Abstract
Background
DNA-damaging drugs constitute standard chemotherapy regimen for advanced colorectal cancer. Here, the interactions between quercetin and 5- fluorouracil (5-FU), etoposide, and camptothecin were examined in cancer cells.
Materials and Methods
HCT116 colorectal or PPC1 prostate cancer cells were treated with quercetin and the drugs. Clonogenicity assays, cell cycle profiles, and expressions of p53, p21, BAX, survivin and cyclin B1 proteins were used to examine the effects of the treatments.
Results
Quercetin synergistically inhibited the clonogenicity of the wild-type cells, but inhibited the cell cycle effects of all the drugs tested. In p53-null cells, the combination of low dose 5-FU with up to 6 μM quercetin promoted clonogenic survival. Treatment of p53-wild-type cells with 50 μM quercetin reduced drug-induced up-regulation of p53, p21 and BAX. The combination of quercetin and the drugs also reduced the levels of cyclin B1 and survivin proteins.
Conclusion
While high doses of quercetin synergize with DNA-damaging agents, the effect of drug combination with quercetin is influenced by the effective doses and the p53 status of the cells.
Keywords: Polyphenol, 5-FU, drug–diet interaction, cancer therapy, colorectal cancer, prostate cancer
Quercetin (C15H10O7) is one of the most abundant dietary flavonoids; for example, it constitutes about 99% of the flavonoids found in apple peel (1). Numerous in vitro and in vivo studies have shown the bioactivity of quercetin in protecting cells from oxidative stress and other types of cell injury (2–4). It is particularly interesting that quercetin has been suggested to have neuroprotective effects against damage induced by drugs and toxic compounds, and against neurovascular insults such as ischemia (5–8).
The cancer chemopreventive activities attributed to the constituents derived from the consumption of fruit and vegetables are considered to be due to diverse bioactive polyphenolic compounds present. Quercetin, as one such constituent, has been studied for its anticancer activities both in vitro and in vivo (9–13). Formulations of quercetin are available as dietary supplements, primarily as antioxidants purported to promote general health. It is tolerated up to one gram/day orally and is regarded as a relatively safe compound (14).
Although quercetin has been well studied for its potential chemopreventive functions, its interaction with cancer chemotherapeutic and other drugs has not been investigated in detail. A few studies have shown the synergistic activities of quercetin with various chemotherapeutic drugs (15–19). Some studies have also suggested precaution in co-administering antioxidants and chemotherapeutic drugs (20, 21). Our recent work also suggested a transient interference of quercetin with the activity of microtubule-targeting drugs to induce arrest of the G2/M cell cycle phase (22).
The nucleotide analog 5-fluorouracil (5-FU) is a component of standard chemotherapy against colon cancer. When converted to its metabolites, 5-FU acts to inhibit cancer cell proliferation by inhibiting thymidylate synthase, by inducing lesions upon incorporation into DNA and RNA, and through RNA-based cytotoxicity (23–25). 5-FU combined with folinic acid and oxaliplatin, known as FOLFOX, is currently one of the standard first-line chemotherapy regimens for stage III and higher colon cancer in humans (26). Camptothecin and etoposide are topoisomerase inhibitors, which also induce DNA lesions during replication, and are used to treat various types of cancer.
Here, we investigated the interaction of quercetin with the chemotherapeutic drugs 5-FU, camptothecin, and etoposide (VP-16).
Materials and Methods
Cells and their culture
Wild-type and p53-null HCT116 colorectal carcinoma cells were generously donated by Dr Bert Vogelstein (Johns Hopkins, Baltimore, MD, USA). Cell cultures were maintained in McCoy’s 5A medium (ATCC, Manassas, VA, USA) supplemented with glutamine, penicillin/streptomycin, and 10% fetal calf serum (FCS). PPC1 prostate carcinoma cells were kindly provided by Dr John Reed (Sanford-Burnham Institute for Medical Research, La Jolla, CA), and were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) from Cellgro (Manassas, VA, USA). Cultures were incubated at 37°C and 5% CO2 in a humidified incubator.
Antibodies and reagent
Antibodies for the following were purchased from Cell Signaling Technologies, Danvers, MA, USA: p53, p21, BCL-2-associated X-protein (BAX), proliferating cell nuclear antigen (PCNA), cyclin B1, survivin, and β-actin. Secondary antibodies against mouse and rabbit primary antibodies were from GE Healthcare (Piscataway, NJ, USA).
Clonogenicity assay
Assessing the clonogenicity of tumor cells is a useful means to determine the effects of candidate agents on the ability of individual cancer cells to proliferate and form visible colonies. Moreover, since most bioactive compounds such as quercetin affect multiple signaling pathways that regulate cell proliferation, clonogenicity was used as an endpoint to evaluate the final outcome of single-cell survival and proliferation under our experimental conditions. The assay was performed as previously described (27) and as modified in prior work (28). About 150 cells were seeded per well of a 6-well dish, allowed to adhere for about 18 hours, and then treated with the compounds simultaneously at the indicated concentrations (0–10 μM for 5-FU, and 0–50 μM for quercetin). To avoid secondary and satellite colony formation, culture media were not changed once treatment was initiated. Treated cells were allowed to form colonies in a volume of culture medium (2 ml per well of a 6-well dish) that was sufficient to allow growth until the end of the experiments (7–10 days). Colonies formed were fixed with 4% formalin for 15 minutes, and stained with 10% crystal violet in methanol for 15–20 minutes. Excess dye was removed by gentle washing with running water. Plates were air dried and colonies were counted using AlphaImager (AlphaInnotech, Santa Clara, CA, USA) in colony-counting mode. The relative clonogenicity of a treatment was computed as a percentage of the number of colonies that formed in the control wells. Dimethyl sulfoxide (DMSO) was used as a solvent control for quercetin or 5-FU alone, and 0.6 μM 5-FU alone was used as baseline control for the combination treatment experiments in clonogenicity assays. In addition to counting the number of HCT116 cell colonies that were formed, we also counted the number of cells per developing colonies, and analyzed the cumulative surface area of the colonies. The cumulative area measurement served as a factor to compare the size of cells in formed colonies.
Immunoblotting
Cell lysates were prepared in NP-40 lysis buffer [20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 10% glycerol and 0.2% NP-40 plus protease inhibitor cocktail] and protein concentrations were determined using detergent-compatible DC protein assay (BioRad Laboratories Inc., Hercules, CA, USA). Samples containing equivalent protein concentrations were mixed with Laemmli buffer, and boiled for 5 minutes. Proteins were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene fluoride (PVDF) membranes (GE Healthcare Life Sciences, Piscataway, NJ, USA) and blocked in 5% non-fat dry milk. Primary antibodies (p53, p21, and cyclin B1) were used at 1:1000 dilution. Peroxidase-conjugated anti-rabbit and anti-mouse IgG secondary antibodies were purchased from GE Healthcare Life Sciences and used at 1:7000 dilution. Chemiluminescent detection was carried out using Classico or Crescendo premixed Chemiluminescent HRP Substrates (Millipore, Billerica, MA, USA).
Flow cytometry
Cells were harvested and prepared for flow cytometry as described elsewhere (28). Cells were harvested by trypsinization using 0.25% trypsin-EDTA (Invitrogen Corp., Carlsbad, CA, USA) and centrifuged. Pellets were resuspended in 300 μl phosphate-buffered saline (PBS; Invitrogen Corp.), fixed by the addition of 700 μl 100% ethanol while vortexing, and stored at −20°C for a minimum of 12 hours. Fixed cells were centrifuged, and stained in FACS staining solution (320 mg/ml RNase A, 0.4 mg/ml propidium iodide) in PBS without calcium and magnesium for 15 minutes at 37°C. Stained cells were filtered through 70-μm pore size filter and analyzed by flow cytometry on a C6 Accuri® flow cytometer (Accuri Cytometers, Ann Arbor, MI, USA). Data was analyzed and histograms were prepared using CFlow™ software (Accuri Cytometers).
Imaging
Phase-contrast images of cells were taken at ×200 magnification using an Olympus inverted microscope fitted with a digital image capture camera (Digital Microscopy Core Lab., School of Veterinary Medicine, Tuskegee, AL, USA). Captured images were stored in TIFF format and subsequently cropped and resized in Microsoft PowerPoint.
Results
Quercetin in combination with 5-FU reduces the clonogenicity of HCT116 cells
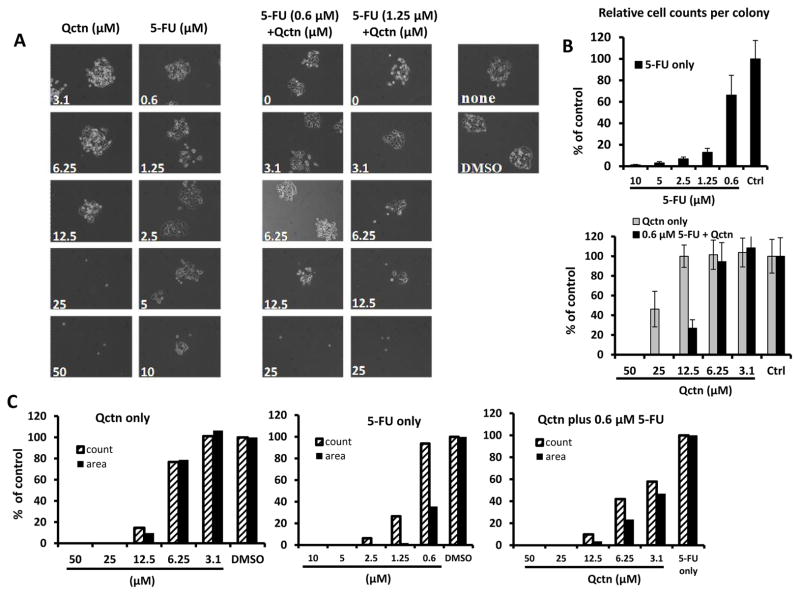
To determine if co-treatment of colon cancer cells with quercetin and 5-FU is more effective than that of the two compounds alone, HCT116 colon cancer cells were exposed to different concentrations of quercetin, 5-FU, or combination of the two. When HCT116 cells were treated with quercetin alone, up to 12.5 μM of the flavonoid allowed for the development of cell colonies. For 5-FU, doses of up to 5 μM allowed colony formation within six days of treatment, during a period in which control HCT116 cells seeded as single cells also formed colonies (Figure 1A). When combined, 0.6 μM 5-FU and 12.5 μM quercetin markedly reduced the formation of colonies by day 6, whereas a similar reduction was found when 6.25 μM quercetin was combined with 1.25 μM 5-FU (Figure 1A). Overall, a single dose of either quercetin at 25 μM or 5-FU at 10 μM was required to reduce HCT116 colony formation to the same extent as the combination treatments above. This suggests a potential synergy between the two compounds in reducing clonogenicity of wild-type HCT116 cells.
Figure 1.
Effects of quercetin and quercetin/5-fluorouracil (5-FU) combination on the clonogenicity of wild-type HCT116 cells. Clonogenic survival was assayed as described in the Materials and Methods. A: Phase-contrast images of wild-type HCT116 cells treated with quercetin (Qctn), 5-FU, or combinations of quercetin with 5-FU. B: Relative counts of cells per colony for wild-type HCT116 cells treated with the indicated doses of 5-FU only (upper panel) or 3.1 – 50 μM quercetin (light bars, lower panel) or 3.1 – 50 μM quercetin in combination with 0.6 μM 5-FU (black bars, lower panel). Control (ctrl) bars represent normalized values from cells treated with DMSO (for 5-FU or quercetin single agent treatments) or 0.6 μM 5-FU (for combination treatments). Error bars indicate standard deviation of counts from ten colonies per treatment condition. C: Relative total number and area for colonies from wild-type HCT116 cells treated with quercetin only, 5-FU only, or a combination of 0.6 μM 5-FU with the indicated doses of quercetin. Relative measurements in both B and C are expressed as a percentage of results from control wells. Representative graphs from two independent experiments are shown.
Since the ultimate purpose of the combination treatment was to reduce the effective dose of 5-FU (eventually to reduce toxic side-effects), we examined the doses of quercetin that could be combined with 0.6 μM 5-FU to suppress clonogenic survival of HCT116 cells. Cells were seeded and treated as above, and at day 8 post-treatment, we counted the number of cells per colony, and analyzed the relative number of cells in comparison to the controls. Comparing cells treated with the individual compounds (quercetin or 5-FU), combination treatment with 0.6 μM 5-FU and 12.5 μM or higher quercetin reduced the number of cells per colony by approximately 75%, demonstrating the synergistic effects of the combination treatment (Figure 1B). Notably, although doses of 5-FU at 2.5 μM or above temporarily permitted limited rounds of cell division and some mini-colony formation (Figure 1A), such cells did not continue to proliferate and form expanding colonies beyond day 6 (Figure 1B).
Next, we determined the total number of colonies that formed in cells treated with the individual compounds or the combination of the two (0.6 μM 5-FU plus 0 to 50 μM quercetin). The cumulative area of all the counted colonies was also measured. The area measurement was useful to assess the relative sizes of the colonies under treatment. If the mini-colonies ceased to expand without total cell loss, then the total colony count may be high but the total area will be low in proportion to the size of the individual colonies. The total colony count was performed 10 days after the start of treatment, when the cell count per colony was too high for a manual count. As shown in Figure 1C, quercetin at doses of 12.5 μM or above markedly reduced the total colony count compared to the DMSO control. Similarly, doses of 5-FU at or above 1.25 μM markedly reduced the total number of colonies formed. Moreover, 0.6 μM or higher 5-FU also markedly reduced the total area of the formed colonies, indicating that the colonies that initially started forming and proceeded through three or more population doublings, further expansion eventually stopping.
Treatment of wild-type HCT116 cells with a combination of 0.6 μM 5-FU and different doses of quercetin also led to a marked dose-dependent decrease in both the number of total colonies formed and the size of the colonies. The combination of 6.25 μM quercetin with 0.6 μM 5-FU was able to reduce the total count by more than 50%, while the cumulative size of the colonies was reduced by almost 80% compared to the cells treated with 5-FU only. Higher doses of quercetin inhibited colony formation even more when combined with 0.6 μM 5-FU (Figure 1C).
Quercetin at high doses interferes with cell cycle arrest phenotype induced by 5-FU
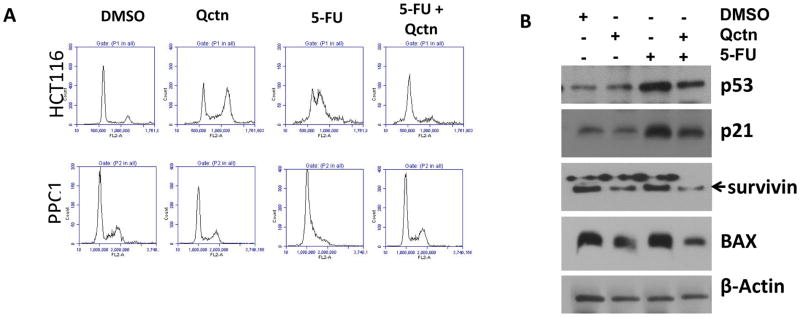
To examine the potential mechanisms through which the combination of 5-FU and quercetin suppresses cell proliferation and colony formation, we examined the cell cycle profiles of wild-type HCT116 (colorectal carcinoma) and PPC1 (prostate carcinoma) cells treated with DMSO, quercetin alone, 5-FU alone, or a combination of quercetin and 5-FU. Unlike colony formation assays where the cells are seeded as single cells, to examine the cell cycle and protein levels, higher doses of the compounds were needed to observe biochemical and cell cycle changes measureable within 24–30 hours. As shown in Figure 2A, while 50 μM quercetin alone moderately enhanced the G2/M population in HCT116 cells, it did not markedly affect the proportion of G2/M cells in the PPC1prostate cancer cells. On the other hand, 5-FU induced G1/S arrest in both cell types. However, in cells treated with a combination of the two compounds, there was a reversal of the G1/S arrest phenotype observed after 5-FU treatment. This phenomenon was similar to our previous observation that cell cycle arrest induced by taxol and nocodazole was abrogated by co-treatment with quercetin (22). Single treatment with quercetin at 25 μM or lower doses did not induce noticeable cell cycle phenotypes, nor interfered with the cell cycle effects of 5-FU (data not shown).
Figure 2.
Cell cycle profiles and regulatory proteins in wild-type HCT116 or PPC1 cells treated with quercetin (Qctn; 50 μM), 5-fluorouracil (5-FU; 10 μM), or a combination of the two. A: Cells were treated for 24 hours as indicated, and cell cycle profiles were determined by flow cytometry. Upper panels show profiles of HCT116 cells, while lower panels show profiles of PPC1 cells. B: HCT116 cells were treated as shown, and cell lysates were prepared about 30 hours post treatment. Levels of p53, p21, survivin, and BAX proteins were determined by immunoblotting. β-Actin immunoblotting is included to show comparable protein loading. The effects of 5-FU on cell cycle and regulatory proteins were antagonized by quercetin.
The p53 protein is a component of the cellular response to 5-FU through the DNA-damaging or other effects of the drug (29). The up-regulation of the p53 targets p21 and BAX serves as biomarker for the transcriptional activity of p53. Therefore, we examined the status of p53 and its targets p21 and BAX in p53 wild-type HCT116 cells treated with the individual compounds (10 μM 5-FU or 50 μM quercetin), or a combination of the two. Interestingly, while 5-FU induced expression and activity of p53, combination of 5-FU with quercetin interfered with the induction of p53 expression (Figure 2B) as compared to the use of 5-FU alone. Moreover, expressions of p53 target proteins p21 and BAX were also reduced compared to that with 5-FU alone, suggesting that the transcriptional activity of p53 was also reduced by quercetin under these circumstances. Additionally, expression of the cell cycle and apoptosis regulatory protein survivin (30) was down-regulated in both quercetin- and combination-treated cells compared to the control, suggesting a broader effect of such a combination treatment. Quercetin alone also induced a moderate reduction in the expressions of p21, survivin and BAX proteins. However, although cells treated with 10 μM 5-FU and 50 μM quercetin showed phenotypic antagonism of cell cycle effects, cells treated at these doses did not survive beyond 72 hours (data not shown).
Effects of combining quercetin with etoposide or camptothecin
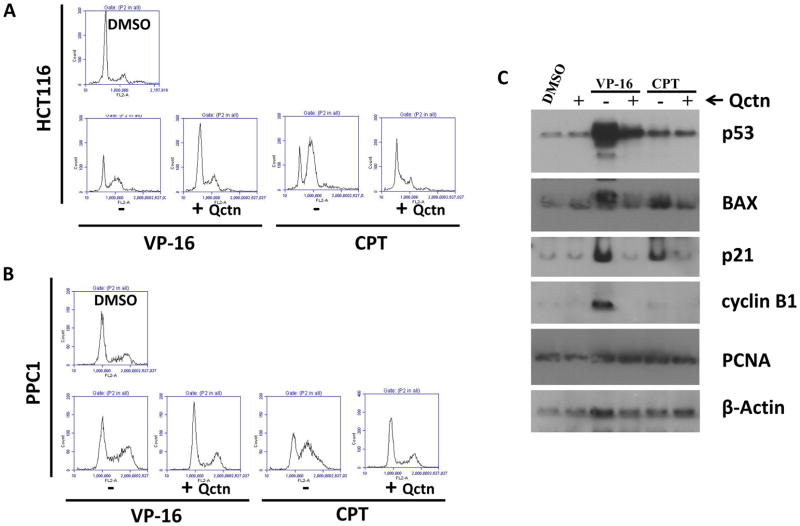
To examine the interaction of quercetin with other drugs, we treated wild-type HCT116 and PPC1 cells with two clinically used anticancer drugs etoposide, (50 μM) and camptothecin (2 μM), either singly or in combination with 50 μM quercetin. As shown in Figure 3A, similar to the treatment with 5-FU, combination of these drugs with quercetin reversed the G2/M-arrest by etoposide and the S-arrest by camptothecin, suggesting a similar outcome of combination treatment to that of 5-FU and quercetin. When the levels of the proteins p53 and BAX were compared among the different treatments, co-treatment of cells with quercetin abrogated the effects of the drugs. In cells treated with combinations, there was no evidence of increase in levels of p53 or its targets BAX and p21. Concurrent with the reversal of etoposide-induced G2 arrest, the level of cyclin B1, a major G2/M regulator, was also reduced in cells treated with etoposide and quercetin. However, there was no effect on the protein level of PCNA with any of these treatments.
Figure 3.
Cell cycle profiles and regulatory proteins in wild-type HCT116 cells and PPC1 prostate cancer cells treated with etoposide (VP-16; 50 μM), camptothecin (CPT; 2 μM), or each drug in combination with quercetin. HCT116 (A) or PPC1 (B) cells were treated with VP-16 or CPT in the presence or absence of 50 μM quercetin for 24 hours, after which cell cycle profiles were analyzed by flow cytometry. Cell cycle histograms are shown. C: HCT116 cells were treated with VP-16 or CPT in the presence or absence of 50 μM quercetin. Cellular levels of the proteins p53, BAX, p21, cyclin B1 and PCNA were determined by immunoblotting. β-Actin immunoblotting is included to show comparable protein loading. In addition to modifying the cell cycle profiles of treated cells, the addition of quercetin inhibited the induction of p53 and its downstream target proteins.
Effects of combining quercetin with chemotherapeutic drugs in p53-null cells
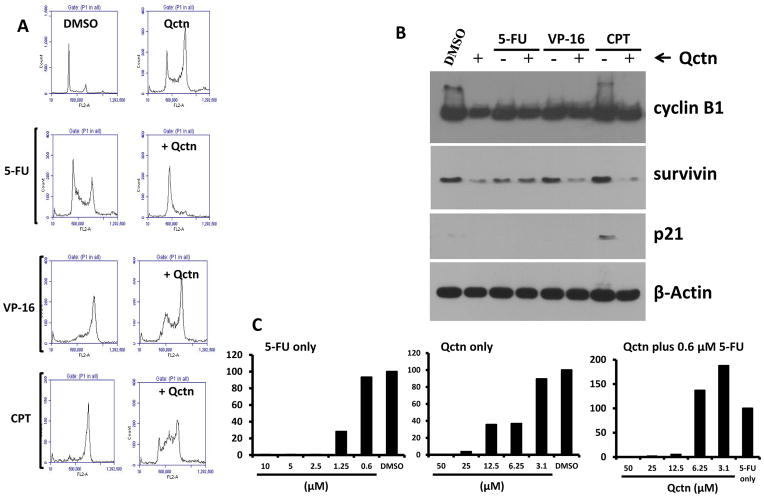
We also examined the interaction of quercetin with 5-FU, etoposide and camptothecin in p53-null HCT116 cells. We observed that, similarly to the wild-type cells, quercetin induced an increase in the proportion of G2/M cells after 24 hours of treatment, and also interfered with the cell cycle effects of the drugs (Figure 4A). There were differences in the cell cycle responses between wild-type and p53-null cells treated with the drugs alone. While etoposide and camptothecin both induced predominantly S-phase arrest in the wild-type cells, the same drugs induced a predominantly G2/M arrest in p53-null cells. Nevertheless, the addition of quercetin to the drugs resulted in the accumulation of cells in the S- and G1-phases (Figure 4A). In addition to the cell cycle effects, there was a moderate reduction in cyclin B1 levels by quercetin and combination treatment. Furthermore, survivin levels were also reduced by quercetin, especially in etoposide- and camptothecin-treated p53-null cells. It is important to note that in these cells, the basal levels of p21 were very low and BAX expression was undetectable (Figure 4B and data not shown). A very low level of p21 induced by camptothecin was reduced to an undetectable level by co-treatment with quercetin.
Figure 4.
Effects of the co-treatment of p53-null cells with DNA-damaging drugs and quercetin. A and B: p53-Null HCT116 cells were treated with 5-fluorouracil (5-FU; 10 μM), etoposide (VP-16; 50 μM), or camptothecin (CPT; 2 μM), in the presence or absence of 50 μM quercetin (Qctn). DMSO was used as a vehicle control. Cell cycle profiles were analyzed after 24 hours by flow cytometry. Cell cycle histograms are shown in A and cyclin B1, survivin, p21 and β-Actin protein levels detected by immunoblotting are shown in B. In C, representative graphs from two independent clonogenic assays of p53-null HCT116 cells treated with 5-FU, quercetin, or 0.6 μM 5-FU in combination with the indicated doses of quercetin are shown. Relative clonogenicity is presented as a percentage of the number of colonies that formed in the control cells. DMSO served as vehicle control for 5-FU or quercetin single agent treatments, whereas 0.6 μM 5-FU was used as a baseline control for the combination treatments. In addition to modifying the cell cycle response of p53-null cells to DNA-damaging drugs, low doses of quercetin appeared to support clonogenicity of the p53-null cells treated with a low dose of 5-FU.
Next, the clonogenicity of p53-null HCT116 cells was examined under treatment with 5-FU or quercetin alone, or with a combination of low-dose 5-FU (0.6 μM) with 0–50 μM quercetin. The relative clonogenicity of treated cells was compared against the solvent (DMSO) for compounds alone, or against 5-FU only for combination-treated cells. Results indicate that while 0.6 μM 5-FU permitted development of colonies comparable to that of the vehicle control cells, combination of the same dose of 5-FU with 12.5 μM or higher doses of quercetin significantly reduced the clonogenicity of p53-null HCT116 cells, which was comparable to the effects seen in wild-type cells (Figure 1C). However, unlike in wild-type cells, the combination of 0.6 μM 5-FU with 6.25 or 3.1 μM quercetin increased the clonogenic survival of p53-null HCT116 cells, as seen by the increased number of colonies in combination-treated cells compared to those treated with 5-FU alone.
Discussion
This study examined the in vitro interaction between chemotherapeutic drugs and the dietary flavonoid quercetin in cancer cell lines. Although several other factors may contribute to the interaction in vivo, the in vitro data reported here reflect the possibility for nutraceutical or higher levels of the dietary flavonoid to potentiate the activity of 5-FU or regimens that include 5-FU. Additionally, our results also indicate a potentially undesirable interaction between the two compounds at lower doses, especially in a p53-null environment.
These results also suggest the need to further investigate in vitro and in vivo conditions under which dietary supplements may be either beneficial or undesirable combined with cancer therapy. It remains largely unknown how dietary compounds or supplements affect the outcomes of cancer chemotherapy and survival. Despite the fact that a large proportion of cancer patients and survivors take dietary supplements in various forms (31–35), there is no established knowledge on how simultaneous presence of drugs and bioactive compounds, such as dietary supplements, interact and influence the outcomes. Since most cancer drugs, including 5-FU, affect the proliferation of normal cells in patients and have severe side-effects, it is most beneficial to be able to significantly reduce the dose of the drug while maintaining the desirable level of anticancer activity. Combination of drugs or other agents that have fewer or less severe side-effects would be ideal alternatives to reduce the toxicity of first-line drugs such as 5-FU. Bioactive nutraceuticals or potent dietary compounds such as quercetin may be considered useful for these purposes.
A few recent studies have compared the combination of quercetin with other cancer chemotherapeutic drugs. In one study, where 100 μM quercetin was combined with tumor necrosis factor-related apoptosis inducing ligand (TRAIL) to treat glioblastoma cell lines, a synergistic effect was observed in induction of apoptosis. The mechanism suggested for this synergistic activity includes the down-regulation of anti-apoptotic proteins X-linked inhibitor of apoptosis protein (XIAP) and survivin (18). In another study where the potentiation of cell killing by 5-FU and platinum compounds was examined in hepatoma cells treated with the drugs in combination with quercetin, it was shown that the inhibition of the expression of heat-shock proteins (HSPs) was a critical function of quercetin. In the studies, doses of quercetin between 50 μM and 200 μM were able to counteract the HSPs induced by the chemotherapeutic drugs and potentiate the drug effects, suggesting the potential use of pharmacologic doses of quercetin in combination with standard anticancer drugs (17, 36). Similarly, Sharma et al. (17) and Kuhar et al. (16) also found that head and neck cancer cells and non-small cell lung carcinoma cells were sensitized to cisplatin by quercetin through mechanisms that possibly involve AKR mouse T-cell lymphoma protein (AKT) inhibition and mitochondrial proteins. Similarly to our results, quercetin was recently reported to enhance the effects of 5-FU in microsatellite instable (MSI) colon cancer cells (37), as well as of doxorubucin in breast cancer cells (15, 19). Differential sensitivities of wild-type and p53-deficient cells to 5-FU has also been documented (37, 38).
Our results suggest that quercetin interacts with chemotherapeutic drugs in a dose-dependent manner to either synergize with or counteract the drugs. While high doses of quercetin clearly synergize with 5-FU in reducing the clonogenicity of both wild-type and p53-null cells, lower doses (below 6 μM) may promote the clonogenic survival of p53-null cells. The results also suggest that the cell cycle modulatory effects of high doses of quercetin are accompanied by reduction in the proteins that regulate cell cycle and apoptosis. Since the cell cycle antagonism by quercetin is also evident in p53-null cells, and is associated with the reduction of p53-target genes in wild-type cells, it is possible that the interaction between the chemotherapeutic drugs and high doses (50 μM) of quercetin is p53 independent. However, since lower doses of quercetin improved clonogenic survival of p53-null cells treated with sub-effective doses of 5-FU, this low-dose effect of quercetin seems to be p53 dependent. The potential mechanisms for this phenomenon are being investigated. Of note, such a dose-dependent effect of quercetin has recently been described as hormesis and synergy outcomes (10), where low doses may serve as antioxidant, while high doses may be pro-oxidant.
Data from this study also suggest that the interaction between quercetin and the drugs may be independent of p53 activation, as the transcriptional activity of p53 was not increased by the combination treatments. Although the exact mechanisms are not known, autophagy, mitochondria-mediated, or other mechanisms (16, 39, 40) could be potential alternatives for the observed synergy between quercetin and chemotherapeutic agents at therapeutic doses.
In previous studies, precautions were suggested when combining bioactive antioxidant compounds with chemotherapeutic drugs. Studies have shown that the antioxidant ascorbic acid (vitamin C) antagonized the activity of a diverse class of chemotherapeutic drugs when co-administered, suggesting a potentially antagonistic interaction between drugs and dietary compounds with antioxidant properties (41). Our prior work also identified an interaction between quercetin and taxol or nocodazole, where the G2/M arrest phenotype induced by the drugs was abolished by co-treatment with quercetin (22). However, our study also demonstrated that the antagonism was only transient, in that the co-treatment did not offer any long-term survival advantage to cells even when the G2/M arrest phenotype was absent. The current study suggests that a similar advantage of co-treatment with quercetin may be true for p53-proficient cells, whereas in p53-null cells, a dose-dependent outcome may be encountered.
There are numerous cellular targets of quercetin that have been implicated in its anticancer activity (13, 42). Depending on the cell type and the dose used, various signaling pathways may be modulated by this flavonoid. However, given the high doses employed in many in vitro studies, it still remains largely unknown how nutritional or nutraceutical levels of quercetin could function to prevent cancer. Potential factors that affect the in vivo bioactivity include interactions between dietary components, metabolism in the body, availability in cellular microenvironments, abundance of targets in the cells, etc. (10). Importantly, the cell and molecular effects of long-term administration of such bioactive compounds as present in the diet and supplementation remain a challenge.
In summary, high doses of quercetin synergistically potentiated the antiproliferative activity of DNA-damaging drugs even when the cell cycle appears to be antagonized such interaction in cancer cells. Moreover, the p53 status of the targeted cells should be taken into account because combination of low doses of quercetin with sub-effective doses of 5-FU may potentially provide a selective advantage to p53-null cells. Furthermore, in vivo studies are recommended to determine the conditions under which adjuvant therapy with combinations of DNA-damaging drugs and nutraceutical or pharmaceutical doses of quercetin might suppress the growth of colon cancer cells. Further work is also needed to delineate the role of p53, its absence and mutations, on the response of cells to such combination treatments.
Acknowledgments
Research for this study was supported by NIH/NCI/NIGMS grant number SC2CA138178, and 2 U54 CA118948-06. We acknowledge the institutional support through the TU CVMANH Center of Excellence grant 5D34HP00001-20-00, and the NCRR/RCMI core facility support through grant #G12RR003059. The funding agencies had no role in the initiation, execution or analysis of this study.
References
- 1.He X, Liu RH. Phytochemicals of apple peels: isolation, structure elucidation, and their antiproliferative and antioxidant activities. J Agric Food Chem. 2008;56:9905–9910. doi: 10.1021/jf8015255. [DOI] [PubMed] [Google Scholar]
- 2.Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 3.de Groot H, Rauen U. Tissue injury by reactive oxygen species and the protective effects of flavonoids. Fundam Clin Pharmacol. 1998;12:249–255. doi: 10.1111/j.1472-8206.1998.tb00951.x. [DOI] [PubMed] [Google Scholar]
- 4.Terao J. Dietary flavonoids as antioxidants. Forum Nutr. 2009;61:87–94. doi: 10.1159/000212741. [DOI] [PubMed] [Google Scholar]
- 5.Hu P, Wang M, Chen WH, Liu J, Chen L, Yin ST, Yong W, Chen JT, Wang HL, Ruan DY. Quercetin relieves chronic lead exposure-induced impairment of synaptic plasticity in rat dentate gyrus in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:43–51. doi: 10.1007/s00210-008-0301-z. [DOI] [PubMed] [Google Scholar]
- 6.Kelsey NA, Wilkins HM, Linseman DA. Nutraceutical antioxidants as novel neuroprotective agents. Molecules. 2010;15:7792–7814. doi: 10.3390/molecules15117792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A, Sehgal N, Kumar P, Padi SS, Naidu PS. Protective effect of quercetin against ICV colchicine-induced cognitive dysfunctions and oxidative damage in rats. Phytother Res. 2008;22:1563–1569. doi: 10.1002/ptr.2454. [DOI] [PubMed] [Google Scholar]
- 8.Ossola B, Kaariainen TM, Mannisto PT. The multiple faces of quercetin in neuroprotection. Expert Opin Drug Saf. 2009;8:397–409. doi: 10.1517/14740330903026944. [DOI] [PubMed] [Google Scholar]
- 9.Murphy EA, Davis JM, McClellan JL, Carmichael MD. Quercetin’s Effects on Intestinal Polyp Multiplicity and Macrophage Number in the Apc(Min/+) Mouse. Nutr Cancer. 2011;63:421–426. doi: 10.1080/01635581.2011.535954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vargas AJ, Burd R. Hormesis and synergy: pathways and mechanisms of quercetin in cancer prevention and management. Nutr Rev. 2010;68:418–428. doi: 10.1111/j.1753-4887.2010.00301.x. [DOI] [PubMed] [Google Scholar]
- 11.Ma ZS, Huynh TH, Ng CP, Do PT, Nguyen TH, Huynh H. Reduction of CWR22 prostate tumor xenograft growth by combined tamoxifen-quercetin treatment is associated with inhibition of angiogenesis and cellular proliferation. Int J Oncol. 2004;24:1297–1304. [PubMed] [Google Scholar]
- 12.Dihal AA, de Boer VC, van der Woude H, Tilburgs C, Bruijntjes JP, Alink GM, Rietjens IM, Woutersen RA, Stierum RH. Quercetin, but not its glycosidated conjugate rutin, inhibits azoxymethane-induced colorectal carcinogenesis in F344 rats. J Nutr. 2006;136:2862–2867. doi: 10.1093/jn/136.11.2862. [DOI] [PubMed] [Google Scholar]
- 13.Araujo JR, Goncalves P, Martel F. Chemopreventive effect of dietary polyphenols in colorectal cancer cell lines. Nutr Res. 2011;31:77–87. doi: 10.1016/j.nutres.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45:2179–2205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Du G, Lin H, Yang Y, Zhang S, Wu X, Wang M, Ji L, Lu L, Yu L, Han G. Dietary quercetin combining intratumoral doxorubicin injection synergistically induces rejection of established breast cancer in mice. Int Immunopharmacol. 2010;10:819–826. doi: 10.1016/j.intimp.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Kuhar M, Sen S, Singh N. Role of mitochondria in quercetin-enhanced chemotherapeutic response in human non-small cell lung carcinoma H-520 cells. Anticancer Res. 2006;26:1297–1303. [PubMed] [Google Scholar]
- 17.Sharma H, Sen S, Singh N. Molecular pathways in the chemosensitization of cisplatin by quercetin in human head and neck cancer. Cancer Biol Ther. 2005;4:949–955. doi: 10.4161/cbt.4.9.1908. [DOI] [PubMed] [Google Scholar]
- 18.Siegelin MD, Reuss DE, Habel A, Rami A, von Deimling A. Quercetin promotes degradation of survivin and thereby enhances death-receptor-mediated apoptosis in glioma cells. Neuro Oncol. 2009;11:122–131. doi: 10.1215/15228517-2008-085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staedler D, Idrizi E, Kenzaoui BH, Juillerat-Jeanneret L. Drug combinations with quercetin: doxorubicin plus quercetin in human breast cancer cells. Cancer chemotherapy and pharmacology. 2011;68:1161–1172. doi: 10.1007/s00280-011-1596-x. [DOI] [PubMed] [Google Scholar]
- 20.Golden EB, Lam PY, Kardosh A, Gaffney KJ, Cadenas E, Louie SG, Petasis NA, Chen TC, Schonthal AH. Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors. Blood. 2009;113:5927–5937. doi: 10.1182/blood-2008-07-171389. [DOI] [PubMed] [Google Scholar]
- 21.Liu FT, Agrawal SG, Movasaghi Z, Wyatt PB, Rehman IU, Gribben JG, Newland AC, Jia L. Dietary flavonoids inhibit the anticancer effects of the proteasome inhibitor bortezomib. Blood. 2008;112:3835–3846. doi: 10.1182/blood-2008-04-150227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samuel T, Fadlalla K, Turner T, Yehualaeshet TE. The flavonoid quercetin transiently inhibits the activity of taxol and nocodazole through interference with the cell cycle. Nutr Cancer. 2010;62:1025–1035. doi: 10.1080/01635581.2010.492087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverstein RA, de Valdivia EG, Visa N. The incorporation of 5-fluorouracil into RNA affects the ribonucleolytic activity of the exosome subunit Rrp6. Mol Cancer Res. 2011;9:332–340. doi: 10.1158/1541-7786.MCR-10-0084. [DOI] [PubMed] [Google Scholar]
- 24.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 25.Brody JR, Hucl T, Costantino CL, Eshleman JR, Gallmeier E, Zhu H, van der Heijden MS, Winter JM, Wikiewicz AK, Yeo CJ, Kern SE. Limits to thymidylate synthase and TP53 genes as predictive determinants for fluoropyrimidine sensitivity and further evidence for RNA-based toxicity as a major influence. Cancer Res. 2009;69:984–991. doi: 10.1158/0008-5472.CAN-08-3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gravalos C, Garcia-Escobar I, Garcia-Alfonso P, Cassinello J, Malon D, Carrato A. Adjuvant chemotherapy for stages II, III and IV of colon cancer. Clin Transl Oncol. 2009;11:526–533. doi: 10.1007/s12094-009-0397-8. [DOI] [PubMed] [Google Scholar]
- 27.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 28.Fadlalla K, Watson A, Yehualaeshet T, Turner T, Samuel T. Ruta graveolens extract induces DNA damage pathways and blocks Akt activation to inhibit cancer cell proliferation and survival. Anticancer Res. 2011;31:233–241. [PMC free article] [PubMed] [Google Scholar]
- 29.Longley DB, Latif T, Boyer J, Allen WL, Maxwell PJ, Johnston PG. The interaction of thymidylate synthase expression with p53-regulated signaling pathways in tumor cells. Semin Oncol. 2003;30:3–9. doi: 10.1016/s0093-7754(03)00119-2. [DOI] [PubMed] [Google Scholar]
- 30.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 31.Naing A, Stephen SK, Frenkel M, Chandhasin C, Hong DS, Lei X, Falchook G, Wheler JJ, Fu S, Kurzrock R. Prevalence of complementary medicine use in a phase 1 clinical trials program: The MD Anderson Cancer Center Experience. Cancer. 2011;117:5142–5150. doi: 10.1002/cncr.26164. [DOI] [PubMed] [Google Scholar]
- 32.Dy GK, Bekele L, Hanson LJ, Furth A, Mandrekar S, Sloan JA, Adjei AA. Complementary and alternative medicine use by patients enrolled onto phase I clinical trials. J Clin Oncol. 2004;22:4810–4815. doi: 10.1200/JCO.2004.03.121. [DOI] [PubMed] [Google Scholar]
- 33.Hlubocky FJ, Ratain MJ, Wen M, Daugherty CK. Complementary and alternative medicine among advanced cancer patients enrolled on phase I trials: a study of prognosis, quality of life, and preferences for decision making. J Clin Oncol. 2007;25:548–554. doi: 10.1200/JCO.2005.03.9800. [DOI] [PubMed] [Google Scholar]
- 34.Boon H, Stewart M, Kennard MA, Gray R, Sawka C, Brown JB, McWilliam C, Gavin A, Baron RA, Aaron D, Haines-Kamka T. Use of complementary/alternative medicine by breast cancer survivors in Ontario: prevalence and perceptions. J Clin Oncol. 2000;18:2515–2521. doi: 10.1200/JCO.2000.18.13.2515. [DOI] [PubMed] [Google Scholar]
- 35.Sparber A, Bauer L, Curt G, Eisenberg D, Levin T, Parks S, Steinberg SM, Wootton J. Use of complementary medicine by adult patients participating in cancer clinical trials. Oncol Nurs Forum. 2000;27:623–630. [PubMed] [Google Scholar]
- 36.Sharma A, Upadhyay AK, Bhat MK. Inhibition of Hsp27 and Hsp40 potentiates 5-fluorouracil and carboplatin mediated cell killing in hepatoma cells. Cancer Biol Ther. 2009;8:2106–2113. doi: 10.4161/cbt.8.22.9687. [DOI] [PubMed] [Google Scholar]
- 37.Xavier CP, Lima CF, Rohde M, Pereira-Wilson C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer chemotherapy and pharmacology. 2011;68:1449–1457. doi: 10.1007/s00280-011-1641-9. [DOI] [PubMed] [Google Scholar]
- 38.Boyer J, McLean EG, Aroori S, Wilson P, McCulla A, Carey PD, Longley DB, Johnston PG. Characterization of p53 wild-type and null isogenic colorectal cancer cell lines resistant to 5-fluorouracil, oxaliplatin, and irinotecan. Clin Cancer Res. 2004;10:2158–2167. doi: 10.1158/1078-0432.ccr-03-0362. [DOI] [PubMed] [Google Scholar]
- 39.Debes A, Oerding M, Willers R, Gobel U, Wessalowski R. Sensitization of human Ewing’s tumor cells to chemotherapy and heat treatment by the bioflavonoid quercetin. Anticancer Res. 2003;23:3359–3366. [PubMed] [Google Scholar]
- 40.Sliutz G, Karlseder J, Tempfer C, Orel L, Holzer G, Simon MM. Drug resistance against gemcitabine and topotecan mediated by constitutive hsp70 overexpression in vitro: implication of quercetin as sensitiser in chemotherapy. Br J Cancer. 1996;74:172–177. doi: 10.1038/bjc.1996.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heaney ML, Gardner JR, Karasavvas N, Golde DW, Scheinberg DA, Smith EA, O’Connor OA. Vitamin C antagonizes the cytotoxic effects of antineoplastic drugs. Cancer Res. 2008;68:8031–8038. doi: 10.1158/0008-5472.CAN-08-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol Nutr Food Res. 2008;52:507–526. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]