Abstract
The molecular basis of vertebrate germ layer formation has been the focus of intense scrutiny for decades, and the inductive interactions underlying this process are well-defined; only recently, however, have studies demonstrated that the regulated inhibition of ectopic germ layer formation is also critical for patterning the early vertebrate embryo. We report here the characterization of Xema (Xenopus Ectodermally-expressed Mesendoderm Antagonist), a novel member of the Foxi-subclass of winged-helix transcription factors that is involved in the suppression of ectopic germ layer formation in the frog, Xenopus laevis. Xema transcripts are restricted to the animal pole ectoderm during early Xenopus development. Ectopic expression of Xema RNA inhibits mesoderm induction, both by growth factors and in the marginal zone, in vivo. Conversely, introduction of antisense morpholino oligos directed against the Xema transcript stimulates the expression of a broad range of mesodermal and endodermal marker genes in the animal pole. Our studies demonstrate that Xema is both necessary and sufficient for the inhibition of ectopic mesendoderm in the cells of the presumptive ectoderm, and support a model in which Fox proteins function in part to restrict inappropriate germ layer development throughout the vertebrate embryo.
Introduction
All tissues in the animal derive from the three primary germ layers: ectoderm, endoderm, and mesoderm. Ectodermal derivatives constitute both the central nervous system and the epidermis, while endodermal derivatives give rise to a number of organs including the lungs, liver, pancreas, thyroid gland, and thymus, as well as to the entire gastrointestinal epithelium. The mesodermal germ layer plays a pivotal role in organizing the vertebrate body axes, and itself gives rise to the muscular, skeletal, and circulatory systems. Much of our understanding of mesoderm formation has come from classical and molecular studies in the amphibian embryo. In the frog, Xenopus laevis, mesoderm is an inducible cell fate: although vegetal pole explants form only endodermal derivatives and animal pole (“animal cap”) explants form only ectodermal derivatives, signals from the cells of the vegetal pole generate the additional formation of mesoderm in overlying animal caps when these explants are recombined (Slack et al., 1984). These observations are consistent with the amphibian fate map: mesoderm forms in the so-called “marginal zone,” at the border between the animal and vegetal poles (Dale and Slack, 1987).
Two classes of secreted molecules have been found to possess mesoderm-inducing activity. Addition of Fibroblast Growth Factor (FGF) or members of the Activin/Nodal-related class of the Transforming Growth Factor β (TGFβ ligand family will induce mesoderm in explants of competent ectoderm, while inhibition of the signaling cascades downstream of these factors blocks mesoderm formation in vivo (Harland and Gerhart, 1997; Slack, 1994). Although considerably less attention has been focused on the signals governing endoderm formation, recent studies have demonstrated that this germ layer is also dependent on TGFβ signaling, as well as on a cascade of transcription factors that act cell autonomously to ensure commitment to an endodermal fate (Shivdasani, 2002). The recognition that similar mechanisms underlie the formation of both mesoderm and endoderm are reflected in the notion of a “mesendodermal” field in the early embryo, containing both mesodermal and endodermal precursors, from which the distinct germ layers emerge only during gastrulation. A second, distinct domain thought to contain only mesodermal precursors has also been described. (Rodaway and Patient, 2001) In this report, we will use the term “mesendoderm” somewhat more loosely, to describe all early mesodermal and/or endodermal cells. Although the mechanisms governing early patterning of the vertebrate ectoderm have been the subject of considerable debate, it has, until recently, been generally assumed that the development of the presumptive ectoderm is governed in large part by the absence of mesoderm- or endoderm-promoting cues.
While growth factor-mediated induction clearly drives mesoderm formation in the vertebrate embryo, recent studies in the mouse and frog suggest that germ layer suppression is also critical in establishing the early vertebrate body plan. For example, the TGFβ ligand Nodal plays an essential role during mesoderm and endoderm formation in the mouse, as nodal mutant mice lack a primitive streak, a posterior structure from which embryonic and extra-embryonic mesoderm, and embryonic endoderm are derived (Conlon et al., 1994; Whitman, 2001; Zhou et al., 1993). Surprisingly, prior to gastrulation, Nodal is transiently expressed throughout the early epiblast (Whitman, 2001). Recent studies suggest that the anterior visceral endoderm (AVE), an extraembryonic tissue overlying the anterior epiblast, is a critical source of Nodal antagonism (Beddington and Robertson, 1999; Perea-Gomez et al., 2002); the AVE expresses at least two secreted Nodal antagonists, Cerberus-like (Cer1) and a TGFβ superfamily molecule, Lefty1 (Perea-Gomez et al., 2001). A subset of compound Cer1−/−;Lefty1−/− mutant embryos develop ectopic primitive streaks (Perea-Gomez et al., 2002); this phenotype is partially rescued in mice containing a single copy of nodal, suggesting that Cer1 and Lefty1, secreted by the AVE, function to restrict ectopic streak formation by inhibiting Nodal activity anteriorly. Recent evidence points to Nodal antagonism at several additional levels during early development: both the transcriptional corepressor DRAP1, and Dpr2, which promotes the lysosomal degradation of Nodal receptors, have been shown to limit Nodal activity in the vertebrate embryo (Iratni et al., 2002; Zhang et al., 2004).
Several recent studies have suggested that inhibition of ectopic germ layer formation is also critical during early development of the frog, Xenopus laevis. For example, an analysis of the Xenopus brachyury promoter found that the restricted expression of this gene in the cells of the mesoderm is in part the result of specific repression in both endoderm and ectoderm; along with one or more as yet undefined homeodomain proteins, the δEF1 repressor family Smad-interacting protein-1 (SIP1) appears to be required for this repression (Lerchner et al., 2000). Recently, Wardle and Smith reported the presence of a significant number of cells found throughout the early gastrula-stage Xenopus embryo that express mesendodermal markers inappropriate to their location and, on occasion, do not correspond to any single regional fate (Wardle and Smith, 2004); these “rogue” cells are largely undetectable by late gastrula stages, suggesting that they are either converted to the fate of their neighbors, or eliminated via as-yet uncharacterized mechanisms. Finally, the Forkhead box (Fox) DNA-binding protein Hepatocyte Nuclear Factor 3β (HNF3β)/FoxA2, expressed throughout the Xenopus deep endoderm at early gastrula stages, also inhibits inappropriate germ layer development in the frog: misexpression of HNF3β in the marginal zone leads to a loss of mesoderm and dorsal mesendoderm, while suppression of HNF3β target genes leads to ectopic axis formation in the endoderm (Suri et al., 2004). Consistently, gene targeting studies have implicated a requirement for murine HNF3β in mesoderm suppression: targeted deletion of both HNF3β and the LIM homeodomain protein Lim 1 in the visceral endoderm leads to the production of ectopic ventral mesoderm in the epiblast, a phenotype not observed following deletion of either gene alone (Ang and Rossant, 1994; Dufort et al., 1998; Perea-Gomez et al., 1999; Weinstein et al., 1994). Thus, the expression of HNF3β in the Xenopus deep endoderm and the mammalian extra-embryonic endoderm appears to be involved in the suppression of ectopic mesendoderm in, respectively, both lower and higher vertebrates. The molecular basis of this requirement, however, remains largely unknown.
We report here that a Fox-mediated, mesendoderm-suppressing mechanism is also active within the Xenopus ectoderm and is required for normal development. We describe the characterization of Xema, a Fox protein expressed exclusively in the ectoderm during early Xenopus development. Misexpression of Xema blocks mesendoderm induction in vivo and by inducing agents; critically, we also find that Xema knockdown promotes mesendoderm development in gastrula stage animal cap explants, demonstrating a requirement for Xema in the inhibition of ectopic germ layer formation in the animal pole. Taken together with studies from our lab and others on HNF3β, this body of research supports a model in which Fox proteins are fundamental mediators of the widespread suppression of ectopic germ layer development during early vertebrate embryogenesis.
Materials and Methods
RNA preparation, explant dissection, and cell culture
RNA was synthesized in vitro in the presence of cap analog using the mMessage mMachine kit (Ambion). Microinjection, explant dissection, and cell culture were performed as described (Hemmati-Brivanlou and Melton, 1994; Wilson and Hemmati-Brivanlou, 1995).
RT-PCR
RT-PCR was performed as described (Wilson and Hemmati-Brivanlou, 1995). Primers designed specifically for this study are as follows. Xema-U: 5′-AGTAGGTCAGTTCCACTTGG; Xema-D: 5′-AAGGACTTTGTCGTGACTGC. All other primer sequences are as described (Suri et al, 2004).
Subtractive hybridization screen
PCR-based differential screens were performed to identify genes with expression enriched in or limited to the gastrula-stage ectoderm, relative to levels found in other regions of the embryo or after treatment with various mesoderm-inducing reagents. In the screen from which Xema was isolated, cDNA was generated from 1μg of mRNA isolated from uninjected animal caps and animal caps injected with EnR-HNF3β RNA; expression of this construct generates ectopic mesoderm in both endoderm and ectoderm (Suri et al., 2004)(data not shown). cDNA pools were hybridized and differentially expressed genes were selected using a PCR-based subtraction method (Clonetech PCR-Select cDNA subtraction kit), both in the lab and using the “Custom PCR-Select Subtraction Analysis” service (Clonetech). Clones whose expression was found to be downregulated in induced explants were selected, amplified, and sequenced. Primers were generated and RT-PCR was performed to confirm differential expression using cDNA derived from independent experimental samples.
Whole-mount in situ hybridization, immunohistochemistry, and β-gal staining
Protocols for whole-mount in situ hybridization were derived from Harland (Harland, 1991) with the following changes: (1) RNase steps were eliminated; (2) BM purple AP substrate (Boehringer Mannheim) replaced BCIP/NBT. The antisense Xema and Xbra probes were synthesized in the presence of digoxygenin-11-UTP (Boehringer Mannheim). Whole-mount β-gal detection was performed as described (Smith and Harland, 1992). Whole-mount antibody staining was performed as described (Hemmati-Brivanlou and Melton, 1994). The 12/101 antibody (ascites, Developmental Studies Hybridoma Bank) was used at a 1:1 dilution. Secondary antibody was a donkey anti-mouse IgG coupled to horseradish peroxidase (Jackson Laboratories), and was used at 1:1000 dilution. Color reactions were performed using the Vector SG and DAB kits (Vector Laboratories).
Preparation of Xema fusion proteins
Xema fusion constructs were generated by PCR. For EnR-Xema, residues 1-298 of the Drosophila Engrailed repressor (Kessler, 1997) were fused upstream of full-length Xema. For VP-Xema, residues 410-490 of the VP16 activator (Kessler, 1997) were fused upstream of full length Xema.
Morpholinos
Morpholino oligos (Gene Tools) were heated for five minutes at 65 degrees then quenched on ice, prior to injection at the 2- or 4-cell stage. Sequences are as follows: control morpholino: 5′-CCTCTTACCTCAGTTACAATTTATA; Xema MO-1: GTGCTTGTGGATCAAATGCACTCAT; Xema MO-2: AGGTCACAAATACACCTGTACTAGC; control morpholino (mismatch-1/MM-1): GTcCTTGTaATgAAATcCACTgAT
Results
Identification of the foxi gene Xema
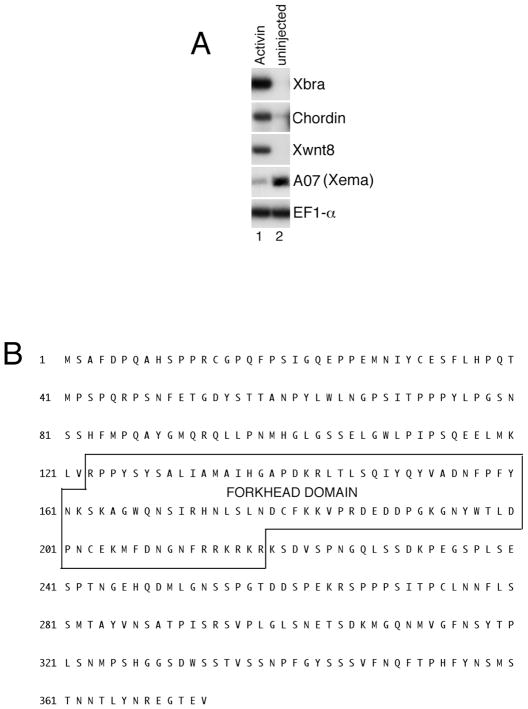
In an attempt to identify factors involved in germ layer suppression, we performed a PCR-based differential screen to select for genes that are downregulated in gastrula stage ectoderm following mesoderm-inducing treatments. cDNA clone A07 was one of several isolates whose expression was strongly suppressed by the TGFβ ligand Activin (Fig. 1A). Database searches revealed that the corresponding full-length transcript encodes a novel Fox transcription factor, of the Foxi class (Fig. 1B)(Kaestner et al., 2000). While A07 shares highest homology with murine Foxi1, a distinct Xenopus gene with lower overall homology has already been designated Foxi1 (Lef et al., 1994). Among lower vertebrate Foxi genes, A07 is most similar to zebrafish Foxi3; furthermore, the DNA-binding domain of A07 appears identical to a partial Xenopus sequence described elsewhere as “Foxi3” (Solomon et al., 2003). Since we cannot currently provide the standard nomenclature for this gene beyond an assignation to the Foxi subclass, we refer to it, for reasons that will become clear, as Xema (Xenopus Ectodermally-expressed Mesendoderm Antagonist, pronounced “zee-ma,”).
Fig. 1.
Identification of the Xenopus Foxi-class gene Xema. (A) Activin inhibits A07 (Xema) expression. RT-PCR analysis of animal cap explants dissected at late blastula stages and cultured until midgastrula stages. Activin (0.5 ng/ml) was added to stage 9 animal caps, as listed. (B) Putative Xema amino acid sequence. The forkhead box (Fox) DNA-binding domain is indicated.
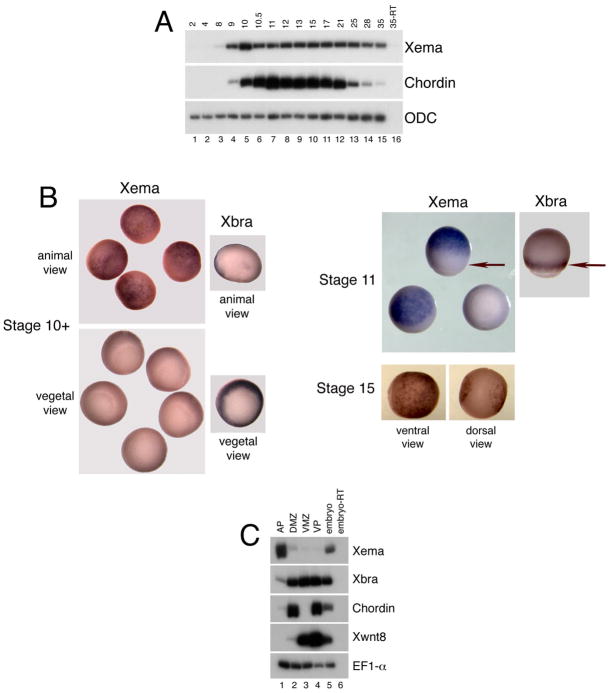
In order to determine the temporal range of Xema expression during early development, we performed reverse transcription polymerase chain reaction (RT-PCR) on RNA harvested from embryos at various embryonic stages. Xema is not expressed maternally, as transcripts are present only after the initiation of zygotic transcription at stage 8.5 (Fig. 2A). Expression is highest at early gastrula stages (stage 10) and is maintained throughout neurula, tailbud, and early tadpole stages. To analyze the spatial distribution of xema transcripts, we performed whole mount in situ hybridization studies. Xema transcripts were observed in the cells of the animal pole at late blastula and gastrula stages; (Fig. 2B and data not shown). Xema transcripts were not detected in the marginal zone during early (stage 10+) or mid (stage 11) gastrula stages; Xbra expression, restricted to the gastrula marginal zone, is shown for comparison (Fig. 2B) (Smith et al., 1991). Xema expression is restricted to the epidermis during early neurula stages, and is excluded from the dorsal ectoderm of the forming neural plate (Fig. 2B). This epidermal-specific expression persists through tailbud stages (data not shown). In order to confirm the regional distribution of Xema transcripts, we performed RT-PCR analysis on early gastrula stage explants. These assays support the in situ analysis: xema is expressed in the animal pole (AP) ectoderm, and is excluded from the ventral and dorsal marginal zone (VMZ and DMZ), as well as the vegetal pole (VP) (Fig. 2C). Thus, Xema is expressed zygotically in the ectoderm during early Xenopus development, and is excluded from regions of the embryo that contribute to mesodermal and endodermal lineages.
Fig. 2.
Expression of Xema during early development. (A) RT-PCR analysis of Xema temporal expression. The ‘-RT’ lane contains all reagents except reverse transcriptase and was used as a negative control. Ornithine decarboxylase (ODC) is used as a loading control (Bassez et al., 1990). Chordin expression is initiated zygotically and is used as a positive control. (B) Whole-mount in situ hybridization of early gastrula (Stage 10+), midgastrula (Stage 11), and early neurula (Stage 15) stage embryos, using antisense Xema and Xbrachyury (Xbra) probes. (B) Xema expression is seen as a blue stain throughout the animal pole of gastrula stage albino embryos; Xema expression is excluded from the marginal zone (denoted by arrows at stage 11) and vegetal pole. Expression of the panmesodermal marker Xbra is found only in the marginal zone of gastrula stage embryos and is used as a control. Xema is expressed in the vental ectoderm (epidermis) of early neurula stage embryos (ventral view); expression is excluded from the neural plate (dorsal view). (C) RT-PCR analysis of Xema expression in early gastrula stage explants. EF1-α is used as a loading control (Krieg et al., 1989). Xbra is a panmesodermal marker at this stage (Smith et al., 1991); chordin is a dorsal endomesodermal marker (Sasai et al., 1994); Xwnt8 is a ventrolateral marker (Christian et al., 1991; Smith and Harland, 1991).
Ectopic Xema suppresses mesendoderm formation
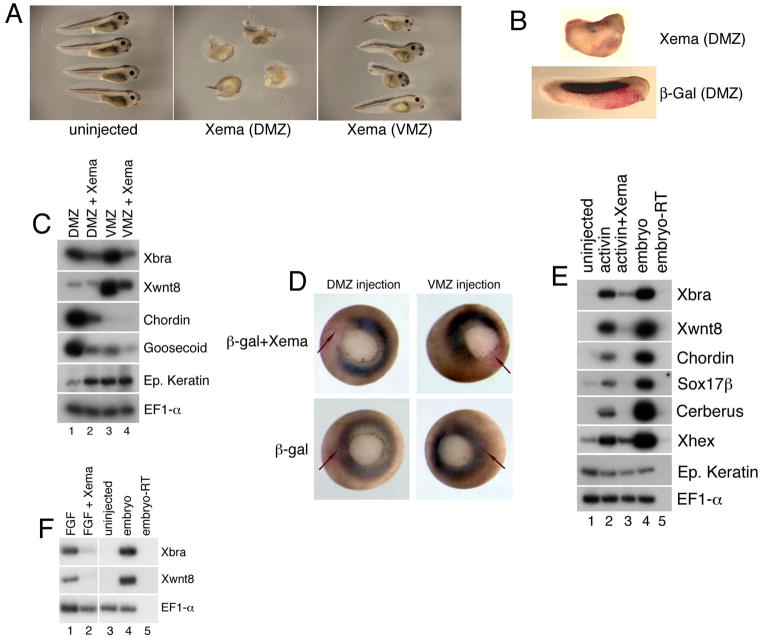
We have previously demonstrated that the fox gene HNF3β is expressed throughout the deep endoderm during Xenopus gastrulation and is involved in suppressing ectopic mesoderm and dorsal mesendoderm in this region (Suri et al., 2004). The gastrula-stage expression of Xema suggested that this fox gene might be involved in the suppression of ectopic germ layer formation in the presumptive ectoderm. Dorsal marginal zone expression of Xema RNA gives rise to embryos, the majority of which lack heads and show little discernable polarity (69%; n=35); ventral injection of Xema RNA results in variable and more modest defects in trunk and tail development (31% with clear abnormalities; n=29) (Fig. 3A). Whole mount immunohistochemistry with the somite-specific antibody 12/101 demonstrated that formation of the somites, a paraxial mesoderm derivative, is dramatically inhibited by dorsal injection of Xema RNA (Fig. 3B) (Kintner and Brockes, 1984). Consistently, Xema misexpression in dorsal marginal zone (DMZ) explants leads to a reduction of Xbra expression, as well as a dramatic decrease in the expression of dorsal endomesodermal markers, including chordin and goosecoid (Fig. 3C, compare lanes 1 and 2)(Cho et al., 1991; Sasai et al., 1994). We found that Xema expression in dorsal cells often leads to a reduction in or absence of dorsal lip invagination (see Fig. 3D and data not shown). Introduction of Xema RNA into ventral cells leads to a strong reduction in the expression of both Xbra and the ventrolateral marker Xwnt8 (Fig. 3C, compare lanes 3 and 4)(Smith and Harland, 1991); VMZ inhibition of Xbra is often more dramatic than that observed in the DMZ (Fig. 3C and data not shown). Marginal zone expression of epidermal keratin is upregulated in dorsal but not ventral explants, suggesting that Xema misexpression may promote ectodermal fates in some contexts, in addition to inhibiting mesendodermal ones (Fig. 3C). Consistent with our explant data, Xbra expression is also blocked by Xema RNA injection in both the dorsal and ventral marginal zones in intact embryos, as demonstrated by whole mount in situ hybridization analysis (Fig. 3D).
Fig. 3.
Ectopic Xema inhibits mesendoderm induction. (A) Xema misexpression disrupts embryonic development. Lateral views of stage 35 embryos, anterior is to right. (B) Whole-mount immunohistochemistry of tailbud stage embryos using the somite-specific antibody 12/101; lateral views, anterior is to right. Embryos were stained with Red Gal as a substrate prior to immunohistochemistry. (C) RT-PCR analysis of dorsal (DMZ) and ventral (VMZ) marginal zone explants from embryos injected with Xema RNA, harvested immediately after dissection at early gastrula stages. (D) Whole-mount in situ hybridization of midgastrula stage embryos, using an antisense Xbrachyury probe; vegetal pole views. Embryos were stained with Red Gal as a substrate prior to in situ hybridization. The embryos shown at the top of the figure were co-injected with β-galactosidase RNA and Xema RNA in two dorsal or ventral blastomeres, at the 4-cell stage, as listed; note the presence of β-galactosidase activity (red) in the gap in Xbrachyury expression (blue). The embryos shown at the bottom of the figure were injected with β-galactosidase RNA in the same regions, as indicated; note the overlap between Xbrachyury expression and β-galactosidase activity. (E) Inhibition of Activin-mediated mesendoderm induction by Xema. RT-PCR analysis of animal cap explants dissected at late blastula stages and cultured until midgastrula stages. Xema RNA was injected into the animal pole region of both blastomeres at the 2-cell stage. Activin (0.5 ng/ml) was added to stage 9 animal caps, as listed. (F) Inhibition of FGF-mediated mesoderm induction by Xema. bFGF (10 ng/ml) was added to stage 9 animal caps, as listed. For all experiments in this Figure, 1 ng of Xema RNA, and/or 100 pg β-Galactosidase RNA was injected, as listed.
We next examined Xema function in animal pole ectodermal explants in the presence or absence of growth factors. Animal caps from uninjected or Xema-injected embryos were first treated with the TGFβ ligand Activin. At the doses used for these studies, Activin induces the expression of a range of mesodermal and endodermal markers at gastrula stages (Fig. 3E, compare lanes 1 and 2), all of which are inhibited by injection of Xema RNA (Fig. 3E, compare lanes 2 and 3), including Xbra, Xwnt8, chordin, the panendodermal marker sox17β, and the anterior endodermal markers cerberus and Xhex (Bouwmeester et al., 1996; Hudson et al., 1997; Jones et al., 1999; Newman et al., 1997). Injection of Xema RNA does not rescue the modest reduction of epidermal keratin expression seen in Activin-treated caps (Fig. 3E, compare lanes 2, 3, and 4). Finally, ectopic Xema also inhibits ventral mesoderm induced by FGF (Fig. 3F). These data demonstrate that Xema can inhibit mesoderm and endoderm formation in a variety of assays and, coupled with the expression data described above, suggest that endogenous Xema may be involved in suppressing non-ectodermal fate in the animal pole.
Activation of Xema target genes phenocopies Xema misexpression
The fox genes encode a large class of nuclear DNA-binding factors; although members of the Foxi-class generally appear to stimulate transcription of target genes, Fox proteins include both transcriptional activators and repressors (Carlsson and Mahlapuu, 2002). To address the mechanism by which Xema inhibits mesendoderm formation, we sought to determine whether this protein functions as a transactivator or repressor during early development. Towards this end, we generated an activated Xema construct, VP-Xema, by fusing the full-length Xema cDNA to the VP16 activation domain (Kessler, 1997). Microinjection of VP-Xema RNA inhibits mesoderm induction by Activin (Fig. 4A). These results mimic the effects seen following expression of wild-type Xema and, coupled with repressor data (see below), suggest that Xema functions as a transcriptional activator during early development.
Fig 4.
Activity of Xema activator and repressor constructs. (A) VP-Xema-mediated inhibition of mesoderm formation. RT-PCR analysis of animal caps dissected at late blastula stages and cultured until midgastrula stages. 1 ng of VP-Xema RNA was injected at early cleavage stages, as listed. Activin (0.5 ng/ml) was added to stage 9 animal caps, as listed. (B) Expression of EnR-Xema induces mesoderm. RT-PCR analysis of animal caps dissected at late blastula stages and cultured until midgastrula stages. 1 ng of EnR-Xema RNA was injected at early cleavage stages, as listed. (C) Expression of EnR-Xema induces ectopic structures. Whole-mount immunohistochemistry of tailbud stage embryos using the somite-specific antibody 12/101; lateral views, anterior is to right. Embryos were stained with X-Gal as a substrate prior to immunohistochemistry. The embryo on the right was co-injected with 100pg β-Galactosidase and 1ng Xema RNA in the animal pole at the 2-cell stage; note the absence of somite staining (red) in the secondary structure containing the β-galactosidase activity (blue). The embryo on the left was injected with 100pg β-Galactosidase RNA in the animal pole at the 2-cell stage and was not probed with the somite-specific antibody.
Repression of Xema target genes induces mesoderm formation
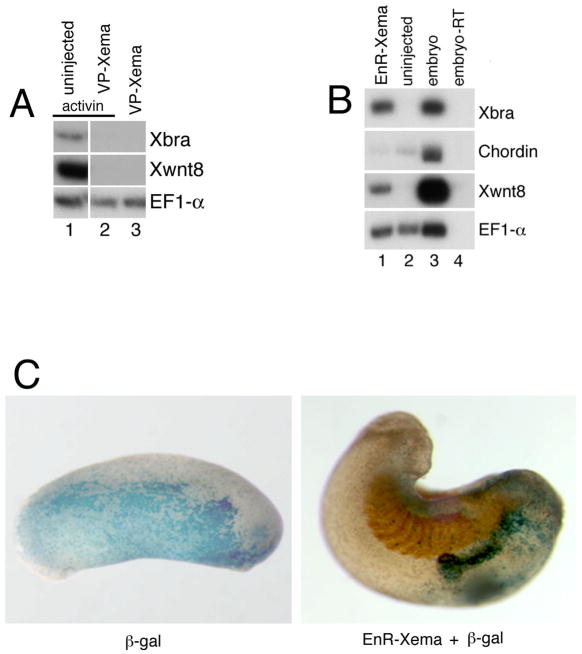
We next sought to determine whether repression of Xema target genes promotes mesendoderm. For these studies, we constructed a Drosophila Engrailed repressor-Xema fusion protein, EnR-Xema (Kessler, 1997). Gastrula stage animal pole explants excised from embryos injected with EnR-Xema express both Xbra and Xwnt8; expression of the dorsal endomesodermal marker chordin is not stimulated by EnR-Xema (Fig. 4B). As expected, EnR-Xema does not inhibit mesoderm induction by Activin and, in fact, modestly synergizes with low levels of Activin (data not shown). These data suggest that the transcriptional repression of one or more genes by EnR-Xema is sufficient to stimulate mesodermal differentiation in the presumptive ectoderm.
Ectodermal EnR-Xema expression in intact embryos leads both to mild defects in head development and to the formation of ectopic lateral structures, often near the heads of affected embryos (62%; n=55) (Fig 4C). Lineage tracing experiments demonstrated that the ectopic structures contain a high proportion of the microinjected cells (Fig. 4C, and data not shown). Although these secondary structures occasionally resemble ectopic tails, they do not usually contain dorsal mesoderm: a “true” secondary axis was seen in only one embryo expressing EnR-Xema, and an antibody against a somite-specific epitope detected the presence of paraxial mesoderm in one additional embryo (data not shown). This phenotype is reminiscent of that seen following modest activation of the fibroblast growth factor (FGF) signaling cascade (Isaacs et al., 1994; Weinstein et al., 1998).
Xema knockdown induces mesoderm
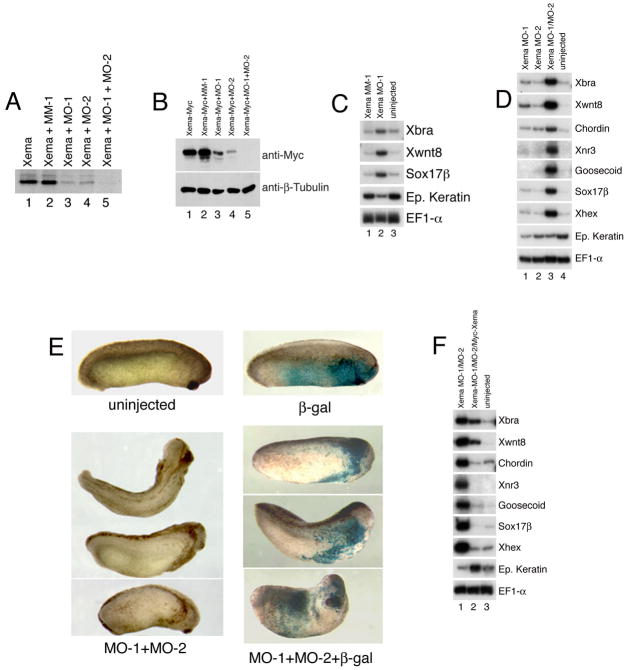
We have shown that Xema misexpression in the marginal zone blocks mesendoderm formation, and that repression of Xema target genes results in ectopic mesoderm. These data, coupled with the localization of Xema transcripts in the early Xenopus ectoderm, suggest that this factor is normally involved in inhibiting ectopic germ layer development in the animal pole. Results with the EnR-Xema construct, however, may differ from loss-of-function studies in several critical regards. First, since EnR-Xema is likely functions as a dominant-negative reagent, other related proteins may normally act redundantly with Xema during mesodermal suppression, in vivo. Second, and perhaps more critically, EnR-Xema may act to repress the transcription of genes in the ectoderm to which Xema can bind but does not normally activate. To determine whether mesendodermal suppression by Xema is required for normal development, we attempted to inhibit Xema function using antisense morpholino oligos. We designed and purchased (from GeneTools, LLC) two morpholinos against Xema, MO-1 and MO-2, both of which effectively block the translation of Xema RNA in vitro (Fig. 5A, compare lanes 1 and 3, 4). Co-expression of MO-1 and MO-2 leads to a more effective inhibition of Xema translation than is observed with either morpholino alone (Fig. 5A, compare lanes 3, 4, and 5). Expression of a control morpholino, identical to MO-1 with the exception of 5 base pair mismatches (mismatch-1/MM-1), has no effect on Xema translation (Fig. 5A, compare lanes 1 and 2). Consistent with the in vitro data, we find that injection of either MO-1 or MO-2 inhibits expression of a 3′-Myc tagged Xema protein (Xema-Myc) in Xenopus embryos (Fig. 5B, compare lane 1 with lanes 2, 3); Co-expression of MO-1 and MO-2 exhibits a greater block to expression than does injection of either morpholino alone and, as expected, Xema MM-1 does not inhibit expression of Xema-Myc (Fig. 5B). These data suggest that Xema MO-1 and MO-2 act specifically to block translation of Xema RNA in vitro and in the context of the early embryo.
Fig 5.
Knockdown of Xema protein induces mesoderm formation. (A) Xema morpholinos block the translation of Xema RNA in vitro. Morpholino oligos (500 ng each) and Xema RNA (1 ng) were simultaneously added to a rabbit reticulocyte lysate mix (Promega), in the presence of [35S]Methionine. (B) Effect of Xema morpholinos on exogenous Xema protein levels. Western blot analysis of whole cell lysates extracted from embryos injected with 1ng Xema-Myc RNA. 10 ng MO-1, 10 ng MM-1, and/or 20 ng MO-2 were injected, as listed. (C) Mesoderm and endoderm are induced by injection of Xema MO-1 (10 ng) but not by Xema MM-1 (10 ng). RT-PCR analysis of animal cap explants from embryos injected with Xema morpholinos in the animal pole at early cleavage stages, dissected at blastula stages and harvested during midgastrula stages. (D) Xema MO-1 (10 ng) and MO-2 (20 ng) potently synergize to induce mesendoderm. (E) Co-expression of Xema MO-1 and MO-2 disrupts normal development. The lower left panels show embryos co-injected with 5 ng of MO-1 and 10 ng of MO-2 in the animal pole of early cleavage stage embryos. The top left embryo is uninjected. Second embryo from top is a dorsal view; all other views are lateral; anterior is to right. Note anterior pigmented ectopic lateral structures. The lower right panels show embryos injected with 5 ng of MO-1, 10 ng MO-2, and 166 pg of β-galactosidase RNA in the animal pole of early cleavage embryos. The top embryo is injected with 166 pg of β-galactosidase RNA only. Note the presence of β-galactosidase staining (blue), primarily in the ectopic structures. All views are lateral; anterior is to right. (F) Rescue of Xema morpholino-mediated mesendoderm formation by injection of 1 ng Myc-Xema RNA. 10 ng MO-1 and 20 ng MO-2 were injected, as listed.
We next attempted to use the Xema morpholino oligos in “knockdown” loss-of-function studies. For these experiments, the same doses of MO-1 and MO-2 were used as for the Xema-Myc assay shown in Fig. 5B. Injection of either Xema MO-1 (Fig. 5C) or MO-2 (see Fig. 5D, and data not shown), stimulates expression of Xbra, Xwnt8, chordin, and the endodermal marker sox17β in animal cap explants, and reduces the expression of the ventral ectodermal marker epidermal keratin (Jonas et al., 1985); critically, injection of the control morpholino MM-1 has no effect on mesodermal or endodermal gene expression (Fig. 5C). Most strikingly, co-injection of MO-1 and MO-2 leads to a strong increase in the expression of a number of endodermal and mesodermal markers, including Xbra, Xwnt8, chordin, Xnr3, goosecoid, sox17β, and Xhex, none of which are expressed in control ectodermal explants (Fig. 5D). It has been difficult to observe the effects of Xema knockdown in intact embryos past the late blastula stage: doses required to generate mesendodermal marker expression in intact caps are lethal in gastrula stage embryos, with toxicity readily apparent by stage 10+; we have thus not been able to score for axial duplications or ectopic expression of marker genes by in situ hybridization. Co-injection of 2-fold lower doses of the Xema morpholinos, however, does result in the formation of secondary lateral structures, resembling those observed following EnR-Xema RNA injection, in 52% of morphant embryos (n=65); co-expressed lineage tracers are heavily concentrated in the ectopic structures (Fig. 5E).
Finally, in an attempt to demonstrate the specificity of the Xema morpholinos, we engineered a 5′-Myc tagged Xema fusion protein (Myc-Xema); this construct, which contains 6 tandem Myc epitopes upstream of the Xema coding sequence, was designed to bypass morpholino-mediated translational repression, which is most effective at or near the translational start site (Summerton, 1999). We find that co-injection of Myc-Xema RNA inhibits Xema knockdown-mediated mesendoderm formation, and rescues expression of epidermal keratin (Fig 5F). Taken together, these data demonstrate that inhibition of Xema function stimulates mesendoderm formation in the animal pole, and strongly suggest that Xema is required to prevent inappropriate mesendodermal differentiation in the cells of the presumptive ectoderm.
Interactions between Xema and pathways regulating mesendoderm development
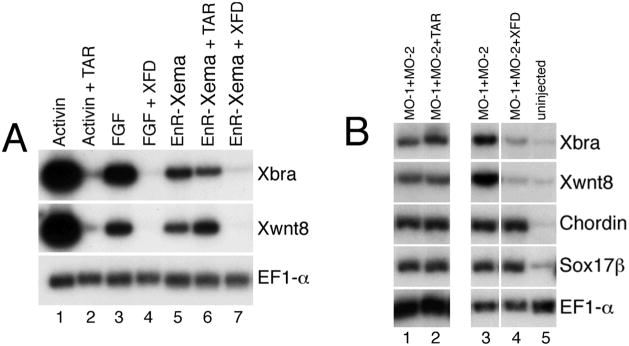
Inhibition of the signaling cascades triggered by either FGF or the Activin/Nodal/Veg1 branch of the TGFβ superfamily block the development of mesoderm in vivo (Amaya et al., 1991; Hemmati-Brivanlou and Melton, 1992). As Xema appears to play a role in preventing ectopic mesoderm formation in the ectoderm, it was of interest to determine the epistatic relationships between Xema and these signaling pathways. As described above, we found that Xema overexpression inhibits mesoderm induction by either FGF or Activin (Fig. 3A, B); these results suggest that Xema may function at a convergence point downstream of the TGFβ and FGF pathways, or in an obligatory parallel pathway. We next addressed whether the activity of either pathway was required for mesoderm induction following a block to Xema function. Inhibition of TGFβ signaling by expression of a truncated activin receptor (TAR) (Hemmati-Brivanlou and Melton, 1992), shown previously to act as a dominant inhibitory reagent, does not potently inhibit mesoderm (Xbra, Xwnt8) induction by EnR-Xema (Fig. 6A, compare lanes 5 and 6) or mesoderm (Xbra, Xwnt8), dorsal endomesoderm (chordin), or endoderm (sox17β) by Xema MO-1/MO-2 co-expression (Fig. 6B, compare lanes 1 and 2). We note that TAR expression did inhibit Xbra and, to a lesser extent, Xwnt8 induction following Xema knockdown, in 3/12 independent trials (data not shown); chordin and sox17β expression were never inhibited by TAR in these assays. Inhibition of FGF signaling by a truncated FGF receptor (XFD)(Amaya et al., 1991), however, strongly inhibits Xbra and Xwnt8 induction by both EnR-Xema (Fig. 6A, compare lanes 5 and 7) and by Xema MO-1/MO-2 co-expression (Fig. 6B, compare lanes 3 and 4). FGF pathway inhibition, like TGFβ pathway inhibition, does not affect the expression of dorsal endomesodermal (chordin) and endodermal (sox17β) marker genes (Figure 6B, compare lanes 3 and 4). These data argue that Xema is not involved solely in the production of a secreted TGFβ antagonist; furthermore, they suggest that FGF signaling contributes to the capacity for mesodermal but not endomesodermal or endodermal development, normally suppressed by Xema, in the animal pole.
Fig 6.
FGF signaling is required for mesoderm induction by either EnR-Xema or Xema morpholinos. (A) EnR-Xema activity is inhibited by co-expression of a truncated FGF receptor (XFD), but is not inhibited by expression of a truncated activin receptor (TAR). RT-PCR analysis of animal cap explants dissected at blastula stages and harvested during midgastrula stages. 1 ng of EnR-Xema RNA, alone or with either 4 ng XFD or 4 ng TAR, was injected at early cleavage stages, as listed. Activin (0.5 ng/mL) or FGF (10 ng/mL) were added to stage 9 animal caps, as listed. (B) XFD inhibits Xema morpholino-mediated Xbra and Xwnt8 expression; TAR did not inhibit Xbra and Xwnt8 expression in 9/12 independent trials. Neither TAR nor XFD inhibit dorsal endomesodermal (chordin) or endodermal (sox17β) marker induction by Xema morpholino injection. 4 ng TAR, 4 ng XFD, 10 ng MO-1, and 20 ng MO-2 were injected at early cleavage stages, as listed.
Discussion
In this study, we describe the characterization of Xema, a novel member of the Foxi-subclass of winged-helix transcription factors that is essential for the development of ectodermal fate. Xema expression is restricted to the cells of the presumptive ectoderm in the Xenopus gastrula embryo; we demonstrate that Xema misexpression is sufficient to inhibit mesendoderm formation in a variety of assays, and that Xema appears to function as a transactivator in this regard. Critically, we find that Xema activity is necessary for the suppression of ectopic mesendoderm in the animal pole. Our data are thus consistent with a model in which transcriptional activation by Xema directly or indirectly stimulates an inhibitor of mesendodermal fate in the presumptive ectoderm. This work, along with previous studies implicating a role for HNF3β in suppressing mesoderm formation in the frog and mouse (Perea-Gomez et al., 1999; Suri et al., 2004), suggests that Fox protein-mediated inhibition of ectopic germ layer development plays a critical role in establishing the early vertebrate body plan.
Our studies of Xema morphants have revealed a unexpected capacity for mesendodermal development in the cells of the presumptive ectoderm. We were surprised to find that morpholino-mediated Xema knockdown stimulates the expression of a wider range of mesendodermal marker genes than does injection of EnR-Xema RNA, particularly since the repressor construct might be expected to also block redundant activation by other Fox proteins on Xema-responsive genes in the animal pole. Since, however, EnR-Xema retains all native Xema sequence, including any putative Foxi transactivation domains, EnR-Xema likely functions as only a modest repressor of Xema target gene expression.
The source and nature of the “mesendodermalizing” signal or signals, suppressed by Xema, are not known. One possibility is that decreased expression of Xema targets in the ectoderm leads to increased sensitivity of these cells to inducing signals from the vegetal pole. In Xenopus, the primary mesendoderm-inducing signal secreted by the vegetal pole is thought to be a TGFβ ligand of the Nodal/Activin class, the expression of which is initiated at the start of zygotic transcription by the actions of the transcription factor VegT (Whitman, 2001). Our data suggest that extracellular TGFβ signaling does not appear to be the primary activity suppressed by Xema, although the occasional, albeit modest, effects seen following TGFβ inhibition suggest some role for “canonical” signals in the mesendodermalizing activity unveiled by Xema knockdown. We also cannot exclude the possibility that the Xema morpholinos promote mesendodermal development indirectly, by affecting cell movements to “reroute” marginal zone and vegetal pole cells to the animal pole.
Alternatively, or additionally, Xema may function to suppress a mesendodermalizing activity native to the animal pole. A role for such a signal is suggested by the demonstration that FGF signaling is required for Xema knockdown-mediated ventral mesoderm development (Fig. 6), as it has been demonstrated that MAP kinase activity is present in the early gastrula ectoderm (LaBonne and Whitman, 1997); Xema may function in part to blunt the mesoderm-inducing potential of this activity. We note also that our studies with the truncated Activin receptor do not rule out the potential contribution of TGFβ pathway stimulation to the mesendodermalizing signal downstream of the receptor and thus autonomous to the animal pole. Finally, it will be important to examine the relationship between Xema and the recently described “rogue cells,” found throughout the early gastrula embryo, that express one or more location-inappropriate molecular markers (Wardle and Smith, 2004). A subset of these cells is found in the animal pole at early gastrula stages and express mesodermal and/or endodermal markers, but are largely undetectable by late gastrula stages (Wardle and Smith, 2004); Xema may thus be involved in the conversion and/or elimination of these cells during gastrulation.
Although our data suggest that transactivation of as-yet uncharacterized Xema target genes leads to the suppression of ectopic mesendoderm, we do not yet have a clear sense of how Xema targets function to suppress inappropriate cell fate nor, in a related issue, do we have a firm understanding of the eventual fate of the misexpressing cells. Our data suggest that, at least in the context of the dorsal marginal zone, Xema expression can promote the conversion of mesendoderm into ventral ectoderm; this effect is not seen in the ventral marginal zone or in Activin-treated animal caps. These assays all involve ectopic Xema, however, and may not correlate precisely with endogenous Xema function in the presumptive ectoderm. Alternatively, Xema may induce or facilitate the elimination of ectopic mesendoderm during gastrulation. We have not observed any increase in TUNEL staining in embryos injected with Xema RNA, however, suggesting that mesendodermal suppression by Xema is not mediated via apoptosis (data not shown).
What relationships, if any, can be drawn between Xema and other proteins involved in the suppression of mesendodermal fate? The secreted Nodal antagonists, described earlier, are likely to play only a limited role in Xema-mediated germ layer inhibition. Sprouty proteins are FGF antagonists involved in the regulation of a number of developmental processes (Kim and Bar-Sagi, 2004). Xenopus Sprouty2 antagonizes FGF-mediated convergent extension movements, but appears to act independently of the Ras/MAPK branch of the cascade, required for mesoderm formation (Nutt et al., 2001). The C-Src Kinase (Csk) functions as an inhibitor of the Src family of non-receptor tyrosine kinases (Brown and Cooper, 1996); these latter proteins are involved in FGF-mediated mesoderm induction in Xenopus (Weinstein et al., 1998). Strikingly, expression of a dominant inhibitory form of Xenopus Csk (Xcsk) in the animal pole synergizes with sub-inducing doses of the Src kinase Laloo to form ventrolateral mesoderm and secondary tails, suggesting that Xcsk is involved in suppressing mesoderm formation in the presumptive ectoderm (Song et al., 2001). Xcsk is widely expressed during early development (Song et al., 2001); thus, regulation of Xcsk by Xema would likely be mediated post-transcriptionally. Since Xema appears to function as a transcriptional activator, however, Xcsk is unlikely to be a direct Xema target. Xenopus SIP1 (XSIP1), is a direct repressor of Xbra, and thus represents a potential target and partial mediator of Xema (Lerchner et al., 2000; Papin et al., 2002). XSIP1transcripts are detected in the dorsal ectoderm of early gastrulae, suggesting that, at least at this stage, XSIP1 expression could be mediated by Xema (Eisaki et al., 2000; van Grunsven et al., 2000), either directly or through stimulation of the recently identified Churchill (Sheng et al., 2003). Neither XSIP1 nor Churchill expression are stimulated by ectopic Xema RNA in the gastrula stage ectoderm, however, suggesting that mesoderm suppression by Xema is independent of XSIP1 (data not shown). Regardless, it is clear that Xema does not function solely as an FGF pathway antagonist. While a role for Xema in the antagonism of intracellular TGFβ signaling remains to be explored, a detailed appreciation of the mechanisms underlying germ layer suppression by Xema and other Fox proteins will likely require the identification and characterization of direct transcriptional targets.
References
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–70. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Ang SL, Rossant J. HNF-3 beta is essential for node and notochord formation in mouse development. Cell. 1994;78:561–74. doi: 10.1016/0092-8674(94)90522-3. [DOI] [PubMed] [Google Scholar]
- Bassez T, Paris J, Omilli F, Dorel C, Osborne HB. Post-transcriptional regulation of ornithine decarboxylase in Xenopus laevis oocytes. Development. 1990;110:955–62. doi: 10.1242/dev.110.3.955. [DOI] [PubMed] [Google Scholar]
- Beddington RS, Robertson EJ. Axis development and early asymmetry in mammals. Cell. 1999;96:195–209. doi: 10.1016/s0092-8674(00)80560-7. [DOI] [PubMed] [Google Scholar]
- Bouwmeester T, Kim S, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature. 1996;382:595–601. doi: 10.1038/382595a0. [DOI] [PubMed] [Google Scholar]
- Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–49. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. 2002;250:1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- Cho KW, Blumberg B, Steinbeisser H, De Robertis EM. Molecular nature of Spemann’s organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991;67:1111–20. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian JL, McMahon JA, McMahon AP, Moon RT. Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development. 1991;111:1045–55. doi: 10.1242/dev.111.4.1045. [DOI] [PubMed] [Google Scholar]
- Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–28. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- Dale L, Slack JM. Regional specification within the mesoderm of early embryos of Xenopus laevis. Development. 1987;100:279–95. doi: 10.1242/dev.100.2.279. [DOI] [PubMed] [Google Scholar]
- Dufort D, Schwartz L, Harpal K, Rossant J. The transcription factor HNF3beta is required in visceral endoderm for normal primitive streak morphogenesis. Development. 1998;125:3015–25. doi: 10.1242/dev.125.16.3015. [DOI] [PubMed] [Google Scholar]
- Eisaki A, Kuroda H, Fukui A, Asashima M. XSIP1, a member of two-handed zinc finger proteins, induced anterior neural markers in Xenopus laevis animal cap. Biochem Biophys Res Commun. 2000;271:151–7. doi: 10.1006/bbrc.2000.2545. [DOI] [PubMed] [Google Scholar]
- Harland R, Gerhart J. Formation and function of Spemann’s organizer. Annu Rev Cell Dev Biol. 1997;13:611–67. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–95. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton DA. A truncated activin receptor inhibits mesoderm induction and formation of axial structures in Xenopus embryos. Nature. 1992;359:609–14. doi: 10.1038/359609a0. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton DA. Inhibition of activin receptor signaling promotes neuralization in Xenopus. Cell. 1994;77:273–81. doi: 10.1016/0092-8674(94)90319-0. [DOI] [PubMed] [Google Scholar]
- Hudson C, Clements D, Friday RV, Stott D, Woodland HR. Xsox17alpha and -beta mediate endoderm formation in Xenopus. Cell. 1997;91:397–405. doi: 10.1016/s0092-8674(00)80423-7. [DOI] [PubMed] [Google Scholar]
- Iratni R, Yan YT, Chen C, Ding J, Zhang Y, Price SM, Reinberg D, Shen MM. Inhibition of excess nodal signaling during mouse gastrulation by the transcriptional corepressor DRAP1. Science. 2002;298:1996–9. doi: 10.1126/science.1073405. [DOI] [PubMed] [Google Scholar]
- Isaacs HV, Pownall ME, Slack JM. eFGF regulates Xbra expression during Xenopus gastrulation. Embo J. 1994;13:4469–81. doi: 10.1002/j.1460-2075.1994.tb06769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas E, Sargent TD, Dawid IB. Epidermal keratin gene expressed in embryos of Xenopus laevis. Proc Natl Acad Sci U S A. 1985;82:5413–7. doi: 10.1073/pnas.82.16.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Broadbent J, Thomas PQ, Smith JC, Beddington RS. An anterior signalling centre in Xenopus revealed by the homeobox gene XHex. Curr Biol. 1999;9:946–54. doi: 10.1016/s0960-9822(99)80421-7. [DOI] [PubMed] [Google Scholar]
- Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–6. [PubMed] [Google Scholar]
- Kessler DS. Siamois is required for formation of Spemann’s organizer. Proc Natl Acad Sci U S A. 1997;94:13017–22. doi: 10.1073/pnas.94.24.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Bar-Sagi D. Modulation of signalling by Sprouty: a developing story. Nat Rev Mol Cell Biol. 2004;5:441–50. doi: 10.1038/nrm1400. [DOI] [PubMed] [Google Scholar]
- Kintner CR, Brockes JP. Monoclonal antibodies identify blastemal cells derived from dedifferentiating limb regeneration. Nature. 1984;308:67–9. doi: 10.1038/308067a0. [DOI] [PubMed] [Google Scholar]
- Krieg PA, Varnum SM, Wormington WM, Melton DA. The mRNA encoding elongation factor 1-alpha (EF-1 alpha) is a major transcript at the midblastula transition in Xenopus. Dev Biol. 1989;133:93–100. doi: 10.1016/0012-1606(89)90300-x. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Whitman M. Localization of MAP kinase activity in early Xenopus embryos: implications for endogenous FGF signaling. Dev Biol. 1997;183:9–20. doi: 10.1006/dbio.1996.8497. [DOI] [PubMed] [Google Scholar]
- Lef J, Clement JH, Oschwald R, Koster M, Knochel W. Spatial and temporal transcription patterns of the forkhead related XFD-2/XFD-2’ genes in Xenopus laevis embryos. Mech Dev. 1994;45:117–26. doi: 10.1016/0925-4773(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Lerchner W, Latinkic BV, Remacle JE, Huylebroeck D, Smith JC. Region-specific activation of the Xenopus brachyury promoter involves active repression in ectoderm and endoderm: a study using transgenic frog embryos. Development. 2000;127:2729–39. doi: 10.1242/dev.127.12.2729. [DOI] [PubMed] [Google Scholar]
- Newman CS, Chia F, Krieg PA. The XHex homeobox gene is expressed during development of the vascular endothelium: overexpression leads to an increase in vascular endothelial cell number. Mech Dev. 1997;66:83–93. doi: 10.1016/s0925-4773(97)00092-0. [DOI] [PubMed] [Google Scholar]
- Nutt SL, Dingwell KS, Holt CE, Amaya E. Xenopus Sprouty2 inhibits FGF-mediated gastrulation movements but does not affect mesoderm induction and patterning. Genes Dev. 2001;15:1152–66. doi: 10.1101/gad.191301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papin C, van Grunsven LA, Verschueren K, Huylebroeck D, Smith JC. Dynamic regulation of Brachyury expression in the amphibian embryo by XSIP1. Mech Dev. 2002;111:37–46. doi: 10.1016/s0925-4773(01)00599-8. [DOI] [PubMed] [Google Scholar]
- Perea-Gomez A, Rhinn M, Ang SL. Role of the anterior visceral endoderm in restricting posterior signals in the mouse embryo. Int J Dev Biol. 2001;45:311–20. [PubMed] [Google Scholar]
- Perea-Gomez A, Shawlot W, Sasaki H, Behringer RR, Ang S. HNF3beta and Lim1 interact in the visceral endoderm to regulate primitive streak formation and anterior-posterior polarity in the mouse embryo. Development. 1999;126:4499–511. doi: 10.1242/dev.126.20.4499. [DOI] [PubMed] [Google Scholar]
- Perea-Gomez A, Vella FD, Shawlot W, Oulad-Abdelghani M, Chazaud C, Meno C, Pfister V, Chen L, Robertson E, Hamada H, et al. Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev Cell. 2002;3:745–56. doi: 10.1016/s1534-5807(02)00321-0. [DOI] [PubMed] [Google Scholar]
- Rodaway A, Patient R. Mesendoderm. an ancient germ layer? Cell. 2001;105:169–72. doi: 10.1016/s0092-8674(01)00307-5. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–90. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng G, dos Reis M, Stern CD. Churchill, a zinc finger transcriptional activator, regulates the transition between gastrulation and neurulation. Cell. 2003;115:603–13. doi: 10.1016/s0092-8674(03)00927-9. [DOI] [PubMed] [Google Scholar]
- Shivdasani RA. Molecular regulation of vertebrate early endoderm development. Dev Biol. 2002;249:191–203. doi: 10.1006/dbio.2002.0765. [DOI] [PubMed] [Google Scholar]
- Slack JM. Inducing factors in Xenopus early embryos. Curr Biol. 1994;4:116–26. doi: 10.1016/s0960-9822(94)00027-8. [DOI] [PubMed] [Google Scholar]
- Slack JM, Dale L, Smith JC. Analysis of embryonic induction by using cell lineage markers. Philos Trans R Soc Lond B Biol Sci. 1984;307:331–6. doi: 10.1098/rstb.1984.0135. [DOI] [PubMed] [Google Scholar]
- Smith JC, Price BM, Green JB, Weigel D, Herrmann BG. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991;67:79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell. 1991;67:753–65. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–40. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Logsdon JM, Jr, Fritz A. Expression and phylogenetic analyses of three zebrafish FoxI class genes. Dev Dyn. 2003;228:301–7. doi: 10.1002/dvdy.10373. [DOI] [PubMed] [Google Scholar]
- Song Y, Cohler AN, Weinstein DC. Regulation of Laloo by the Xenopus C-terminal Src kinase (Xcsk) during early vertebrate development. Oncogene. 2001;20:5210–4. doi: 10.1038/sj.onc.1204672. [DOI] [PubMed] [Google Scholar]
- Summerton J. Morpholino antisense oligomers: the case for an RNase H-independent structural type. Biochim Biophys Acta. 1999;1489:141–58. doi: 10.1016/s0167-4781(99)00150-5. [DOI] [PubMed] [Google Scholar]
- Suri C, Haremaki T, Weinstein DC. Inhibition of mesodermal fate by Xenopus HNF3beta/FoxA2. Dev Biol. 2004;265:90–104. doi: 10.1016/j.ydbio.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Grunsven LA, Papin C, Avalosse B, Opdecamp K, Huylebroeck D, Smith JC, Bellefroid EJ. XSIP1, a Xenopus zinc finger/homeodomain encoding gene highly expressed during early neural development. Mech Dev. 2000;94:189–93. doi: 10.1016/s0925-4773(00)00318-x. [DOI] [PubMed] [Google Scholar]
- Wardle FC, Smith JC. Refinement of gene expression patterns in the early Xenopus embryo. Development. 2004;131:4687–96. doi: 10.1242/dev.01340. [DOI] [PubMed] [Google Scholar]
- Weinstein DC, Marden J, Carnevali F, Hemmati-Brivanlou A. FGF-mediated mesoderm induction involves the Src-family kinase Laloo. Nature. 1998;394:904–8. doi: 10.1038/29808. [DOI] [PubMed] [Google Scholar]
- Weinstein DC, Ruiz i Altaba A, Chen WS, Hoodless P, Prezioso VR, Jessell TM, Darnell JE., Jr The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell. 1994;78:575–88. doi: 10.1016/0092-8674(94)90523-1. [DOI] [PubMed] [Google Scholar]
- Whitman M. Nodal signaling in early vertebrate embryos: themes and variations. Dev Cell. 2001;1:605–17. doi: 10.1016/s1534-5807(01)00076-4. [DOI] [PubMed] [Google Scholar]
- Wilson PA, Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995;376:331–3. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou H, Su Y, Sun Z, Zhang H, Zhang Y, Ning Y, Chen YG, Meng A. Zebrafish Dpr2 inhibits mesoderm induction by promoting degradation of nodal receptors. Science. 2004;306:114–7. doi: 10.1126/science.1100569. [DOI] [PubMed] [Google Scholar]
- Zhou X, Sasaki H, Lowe L, Hogan BL, Kuehn MR. Nodal is a novel TGF-beta-like gene expressed in the mouse node during gastrulation. Nature. 1993;361:543–7. doi: 10.1038/361543a0. [DOI] [PubMed] [Google Scholar]