or more drugs can be delivered to the tumor site. LiquoGel is a tetrameric copolymer of thermogelling N-isopropylacrylamide; biodegrading macromer of poly(lactic acid) and 2-hydroxyethyl methacrylate; hydrophilic acrylic acid (to maintain solubility of decomposition products); and multifunctional hyperbranched polyglycerol to covalently attach drugs. LiquoGel is formulated as a 16.9 wt% copolymer solution in aqueous media. The solution gels at physiological conditions and degrades to release drug contents within 1–6 days.
GnRHa Agonists and Antagonists
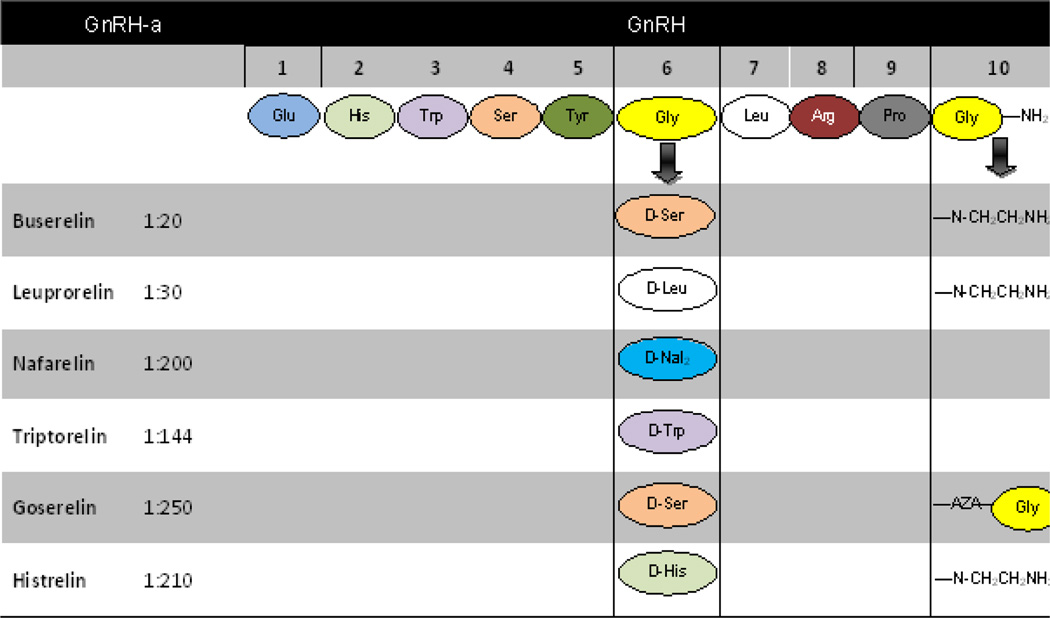
Gonadotrophin-releasing hormone analogues (GnRHa) target reduced production or hinderance of estrogen action on the smooth muscle cells of the uterus. Pituitary down-regulation suppresses the reproductive endocrine axis and can be exploited therapeutically to reduce circulating sex steroid levels. On the basis of this mechanism, several GnRH agonist peptides are commercially available in long-acting injectable depot formulations to suppress estrogen and thus create a menopause-like state. The agonistic response is produced by D-amino acid alterations in position 6 and /or ethyl-amide substitutions for carboxyl-terminal Gly10-amide (see Figure 1). These analogues are more resistant to proteolysis and increase GnRH receptor binding affinity in the circulation, thus increasing the half-life of the analogues. As an example, triptorelin is used prior to surgery via injection for fibroid removal and, more recently, for preventative measures against permanent ovarian failure associated with chemotherapy.[12]
Figure 1.
GnRH Agonist Structures. Modifications at positions 6 and 10 are the basis for development of variety of GnRHa.
In contrast, antagonists compete with endogenous GnRH for pituitary binding sites. Antagonistic analogues are formed by alterations in position 2 and/or 3.The first generation analogs were hydrophilic, and contained replacements for His at position 2 and for Trp at position 3. Third generation antagonists including cetrorelix and ganirelix were developed to eliminate anaphylactic reactions. One of the major remaining limitations to the wide use of the GnRH antagonists in leiomyoma treatment is the short half-life of these agents and the non-availability of depot formulations (necessitating repetitive dosing).[13]Depot preparations of the next generation peptide antagonists degarelix and ozarelix are currently in late stage clinical development.
GnRHa treatment results in marked diminution of fibroid volume.[14] One mechanism of fibroid shrinkage by pharmacological concentrations of GnRHa was provided recently when decreased expression of nuclear factor of activated T cells 5 (NFAT5), a hyperosmolarity gene expressed higher in fibroid cells than myometrial cells, was noted.[15] This basal level expression is increased under hyperosmolar conditions, thus supporting the view that water flows out of fibroid cells at pharmacologic doses. GnRH agonists are widely used to prevent bleeding in preparation for surgical procedures in women with symptomatic fibroids. Long-term use increases risks of side effects including osteoporosis, vaginal dryness, impotence, reduced breast size, emotional instability, depression, hair loss, and musculoskeletal stiffness.[7] These side effects lessen with combinations of GnRHa and “add back” therapies. Additionally, when GnRH agonist treatment by monthly depots is suspended, menses return after 8–12 weeks leading to a rapid increase of the uterus and fibroid size.
Aromatase Inhibitors as a Therapetuic Drug Strategy
Aromatase inhibitors (AIs) offer reversibility, total blockage of aromatase (CYP19), and possibly reduced side effects. AI medicines are often categorized as steroidal or non-steroidal aromatase inhibitors (NSAIs) based on their structural similarity with steroids, or as 1st and 2nd generations based on their evolution in time. Third generation AIs such as letrozole, anastrozole, and exemestande are used clinically. The half-lives are 48 h, 72 h, and 27 h respectively. Active research is ongoing to develop NSAIs that are more potent, selective and even multipotent.[16] Aromatase inhibitors are generally well tolerated with a low incidence of serious short-term adverse effects. Long-term use with the consequent hypo-estrogenaemia could result in loss of bone mineralization and an increased fracture risk
The vast majority of women presenting with fibroid disease who would benefit from medical therapy are premenopausal, and aromatase inhibitors are unlikely to be effective. It should be noted, however, that in the obese menopausal woman presenting with fibroids, AIs may be preferable to progestin therapy as the latter has the potential to exacerbate the potential lipid disorder in the obese, often hypertensive woman.
Anastrozole and Letrozole
This drug is associated with a reduction in fibroid size, thinning of endometrium and cessation of bleeding. Anastrozole has a half-life of ~48 h and is effective with daily oral administration. Mild side-effects of hot flashes, vaginal dryness and musculoskeletal pain are reported. The LEAP (letrozole, exemestane, and anastrozole pharmacodynamics) trial was a phase I pharmacodynamic study comparing the effects of these three AIs on safety parameters such as serum markers of bone formation and resorption, in healthy postmenopausal women with normal bone mineral density. The results demonstrated that all three inhibitors administered for 24 weeks caused incremental increases in bone resorption markers such as C-telopeptide crosslinks.
Selective Estrogen Receptor Modulators (SERMs)
SERMs, like estrogen, are agents that elicit tissue-specific responses by intensely interacting with two kinds of estrogen receptors (ERs), ERα and ERβ, inhomogenously distributed throughout the body. SERMs have complex pharmacokinetics properties due to their vaguely understood physicochemical properties and low solubility in blood (1–200 ng/ml). In addition to analytical detection limitations at such low concentrations, several of the compounds have sufficiently long half lives that impede protocol development. Since SERM are not administered to humans intravenously, the exact bioavailability of any of these drugs has not been evaluated properly. Frequently, differences in SERM activity depend upon the target gene promoter, as well as the background of a desired cell or tissue.
SERMs are characterized by their diverse range of agonist/antagonist actions on ER-mediated processes. SERMs belong to several different chemical classes such as benzopyran, benzothiophenes, chromane, indoles, naphtalenes, and triphenylethylenes compounds, all of which are not steroidal compounds. Many are available for clinical usage including raloxifene and tamoxifen discussed below. Novel SERMs are currently being tested in clinical trials such as LY353381 (arzoxifene), EM-652 and CP 336,156 and their structures are very similar to known SERMs.[17] The true breakthrough for SERMs could occur when ERα and ERβ are modulated independently with receptor-specific agents.
Raloxifene and Tamoxifen
Two of the best characterized SERMs are tamoxifen and raloxifene, which are both considered to act predominantly as estrogen antagonists, blocking the effects of estrogens. Both raloxifene and 4-hydroxytamoxifen (a tamoxifen metabolite) fit into the hydrophobic pocket of the ligand binding domain of ER but the antiestrogen side chain prevents reorientation of helix 12 that must seal the ligand into the receptor before co-activators bind and produce a transciprtion complex. Both drugs interact through phenolic hydroxyls with Glu353 and Arg394 to correctly position the ligands in the binding domain. However, in the case of raloxifene, the side chain (critical for antiestrogenic activity) interacts with Asp351. This same interaction is very weak in the case of 4-hydroxytamoxifen.
Raloxifene is a more complete uterine antagonist than tamoxifen, significantly reducing fibroid size in postmenopausal women yet is less efficacious at reducing tumor volume in premenopausal women.[18] Clinical outcomes in premenopausal women treated with raloxifene suggest that this compound, like tamoxifen, can affect the ovaries via the HPO axis. Tamoxefin is associated with insidious side effects, such as thromboembolic events, vasomotor symptoms and an increased risk of developing endometrial cancer and cataracts.
Progesterone Receptor Agonists and Modulators
The apparent, albeit unclear, role of progesterone (P4) in the growth of fibroids has encouraged the development of synthetic progesterone ligands with either progesterone receptor (PR) agonist (progestins) or mixed agonist/antagonist activity.[19] It has been shown that P4 and PR complexes reduce apoptosis and promote proliferation of fibroid cells.[20]PRs primarly exist in two isoforms, hPR-A and hPR-B. Both isoforms are acquired from the same gene by action of two different promoters.[21] Another PR isoform, PR-M has nongenomic activity and thought to participate in cellular respiration and protection from apoptosis. Recently, an increase in PR-M expression and mitochondria numbers was reported in fibroid edge compared to myometrium. Thus, a non-genomic progestin –induced increase in cellular respiration may be a major factor in the growth of fibroids.[22]
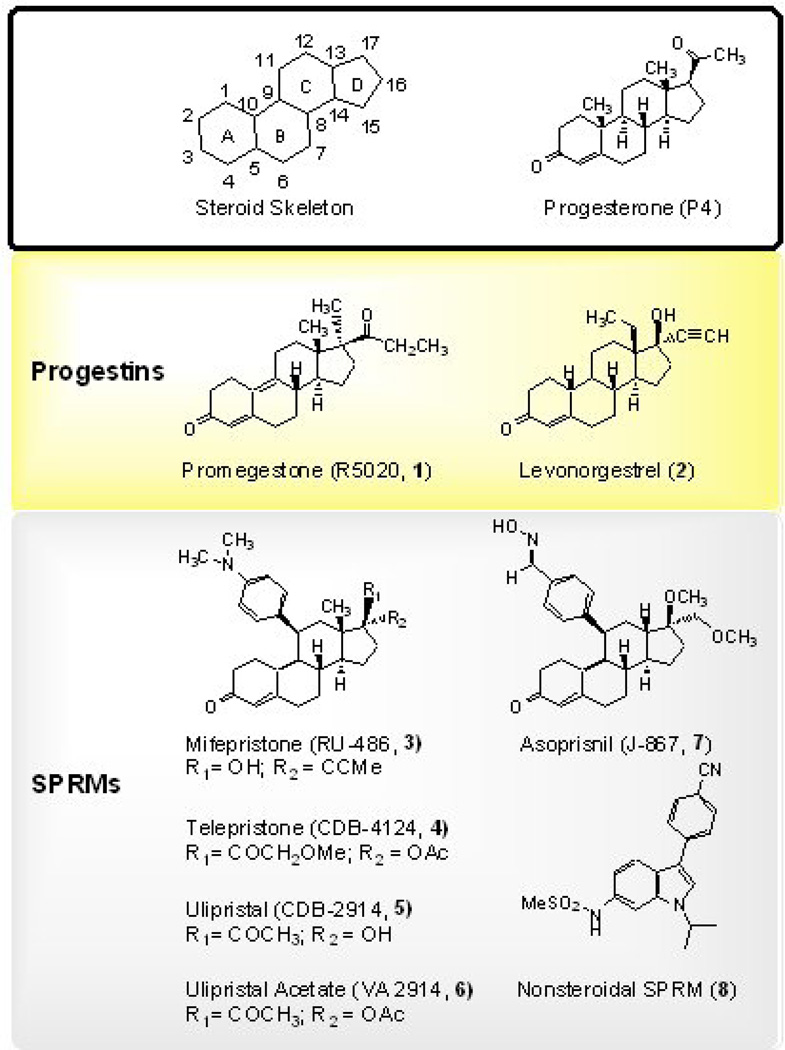
Figure 2 shows the chemical structure of various ligands that bind to PR ranging from full agonists on the PR to SPRMs. The progestins shown are derived from the parent molecule P4, although testosterone derived progestins also exit. Very small structural changes in the parent molecule may induce considerable differences in the activity of the derivative. Nonsterodal SPERMs are actively being developed such as the 3-aryl indoles recently reported.[23] The alkyl substituent at N1 plays a very important role in binding potency and increasing the number of alkyl chains in this substituent group from methyl to ethyl or isopropyl (8) improved the binding affinity 7- and 5-fold, respectively. The functional activities follow this same trend.
Figure 2.
Structures of Progestins and SPRMs. Comparison of the chemical structures of progesterone and Progesterone receptor (PR) agonists (progestins) and selective PR modulators (SPRMs;PR anatagonists with PR agonisitic properties). Small structural changes account for important differences in the effects of compounds on PR. As a point of reference, the steroid skeleton is also shown with the rings named and the positions numbered according to commonly used nomenclature.
Mifepristone is a 19-nortestosterone-derived compound that binds with high affinity to PR (slightly higher than P4) yet does not make the same binding contacts as progestins.[24] The side chains at the C11 position of the steroidal skeleton (R1 and R2) are the main structural features that correlate with mifepristone’s antagonist activity (see Figure 2). Two clinical trials of mifepristone reported a reduction in fibroid volume and uteri size.[24; 25] Side effects of mifepristone therapy include vasomotor symptoms and hot flashes (increased over baseline in the 10-mg group). Though promising, patients experienced slow regrowth of fibroid tumors following cessation of drug treatment.
Levonoergestrel is a hormonally active levorotatory enantiomer of a racemic mixture of norgestrel, a progestin derived from 19-nortestosterone. The drug produces an antiproliferative action on the endometrium. Levonoergestrel is formulated within an intrauterine device (IUS) and commercially sold as Minera®. The device is inserted directly into the uterus and its use is attributed to significant reduction of uterine bleeding.[26; 27] While uterine volume decreased, there is controversy as to whether or not significant fibroid shrinkage occurs.[28] There is a high expulsion rate when the IUS device is used for the treatment of heavy menstrual bleeding associated with fibroids (9.8%) or in the presence of submucous fibroids (15.4%).[28]
Asoprisnil is a hydrophobic oxime with substitutions at the C11 position of its steroid-like skeleton. Clinical trials involving asoprisnil have reported reversible suppression of menstruation and variable effects on ovulation (Phase I);[29] reduced fibroid size in a dose-dependent manner (5, 10 and 25 mg), and a similar effect on improvement with menorrhagia with a 28–83% amenorrhea rate (Phase II);[29]improved dysfunctional bleeding and reduce fibroid size (Phase III).[19] These results are again promising, however, SPRMs like asoprisnil have not yet received FDA approval due to concerns over the effect of these agents on the endometrium.
Ulipristal (5) and its acetate analog (6)were developed as SPRMs with pure PR antagonistic activity. They both have minimal antiglucocorticoid effects and are under development for use in symptomatic fibroids. In vitro studies and small randomized trials involving ulipristal report fibroid size and related sympostoms were significantly reduced (P = 0.01 and P = 0.04) over placebo with no adverse effects observed. [30] Ulipristal acetate treatment administered for 13 weeks on randomly controlled women with symptomatic fibroids, menometrorrhagia, and anemia, successfully controlled excessive bleeding due to uterine fibroids and reduced the size of the fibroids.[31] Changes in total fibroid volume calculated by MRI have also been used as primary outcome measure for success of ulipristal therapy[32] Ulipristal acetate is currently in a Phase III clinical trial.
Telepristone has mostly progesterone antagonist activity and low antiglucorticoid activity. It was specifically developed for use as an antiprogestin in the treatment of uterine fibroids and endometriosis. It has been shown to reduce fibroid size by up to 40% and significantly decreases vaginal bleeding. While one study reported that telepristone induced apoptosis in fiboird cells[33] another studies did not confirm this fact.[34; 35] The US FDA placed a full clinical hold on telepristone in August 2009 because of elevated liver enzymes associated with drug treatment. Since the partial clinical hold status granted in June 2010, one clinical trial with low-dose telepristone designed to test the safety of the drug and efficacy of escalating doses.
Chemical Inhibition of Growth Factors
TGFβ is the main profibrotic cytokine which regulates fibrosis by stimulating activation, survival and collagen synthesis of collagen producing cells. This dimeric polypeptide, composed of identifical 112-amino acid subunits, is over expressed in uterine fibroids compared with myometrium from the same individual.[2] Possible therapies could develop from TGFβ neutralizing antibodies, soluble analogues of TGFβ receptor to act as a decoy receptor, or orally administered TGFβ receptor inhibitors.[36] However, targeting TGFβ is problematic, requiring progteins as therapetuics (cost, purity, stability, immune response) and manipulating TGFβ signaling inhibition, preventing its widespread usage.
Anti-fibrotics
Fibroids are characterized by altered collagen fibrils, fibrosis and tissue stiffness,[37; 38]as well as increased amounts of type I and V collagen,[39] Selective elimination of collagen producing cells or reducing their state of activation is currently limited to experimental trials. Medical strategies that interfere with collagen formation (e.g. anti-fibrotic drugs) should be efficacious in fibroid treatment. A high throughput screening assay has been developed to indentify drug candidates that show anti-fibroitc activity.[40] Two promising anti-fibrotic candidates are highlighted below, but in general this class of pharmaceuticals presents undesirable side effects when systemically administered.
Pirfenidone
Pirfenidone is an orally bioavailable antifibrotic agent that has been shown to regulate fibrosis.[41] Pirfenidone inhibits fibroblast proliferation, diminishes the messenger RNA levels of collagen types I and III in a dose dependent manner, and effectively inhibits myometrial and fibroid cell proliferation in vitro.[42] The effect of this antifibrotic drug in fibroid cells is specific only for collagen type I. The drug is currently in phase III clinical trials for the treatment of pulmonary fibrosis.[43]
Pirfendione is relatively insoluble in aqueous solutions (<2%) but very soluble in alcohol and chloroform. This drug penetrates the cell membrane without requiring a receptor. When administered orally, PFD is easily absorbed in the gastrointestinal (GI) tract, reaching most tissues and crossing the blood-brain barrier. Pirfendione displays rapid absorption (tmax = 0.33–1 hr) and clearance (t1/2 = 2–2.5 hr).[41] Two metabolites were identified, which seem to be produced from oxidation of the methyl group on the pyrilidone ring followed by subsequent formation of the carboxylic acid. Oral administration of pirfenidone in clinical trials is associated with undesirable side effects including vomiting, fever, abnormality of hepatic function, dizziness, facial paralysis, hepatoma, and skin photosensitivity.
Halofuginone
As an extract from hydrangeas, halofuginone is a small organic molecule exhibiting coccidiostat benefits in birds and more recently anti-fibrotic activity against fibroid cells. Halofuginone inhibits both fibroid and myometrial smooth muscle cell proliferation by rapidly inhibiting DNA synthesis and later inducing apoptosis.[44] In addition, halofuginone significantly suppressed TGFβI mRNA production.
After single intravenous and subcutaneous administration, halofuginone rapidly distributes out of the plasma compartment and into the tissues resulting in a large volume of distribution. Renal clearance was limited to 7.5– 16.7% of total body clearance and only 15–16% of the parent compound was excreted in the urine within 48 h after oral administration. Plasma-half life varies between 5 and 17 h. At 3.5 mg/day, nausea, vomiting, and fatigue were reported. Several patients experienced bleeding complications on treatment with halofuginone in which a causal relationship could not be excluded. The PKs of halofuginone were linear over the dose range studied with a large interpatient variability. This medication is not currently used in humans and its toxicity is unknown.[45] Reported side effects of halofuginone when taken in patients with advanced solid tumors were nausea, vomiting and fatigue.
Purified collagenase
Collagenase clostridium histolyticum is a FDA approved drug targeting Dupuytren’s Contracture in adults with a palpable cord. This drug comprises a fixed –ratio mixture of two classes of purified collagenases; clostridial type I and type II collagenase. The type I enzyme is 114 KDa 1000 amino acid single polypeptide chain and type II collagenase is about 1000 amino acids long with a molecular weight of 113 KDa. Both are metalloproteinases requiring zinc and calcium for full activity and have selective activity against collagen. These two classes of enzymes are not immunologically cross-reactive and differ from each other in domain structure substrate affinity and catalytic efficiency. When combined, they demonstrate synergistic collagenolytic activity.
Class I collagenase enzymes attach to the C and N termini of the mature triple helix collagen while class II enzymes attach to bonds within the collagen molecule. For catalytic activity, zinc is required and calcium is necessary to maintain the conformation of the collagen binding sites in a state that allows the enzyme to bind to the triple helical collagen fibril. Both classes are capable of degrading small collagen fragments. The concerted effort of both enzymes degrade the entire collagen triple helix as opposed to mammalian matrix metalloproteinases which degrade only specific bonds in the collagen molecule and do not degrade collagen completely. In vivo, clostridial histolyticum collagenase is not effective in degrading type IV collagen. Thus in clinical studies, purified clostridial histolyticum collagenase did not degrade large blood vessel membranes or nerves.
In mammals, including humans, clostridial histolyticum collagenase is inhibited rapidly in the blood stream by serum proteins which forms a complex with the collagenase. These complexes containing the inactive enzyme are then removed from circulation in the liver. Purified clostridial collagenase is not a reproductive toxin.
The dose used for treatment of a Dupuytren’s contracted joint is 0.58mg. per direct injection into the cord. Up to three injections may be given per affected joint. This drug is approved by FDA for the treatment of Dupuytren’s Contracture of the adult hand with a palpable cord present1 and has not yet been used in clinical trials for fibroids. Our laboratory is conducting proof of principle studies. 2
Low-dose Oral Contraceptive Pill as a Therpaeutic Drug Strategy
Combined oral contraceptive pills and progestin-only pills are effective for the treatment of abnormal uterine bleeding.[46] These therapies can induce endometrial atrophy and stabilize the endometrium. However, the myoma size does not change. Further, evidence shows that estrogens and progestins can act as growth stimulators for uterine myomas.
Herbals as a Therpetuic Drug Strategy
Herbal therapies are recognized in Eurasia for their benefits to human well being. Traditional Chinese Medicines (TCM) are documented for modern pharmacological characteristics in a recent book chapter.[47] Kue-chin-fu-ling-man (KBG) improved clinical symptoms of hypermenorrhea and dysmenorreha in 110 premenopausal women with uterine fibroids and fibroid shrinkage was observed in ~60% of the women. TCM relate fibroid formation to stagnated blood (“ yu zheng ”) accumulation. Thus, blood circulation enhancers are often added to the herbal formulation.
Other Chinese formulations (Augmented Rambling Powder, Cinnamon Twig and Poria Pill) are reported to lower estrogen levels.[48] Dong Quai and Peony Powder reduce blood estrogen levels and inflammatory compounds in the uterus. Four Substance Decoction treats both fibroids and endometriosis in women who are affected by poor diet or overfatigue.[48] Western herbal remedies are also highlighted.[48] Cinnamon oil (5–10 drops) administered 16 times in 4 hours is reported to subside bleeding due to fibroids. Reishi stops pelvic inflammation when administered daily in triplicate dosages of 12 mL/dose.
Green tea is a powerful antioxidant touted as a remedy for various symptoms including fibroids.[13] Approximately 90 – 126 mg dry weight of the active ingredient, catechins, is typically contained in 1 g leaf brewed for 3-min in 100 mL water. Epigallocatechin-3-gallate (EGCG) is the major component of catechin (>40% of the total polyphenolic mixture of green tea catechins). The mechanism of EGCG action appears to be blockage of tumorgenesis by modulating signaling pathways involved in cell proliferation, transformation, inflammation, and oxidative stress. All these processes contribute to the pathogenesis of uterine fibroids. EGCG induced apoptosis and inhibited proliferation of fibroid cells.[13] This suggests that EGCG may be a potential anti-fibrotic agent acting through multiple signal transduction pathways. An ongoing double-blind placebo-controlled clinical trial is currently in phase II of investigations to evaluate the clinical role of EGCG in women with symptomatic fibroids.[13]
Conclusions
If the molecular basis for fibroid development and of myometrial proliferation is understood, additional nonsurgical therapeutic interventions may be forthcoming. Current clinical needs are stated to a) include an effective prevention strategy if possible b) improve early detection; c) slow the growth of fibroids; d) determine the mechanisms of infertility due to fibroids; e) develop better treatment modalities; f) reduce recurrences after treatment; g) evaluate long-term results.
Drug therapies for uterine fibroids are either short-lived or have significant side effects. Leuprolide acetate is presently used for only short term treatment prior to surgery. SPRMs have not yet received FDA approval, due to concern over the effect of these agents on the endometrium, despite the intensive developmental efforts by several pharmaceutical companies and clinical trials which demonstrated significant shrinkage of fibroids and symptomatic improvement. Other medical therapies have been suggested in the recent past such as SERMs, AIs, and anti-fibrotics but clinical trial results for efficacy treating fibroids has been limited or disappointing. TGFβ inhibitors effectively inhibit fibrosis in different animal models; however, systemic inhibition of TGFβ raises important safety issues because of the pleiotropic physiological effects of this factor. Local delivery of such agents allows the delivery of intact peptide molecules and promises to reduce fibrosis, while avoiding systemic side effects.
Questions arise as the knowledge about uterine fibroids continues to develop. Patients and their care givers will wonder: Is there a drug therapy that rivals the effectiveness of surgical procedures yet preserves the uterine child bearing function? If realized, could such a therapy be administered under ultrasound guidance in an outpatient setting? Addressing these questions presents unique opportunities at the interface of molecular medicine and clinical care.
With the advent of the means to deliver drugs or drug combinations directly to fibroid tumors, the potential of reduction and perhaps eradication of fibroids prior to the need for surgical intervention could be realized. Multiple drugs could be given with the LiquoGel platform as a drug cocktail or sequentially for the benefit of patient care. A number of the more conventional drug therapies for uterine fibroids could be potentially entrapped or covalently linked to LiquoGel to afford delivery with potentially reduced side effects, improved efficacy, and controlled release profiles. The treatment could be administered by skilled personnel in a doctor’s office under ultrasound guidance.
Ultimately, development in uterine fibroid therapy stems from the need for viable choices to reduce sympotoms associated with this disease. In light of rising health care costs, patients looking for economical approaches to health may consider a combination of conventional medicine supplemental approaches. Both care giver and patient should be educated about herbal treatment options for uterine fibroids. Although further studies and chemical investigations are needed to determine and validate the herbal therapy efficacy for uterine fibroid treatment, patient interest in herbal treatments may excite more investigation in this field.
Highlights.
-
➢
We present developments in seven drug classes typically used in fibroid therapy
-
➢
Natural drug remedies are presented
-
➢
Chemical and pharmacological drug properties are described
-
➢
Drug side effects and clinical efficacy for fibroid therapy are reviewed
-
➢
We present novel therapeutic interventions enabled by polymeric drug delivery systems
Acknowledgements
This work was supported by National Institute of Health K12HD043446. NIH was not involved with any aspects of preparing this manuscript. The authors would like to thank Friederike Jayes and Aletheia Burrell for assistance with developing this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Jayes FL, Ma X. Leppert PC. Purfied clostridial histolyticum collagenase degrades uterinefibroid tissue ex vivo: potential treatment for uteine fiboirds. Submitted to American Society forReproductive Medicine (ASRM)
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Peddada SD, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci U S A. 2008;105(50):19887–19892. doi: 10.1073/pnas.0808188105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leppert PC, et al. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol. 2006;195(2):415–420. doi: 10.1016/j.ajog.2005.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catherino W, et al. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosomes Cancer. 2004;40:204–217. doi: 10.1002/gcc.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carrino DA, et al. Proteoglycans of uterine fibroids and keloid scars: Similarity in their proteoglycan composition. Biochem. J. 2012;443:361–368. doi: 10.1042/BJ20111996. This report presents evidence that uterine fibroids are characterized by disarrayed collagen fibrils strikingly similar to those of keloid scars. The authors find that keloids are a form of excessive dermal fibrosis demonstrating abnormal wound-healing response in certain individuals; they form after skin trauma such as surgery; they tend to develop during and after puberty and in women symptoms of keloids disappear after menopause; they produce high levels of collagen, and fibronectin; they are characterized as resisting apoptosis and thus continue to produce collagen.
- 5.Cook H, et al. The impact of uterine leiomyomas on reproductive outcomes. Minerva Ginecol. 2010;62(3):225–236. [PMC free article] [PubMed] [Google Scholar]
- 6.Bouwsma EV, et al. Comparing focused ultrasound and uterine artery embolization for uterine fibroids-rationale and design of the fibroid interventions: Reducing symptoms today and tomorrow (firstt) trial. Fertil Steril. 2011;96(3):704–710. doi: 10.1016/j.fertnstert.2011.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsanezhad M, et al. Medical management of uterine fibroids. Current Obstetrics and Gynecology Reports. 2012:1–8. [Google Scholar]
- 8. Cardozo ER, et al. The estimated annual cost of uterine leiomyomata in the united states. American journal of obstetrics and gynecology. 2011 doi: 10.1016/j.ajog.2011.12.002. The article reports than annual societal costs for uterine fibroid therapy apporach $34.4 billion not accounting for those women with symptomatic fibroids and no access to medical care.The authors use pooled odds ratios when possible and reflect a large number of studies, to arrive at numbers for direct, indirect, and associated pregnancy and obstetrical related costs in the assessment of cost of uterine fibroids.
- 9.Wikland M, et al. A randomized controlled study comparing pain experience between a newly designed needle with a thin tip and a standard needle for oocyte aspiration. Hum. Reprod. 2011;26(6):1377–1383. doi: 10.1093/humrep/der100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright JC, et al. In situ forming systems (depots) In: Wright JC, Burgess DJ, editors. Long acting injections and implants. New York: Springer US; 2012. pp. 153–166. [Google Scholar]
- 11. Taylor DK, et al. Temperature-responsive biocompatible copolymers incorporating hyperbranched polyglycerols for adjustable functionality. Journal of Functional Biomaterials. 2011;2(3):173–194. doi: 10.3390/jfb2030173. The authors describe the design, synthesis, and characterization of a four component copolymer that responds to elevation in temperature by undergoing a phase transition from liquid to solid. This is the first thermoresponsive injectable that has the potential to deliver multiple drugs by mechanistically different approaches: covalent attachment of drug and physical entrapment of drug.
- 12.del Mastro L, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer. JAMA: The Journal of the American Medical Association. 2011;306(3):269–276. doi: 10.1001/jama.2011.991. [DOI] [PubMed] [Google Scholar]
- 13. Sabry M, Al-Hendy A. Innovative oral treatments of uterine leiomyoma. Obstetrics and Gynecology International. 2012;2012 doi: 10.1155/2012/943635. This review of drug therapies covers conventional drugs in a very succinct manner. In addition, the authors include a section on herbal therapies.
- 14.Khan KN, et al. Changes in tissue inflammation, angiogenesis and apoptosis in endometriosis, adenomyosis and uterine myoma after gnrh agonist therapy. Hum. Reprod. 2010;25(3):642–653. doi: 10.1093/humrep/dep437. [DOI] [PubMed] [Google Scholar]
- 15. McCarthy-Keith DM, et al. Gonadotropin-releasing hormone agonist increases expression of osmotic response genes in leiomyoma cells. Fertil Steril. 2011;95(7):2383–2387. doi: 10.1016/j.fertnstert.2011.03.084. The authors characterize hyperosmolarity-responsive genes in leiomyoma cells and determine whether gonadotropin-releasing hormone (GnRH) agonist treatment altered their expression. This report is timely because it offers insight into yet another mechanism of fibroid shrinkage.
- 16. Gobbi S, et al. Novel highly potent and selective nonsteroidal aromatase inhibitors: Synthesis, biological evaluation and structure−activity relationships investigation. J. Med. Chem. 2010;53(14):5347–5351. doi: 10.1021/jm100319h. This is one of a series of papers that reports on some molecules featuring different oxygenated heterocycles usch as flavone, chromone, and xanthone as aromatase inhibitors. The authors are focusing on development of novel AI constructs with the aim of increasing potency and selectivity versus other CYP enzymes.
- 17.Saudagar RB, et al. Serm’s in treatment of breast cancer. Asian J. Pharm. Res. 2011;1(4):81–86. [Google Scholar]
- 18. Dai X, Wu J. Selective estrogen receptor modulator: Raloxifene. Journal of Reproduction and Contraception. 2011;22(1):51–60. This paper compiles a lot of the previously reported findings on raloxifene. It also has general discussion about SERMs as a drug class.
- 19. Bouchard P, et al. Selective progesterone receptor modulators in reproductive medicine: Pharmacology, clinical efficacy and safety. Fertil. Steril. 2011;96(5):1175–1189. doi: 10.1016/j.fertnstert.2011.08.021. This paper serves as the first recent review of SPRMs and compiles a lot of the previously reported findings for this drug class. Thus, it is a timely contribution to the field.
- 20.Catherino WH, et al. Proceedings from the national institute of child health and human development conference on the uterine fibroid research update workshop. Fertil. Steril. 2011;95(1):9–12. doi: 10.1016/j.fertnstert.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chai SY, et al. Term myometrium is characterized by increased activating epigenetic modifications at the progesterone receptor-a promoter. Mol. Hum. Reprod. 2012 doi: 10.1093/molehr/gas012. [DOI] [PubMed] [Google Scholar]
- 22. Crochet JR, et al. Mitochondrial progesterone receptor (pr-m) distribution in uterine leiomyoma and myometrium and impact on mitochondrial membrane potential (mmp) in immortilized human myometrial cells (htert-hm) Fertil. Steril. 2011;96(4):S96. In providing evidence of increase PR-M expression and number of mitochondria in the fibroids edge, these authors present evidence that a non-genomic progestin-induced increase in cellular respiration may be a major factor in the growth of fibroids.
- 23.Richardson TI, et al. Novel 3-aryl indoles as progesterone receptor antagonists for uterine fibroids. Acs Medicinal Chemistry Letters. 2011;2(2):148–153. doi: 10.1021/ml100220b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spitz IM. Mifepristone: Where do we come from and where are we going? Clinical development over a quarter of a century. Contraception. 2010;82(5):442–452. doi: 10.1016/j.contraception.2009.12.012. This paper serves as the current review of milepristone compiling results over 25 years.
- 25.Esteve JLC, et al. Treatment of uterine myoma with 5 or 10 mg mifepristone daily during 6 months, post-treatment evolution over 12 months: Double-blind randomised clinical trial. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2012;161(2):202–208. doi: 10.1016/j.ejogrb.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Heikinheimo O, Gemzell-Danielsson K. Emerging indications for the levonorgestrel-releasing intrauterine system (lng-ius) Acta Obstet. Gynecol. Scand. 2012;91(1):3–9. doi: 10.1111/j.1600-0412.2011.01303.x. [DOI] [PubMed] [Google Scholar]
- 27.Sayed GH, et al. A randomized clinical trial of a levonorgestrel-releasing intrauterine system and a low-dose combined oral contraceptive for fibroid-related menorrhagia. International Journal of Gynecology & Obstetrics. 2011;112(2):126–130. doi: 10.1016/j.ijgo.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Kriplani A, et al. Efficacy of the levonorgestrel-releasing intrauterine system in uterine leiomyoma. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 116(1):35–38. doi: 10.1016/j.ijgo.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 29.Stovall DW, Mikdachi HE. Treatment of symptomatic uterine leiomyomas with selective progesterone receptor modulators. Expert Review of Obstetrics & Gynecology. 2011;6(6):579–582. [Google Scholar]
- 30.Rodriguez MI, et al. Intrauterine progestins, progesterone antagonists, and receptor modulators: A review of gynecologic applications. Am. J. Obstet. Gynecol. 2010;202(5):420–428. doi: 10.1016/j.ajog.2009.10.863. [DOI] [PubMed] [Google Scholar]
- 31.Donnez J, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N. Engl. J. Med. 2012;366(5):409–420. doi: 10.1056/NEJMoa1103182. [DOI] [PubMed] [Google Scholar]
- 32.Nieman LK, et al. Efficacy and tolerability of cdb-2914 treatment for symptomatic uterine fibroids: A randomized, double-blind, placebo-controlled, phase iib study. Fertility and Sterility. 2011;95(2):767–U769. doi: 10.1016/j.fertnstert.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo X, et al. The selective progesterone receptor modulator cdb4124 inhibits proliferation and induces apoptosis in uterine leiomyoma cells. Fertility and Sterility. 2010;93(8):2668–2673. doi: 10.1016/j.fertnstert.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roeder H, et al. Cdb-4124 does not cause apoptosis in cultured fibroid cells. Reproductive Sciences. 2011;18(9):850–857. doi: 10.1177/1933719111399929. This report is a major finding as it corrects previous thoughts on the role of CDB-4124.
- 35.Wade HE, et al. Multimodal regulation of e2f1 gene expression by progestins. Mol. Cell. Biol. 2010;30(8):1866–1877. doi: 10.1128/MCB.01060-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leask A. Potential therapeutic targets for cardiac fibrosis: Tgfbeta, angiotensin, endothelin, ccn2, and pdgf, partners in fibroblast activation. Circ. Res. 2010;106(11):1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 37.Malik M, et al. Why leiomyomas are called fibroids: The central role of extracellular matrix in symptomatic women. Semin. Reprod. Med. 2010;28(3):169–179. doi: 10.1055/s-0030-1251475. [DOI] [PubMed] [Google Scholar]
- 38.Norian JM, et al. Characterization of tissue biomechanics and mechanical signaling in uterine leiomyoma. Matrix Biol. 2012;31(1):57–65. doi: 10.1016/j.matbio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwahashi M, Muragaki Y. Increased type i and v collagen expression in uterine leiomyomas during the menstrual cycle. Fertil. Steril. 2011;95(6):2137–2139. doi: 10.1016/j.fertnstert.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 40. Fritz D, et al. Progress towards discovery of antifibrotic drugs targeting synthesis of type i collagen. Curr. Med. Chem. 2011;18(22):3410–3416. doi: 10.2174/092986711796504691. This paper serves as the first recent review of antifibrotic drugs. The authors compile a lot of the previously reported findings within this drug class.
- 41.Cho ME, Kopp JB. Pirfenidone: An anti-fibrotic therapy for progressive kidney disease. Expert Opinion on Investigational Drugs. 2010;19(2):275–283. doi: 10.1517/13543780903501539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Macias-Barragan J, et al. The multifaceted role of pirfenidone and its novel targets. Fibrogenesis & Tissue Repair. 2010;3(1):16. doi: 10.1186/1755-1536-3-16. This is concise review of the chemical and biological properties of pirfenidone. The authors have captured not only major previous reports on this drug, but in addition, given insight into what we can expect from pharmaceutical development within this drug class.
- 43.Inc I. Efficacy and safety of pirfenidone in patients with idiopathic pulmonary fibrosis (ipf) (ascend). City, 2011 [Google Scholar]
- 44.Grudzien MM, et al. The antifibrotic drug halofuginone inhibits proliferation and collagen production by human leiomyoma and myometrial smooth muscle cells. Fertility and Sterility. 2010;93(4):1290–1298. doi: 10.1016/j.fertnstert.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tantibhedhyangkul JA, Behera M. Non-surgical treatment options for symptomatic uterine leiomyomas. Current Women's Health Reviews. 2010;6(2):146–160. [Google Scholar]
- 46.Su Y, et al. Contraceptives with novel benefits. Expert Opin. Investig. Drugs. 2012;21(1):83–90. doi: 10.1517/13543784.2012.642368. [DOI] [PubMed] [Google Scholar]
- 47.Liu WJH. Traditional herbal medicine research methods. John Wiley & Sons, Inc; 2011. Introduction to traditional herbal medicines and their study; pp. 1–26. [Google Scholar]
- 48.Balch PA, Bell S. Prescription for herbal healing, 2nd edition: An easy-to-use a-to-z reference to hundreds of common disorders and their herbal remedies. New York, NY: Penguin Group; 2012. [Google Scholar]