Abstract
The design of sulfated, small, non-saccharide molecules as modulators of proteins is still in its infancy as standard drug discovery tools such as library of diverse sulfated molecules and in silico docking and scoring protocol have not been firmly established. Databases, such as ZINC, contain too few sulfate-containing non-saccharide molecules, which severely limits the identification of new hits. Lack of a generally applicable protocol for scaffold hopping limits the development of sulfated small molecules as synthetic mimetics of the highly sulfated glycosaminoglycans. We explored a sequential ligand-based (LBVS) and structure-based virtual screening (SBVS) approach starting from our initial discovery of monosulfated benzofurans to discover alternative scaffolds as allosteric modulators of thrombin, a key coagulation enzyme. Screening the ZINC database containing nearly 1 million non-sulfated small molecules using a pharmacophore developed from the parent sulfated benzofurans followed by a genetic algorithm-based dual-filter docking and scoring screening identified a group of 10 promising hits, of which three top-scoring hits were synthesized. Each was found to selectively inhibit human alpha-thrombin suggesting the possibility of this approach for scaffold hopping. Michaelis-Menten kinetics showed allosteric inhibition mechanism for the best molecule and human plasma studies confirmed good anticoagulation potential as expected. Our simple sequential LBVS and SBVS approach is likely to be useful as a general strategy for identification of sulfated small molecules hits as modulators of glycosaminoglycan–protein interactions.
Keywords: Allosteric Inhibition, Anticoagulants, Scaffold Hopping, Thrombin, Virtual Screening
Thrombin is a plasma serine protease that plays important roles in blood clotting.1 It cleaves fibrinogen to fibrin resulting in clot formation, which is a natural defense mechanism to prevent excessive loss of blood from injury. Yet, under abnormal conditions, internal clot formation can results in heart attacks and strokes, which is typically prevented through the use of anticoagulants. Heparin, a highly sulfated glycosaminoglycan (GAG), is one such anticoagulant and has been in use essentially unchanged since the 1930s. It is also an anticoagulant with numerous adverse consequences, which has catalyzed the search for better anticoagulants.2
Majority of anticoagulant search efforts have focused on the discovery of direct inhibitors of coagulation enzymes, including thrombin and factor Xa. Dabigatran and rivaroxaban, small peptidomimetics, have been realized through these discovery efforts. Both of these are active site inhibitors. Major efforts are also in progress to discover active site inhibitors of other coagulation enzymes including factor VIIa and factor XIa.
A paradigm shifting idea is to discover small molecules that allosterically modulate coagulation enzymes. Allostery can offer major advantages such as fine control over a protein’s activity and better specificity of action. Whereas active site inhibitors typically display an efficacy of 100%, allosteric inhibitors can induce less than quantitative inhibition, which in principle can afford greater tunability and regulatory control. With regard to the second point, higher specificity of action is possible from allostery because of greater structural differences in allosteric binding sites than in catalytic active sites.
Despite these advantages, the design of small molecule, allosteric inhibitors of coagulantion remains uncharted. In fact, the possibilities for discovery of such molecules is high because most coagulation enzymes contain allosteric binding sites. For example, thrombin contains two anion-binding exosites, called exosites I and II, that are characterized by clusters of basic residues. Various co-factors and proteins interact with these exosites and regulate the function of thrombin.3–5 Evidence accumulated over the past several decades indicates that the proteolytic function of thrombin can be altered through appropriate interaction with these exosites.5 For example, heparin, a natural sulfated glycosaminoglycan, binds in exosite II and alters the active site of thrombin.6 Likewise, thrombomodulin, an endothelial cell surface receptor, engages both exosites I and II to alter thrombin’s specificity from fibrinogen to protein C.1,7 Other naturally occurring allosteric modulators of thrombin include hirudin,8 chondroitin sulfate,9 and haemadin.10 Despite the availability of this natural avenue, few small molecules have been designed that exploit the natural allosteric mechanism of modulation of thrombin’s activity.
Recently, our laboratory designed the first small molecules acting as allosteric inhibitors of thrombin.11 A group of sulfated benzofurans containing a single sulfate group exhibited good inhibition of human α-thrombin under physiological conditions (Fig. 1). Enzyme kinetic studies showed an allosteric inhibition phenomenon, while later studies with site-directed thrombin mutants identified the site of binding to a hydrophobic region close to Arg173, a site encompassed within exosite II.12 This site of binding is a new discovery and is likely to be a promising avenue for anticoagulant drug discovery.
Figure 1.
Structures of some monosulfated benzofuran dimers discovered as first small molecular allosteric inhibitors of thrombin (Sidhu et al. J. Med. Chem. 2011, 54, 5522). Key units of this scaffold include: two aromatic rings, hydrophobic substituents at 3 positions and monosulfate at the 5 position. Pharmacophore query designed on the basis of this SAR is as shown in Fig. 2.
To date monosulfated benzofuran scaffold is the only scaffold available for allosteric modulation. This is a serious limitation. It is important to discover alternative scaffolds to enhance structure – activity studies and improve the chance of clinical viability. Yet, how do we discover alternative sulfated small molecule scaffolds? A traditional technology is high-throughput screening (HTS) of a library of diverse molecules. Yet, such a library of molecules containing sulfate groups has not been developed or is not commercially available. Another approach is virtual screening (VS). The primary goal here is to discover novel chemical structures that exhibit efficacy at the desired target. This is a more cost-effective solution with a reasonable level of hit discovery.13,14
Nearly all VS approaches can be classified into either structure-based (SBVS) or ligand-based (LBVS) methods.14–16 While a majority of discovery efforts have focused on one of the approaches, a growing trend has been to utilize both to enhance the probability of hit discovery.17–22 We have previously explored both SBVS and LBVS, individually, to discover sulfated small molecules and understand their interaction with proteins.11,12,23,24 Whereas SBVS of sulfated small molecules has proved to be challenging because of lack of robust scoring functions used in the docking, LBVS has been found to be beset with inability to consider the shape of the binding site.25 LBVS is useful when applied to large libraries but such libraries are not available for sulfated small molecules. Likewise, SBVS with large libraries of sulfated small molecules, if available, is also difficult because it is computationally intensive and very time consuming.26
Despite these difficulties, VS is likely to be a useful approach to rapidly discover diverse sulfated small molecule scaffolds. To assess whether a VS-based approach can rapidly provide alternative allosteric thrombin inhibitors, we explored a sequential LBVS and SBVS approach. First we performed LBVS on a library of nearly 1 million non-sulfated molecules using a pharmacophore query developed on the basis of our monosulfated benzofuran results.11,12 The hits generated from the LBVS study were then screened by docking onto thrombin to identify a smaller group of 10 hits. Of these, three top-scoring molecules were selected for thrombin inhibition studies. All three molecules based on a scaffold significantly different from that of benzofuran, except for the presence of sulfate group(s), were found to inhibit thrombin. The hits displayed allosteric inhibition mechanism in a manner similar to monosulfated benzofurans and plasma anticoagulation, as would be expected. The success achieved with sequential LBVS and SBVS bodes well for discovery of diverse sulfated small molecules as modulators of protein function.
Pharmacophore Query Generation
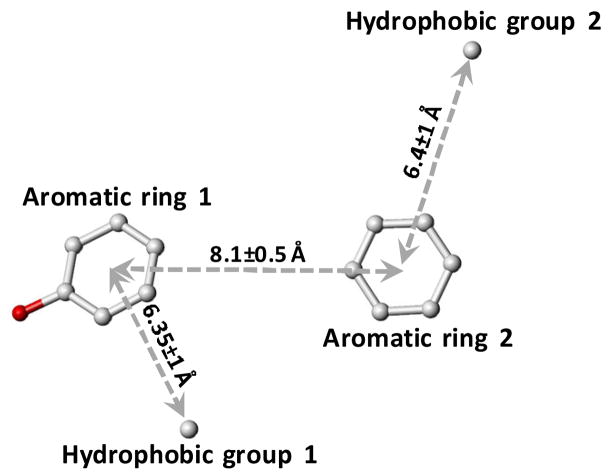
A pharmacophore was generated based on the SAR of monosulfated benzofuran library previously studied in our laboratory (Figs. 1 and 2).11,27 Briefly, the potency of monosulfated benzofurans increased nearly a 1000-fold from a monomeric to a dimeric scaffold. Thus, at least two aromatic units were deemed as essential. The average distance between these aromatic units from their centroids was found to be 8.1±0.5 Å. The SAR also indicated a strong requirement of a hydrophobic substituent at the 3 position of both aromatic units. These hydrophobic substituents were found to be 6.4±1 Å from the aromatic centroid. Finally, the most difficult condition to be implemented in LBVS was the presence of a sulfate group at the 5 position of the first benzofuran ring (see Fig. 1). Commercial libraries do not contain molecules with a sulfate group. Hence, the pharmacophore query was devised with a hydroxyl group, instead of a sulfate group, under the assumption that it can be added later, both in silico and by synthesis. These features constituted the pharmacophore query for LBVS, which is shown in figure 2.
Figure 2.
Three-dimensional (3D) pharmacophore query developed on the basis of SAR study with monosulfated benzofuran dimers (see Fig. 1).11,27 The atom in red represents an oxygen of a hydroxyl group. See text for details.
Ligand-Based Virtual Screening
LBVS was performed using UNITY on ZINC database (Shoichet Laboratory, USCF, CA), which contains structural information on nearly 1 million compounds (2006 version) that are all non-sulfated. The LBVS hits were also expected to satisfy three of four Lipinski’s rules (MW ≤ 500, H-Bond donors ≤ 5, H-Bond acceptors ≤ 10, and −0.4 < LogP < +5.0). In addition, no limit was placed on the number of rotatable bonds present in a hit. Using this 3D pharmacophore query, LBVS on the ZINC database identified 4560 hits. These hits were then processed computationally to introduce sulfate group(s) using an in-house programming script. The script identified free phenolic group(s) on the molecule and performed an in silico sulfation.
Structure-Based Virtual Screening
Molecular docking studies were performed using the genetic algorithm-based dual-filter protocol developed earlier for heparin-based sequences.28 In this approach, the first filter identified structures that interacted with the allosteric site on thrombin with a high GOLDScore, while the second filter was used to identify molecules that bound in the same site with high consistency. Briefly, a single docking run was performed with each member of the library derived from LBVS, i.e., the 4560 hits. The molecules were docked onto the hydrophobic pocket of ~22 Å radius near Arg173 of thrombin (PDB: 3EQO). This filter led to the identification of 138 molecules with a GOLDScore greater than 70. These molecules were then subjected to the consistency filter, in which docking was allowed to evolve for greater number of iterations (~100,000) in triplicate. The structures that bound consistently in the same binding pose (RMSD<2.0 Å) between the three independent runs were considered as the final hits of the SBVS approach. It is important to recognize that such a dual-filter screening algorithm has not been utilized earlier to discover sulfated non-heparin scaffolds.
Only ten molecules of 4560 screend were found to satisfy the requirements of both the filters (Fig. 3). These molecules possessed both high GOLDScore as well as high consistency of binding geometry. Although each structure is different, the molecules can be broadly grouped into two major categories – linear and bifurcated.1 Molecules 1, 3 and 7 can be classified as linear, while 2, 4–6, and 8–10 belong to the bifurcated category. Majority of these molecules contain two sulfate groups, except for 2, 6 and 7, which are monosulfated. The GOLDScores for 1, 2 and 3 were 80.4, 74.0 and 75.6, respectively, which were significantly higher than that for the next best hits. Hence, these were targeted for synthesis and biochemical study.
Figure 3.
Virtual screening algorithm (combination of LBVS and SBVS) used in the discovery of sulfated allosteric thrombin modulators (A) and the final 10 molecules proposed by the algorithm as the top 10 hits (B).
Synthesis of 1, 2 and 3
Appropriate phenolic precursors of 1, 2, and 3, i.e., 4-(4-(benzyloxy)phenyl)-2-((2-(2,3-dihydroxyphenyl)-2-oxoethyl) thio)-6-nitropyridine-3,5-dicarbonitrile,1-(4-hydroxyphenyl)-7-methyl-2-(4-methylbenzyl)-1,2-dihydrochromeno[2,3-c]pyrrole-3,9-dione and N-(4-hydroxyphenyl)-2-(3-{[(4-hydroxyphenyl) amino]carbonyl}phenyl)-1,3-dioxo-5-isoindolinecarboxamide, respectively, were purchased from Spec (Wakefield, RI), ChemBridge Corporation (San Diego, CA) and ChemDiv (San Diego, CA), respectively. Sulfation of these precursors was performed using the microwave-based sulfation method previously developed in our laboratory,29 which involved using the sulfating agent trimethylamine-sulfur trioxide complex in the presence of the base using the microwave radiation for 40 minutes at 100°C. Sephadex C-25 cation exchange resin was used to exchange the ammonium ion to sodium cation to improve the water solubility of the molecules. The final molecules were purified using HPLC on a C–4 column to obtain sulfated compounds in greater than 98% purity (see Supplementary Information). The formation of sulfated products was confirmed by 1H-NMR spectroscopy through a characteristic downfield shift of protons ortho to the sulfate group(s). In addition, high resolution ESI-MS was also used to confirm the structure of molecule (see Supplementary Information).
Inhibition of human α-thrombin
A chromogenic substrate hydrolysis assay was used for evaluating the inhibition potential of the best VS hits. Briefly, the fractional decrease in the initial rate of Spectrozyme TH hydrolysis as a function of the concentration of the inhibitor was plotted on a semi-log plot and fitted by the logistic dose–response equation 1 to derive the IC50 (Fig. 4, see Supplementary Information). All three hits showed reasonable inhibition of α-thrombin at pH 7.4 and 25°C. Inhibitors 1, 2, and 3 exhibited IC50 of 65.9±7.3 μM, 743±66 μM and 286±20 μM, respectively. These potencies are somewhat lower (~5 to 10-fold) than that observed for monosulfated benzofuran dimers (Fig. 1) from which the pharmacophore query was designed. Considering that primary objective was to derive scaffolds different from benzofurans, the success achieved with each of three best hits is encouraging. The primary reasons for attempting to discover scaffolds different from the benzofuran scaffold were to study whether additional hydrogen-bonding atoms would reduce chances of aggregation, introduce synthetic ease and provide access to expanded structure-activity relationships. Thus, the discovery of alternative scaffolds bodes well for development of synthetic analogs that may display higher potency.
Figure 4.
Direct inhibition of human α-thrombin by VS hits 1, 2 and 3. The inhibition of thrombin was determined spectrophotometrically through Spectrozyme TH hydrolysis assay at pH 7.4 and 25 °C. Solid lines represent sigmoidal fits to the data to obtain IC50, as described in the experimental section.
It is important to note that the potency of these GAG mimetics is not too far from that reported in the literature on potencies of related sulfated molecules. A large number sulfated GAG mimetics display potencies in the low to high μM range for their targets. For example, rationally designed sulfated flavonoids and sulfated tetrahydroisoquinolines displayed affinities in the range of 10 to 1000 μM for their targets.24,30,31 Likewise, the affinity of a highly sulfated octasaccharide for glycoprotein D of herpes simplex virus is 19 μM32 and that of a dermatan sulfate hexasaccharide for heparin cofactor II is 20 μM.33 The only system with high potency of smaller sulfated molecule is the antithrombin–heparin pentasaccharide system for which the affinity is 50 nM.34 Thus, the potency of initial hits 1 – 3 is reasonable in context of sulfated molecules. The more important point is scaffold hopping, which should expand anticoagulant search.
Mechanism of Thrombin Inhibition
To assess whether these hits utilize an allosteric mechanism in a manner similar to monosulfated benzofurans,11 the kinetics of Spectrozyme TH hydrolysis in the presence of 1 was measured (Fig. 5, see Supplementary Information for details). Whereas KM remained essentially invariant at 0, 20, 70 and 185 μM of 1, VMAX decreased steadily from 35.0 mAUmin−1 μM−1 in the absence of 1 to 15.0 mAUmin−1μM−1 in the presence of 185 μM of 1. This suggested that the presence of 1 does not significantly affect the binding of the substrate in the active site of thrombin, while significantly reducing its forward rate constant. This behavior is characteristic of a non-competitive, allosteric mechanism. However, it is important to recognize that this does not prove that the molecules bind in the targeted site of thrombin, as hypothesized in the design of scaffold hopping query. This confirmation will require co-crystal structure studies.
Figure 5.
Michaelis – Menten kinetics of Spectrozyme TH hydrolysis by human α-thrombin in the presence of 0 (○), 20 (▲), 70 (□) and 185 (◆) μM 1 (see Supplementary Information). Solid lines represent non-linear regression fits to the data to yield KM and VMAX.
Screening Against Other Serine Proteases
To assess the selectivity of thrombin inhibition, the three sulfated inhibitors 1, 2 and 3 were screened against a panel of related coagulation proteases including factors VIIa, IXa, Xa, XIa and XIIa. The screening was performed at a single high concentration (365 μM) of inhibitors using appropriate substrates in chromogenic substrate hydrolysis assays. Figure 6 shows the fractional residual activity of each enzyme calculated as the ratio of initial rates of hydrolysis with and without the inhibitor. None of the inhibitors decreased the activity of the coagulation factors, except for factor XIa, by more than 20%. In the case of factor XIa, 1 and 2 were found to display higher potency than that of 3. This implies that our sequential LBVS and SBVS may have also led to identification of novel factor XIa inhibitors. The result suggests that factor XIa possesses an allosteric site similar to that near Arg173 in thrombin, an interesting possibility worth pursuing. Interestingly, the order of potencies against thrombin and factor XIa is different. This implies that binding site may be similar but subtle differences could be exploited to discover molecules with higher level of selectivity.
Figure 6.
Residual activity of factors VIIa, IXa, Xa, XIa and XIIa at a fixed concentration of 365 μM of inhibitors 1 (grey bars), 2 (black bars) and 3 (white bars). Residual activity was measured in a spectrophotometric assay as described in Supplementary Information. See text for details.
Prolongation of Plasma Clotting Time
To further test whether the hits are useful in preventing coagulation in a more biologically relevant system, we utilized prothrombin (PT) and activated partial thromboplastin time (APTT) coagulation assays. In these assays, the concentration of an anticoagulant required to double the clotting time of human plasma is measured using a fibrometer, as described in our earlier work.12 The best hit, 1, was selected for these studies. A 2-fold increase in PT and APTT required 606 and 550 μM concentrations of 1, respectively suggesting useful anticoagulant potency of this initial hit (Fig.. 7).
Virtual screening strategies that reliably yield promising small molecule heparin mimetics are expected to be extremely useful in the design or discovery of modulators of processes in which heparin or heparan sulfate molecules play a role. The processes in which these GAGs play critical roles are many including angiogenesis, immune regulation, viral invasion and others, in addition to coagulation.35–38 Yet, few sulfated small molecules have been designed or discovered to date. A key reason for this state of art is that fundamental problems beset VS strategies, especially molecular docking and scoring, with highly sulfated molecules. Modeling such sulfated molecules is challenging because their charge density is high, which results in recognition of any group of arginines and lysines. In addition, heparin- or heparan sulfate binding site on proteins are surface-exposed and shallow. The strategy developed in this work, sequential LBVS and SBVS, attempts to overcome these challenges. The reasonable success rate achieved using this approach bodes well for discovery of GAG and/or heparin mimetics. It is possible that the sequential approach for sulfated small molecules works because the drawbacks of one VS approach were overcome by the strengths of the other.
The three sulfated small molecules identified by the dual VS approach may help develop more promising leads as inhibitors of coagulation. The potency of the initial hit 1 is reasonable when compared to highly sulfated saccharides30 and sulfated small molecules11 studied so far. The observation that hit 1 retains anticoagulant activity in human plasma is highly encouranging. Additionally, the selectivity observed against thrombin (and possibly factor XIa) is likely to drive further structure activity studies in these novel scaffolds, thereby expanding anticoagulant search. In fact, factor XIa activity of these hits is being investigated in detail and will be reported in due course.
Supplementary Material
Figure 7.

Prolongation of clotting time as a function of concentration of 1 in either APTT (A) or PT (B) assay. Solid lines are trend lines from which the concentration of 1 needed to double clotting times, i.e., 2×APTT and 2×PT, was derived as shown by the dotted lines. See Supplementary Information for detailed protocol.
Acknowledgments
This work was supported by grants HL090586 and HL107152 from the National Institutes of Health to URD.
Footnotes
Linear and bifurcated refer to the presence or absence of branching with regard to the scaffold. A scaffold devoid of a major branch point is defined as linear, while that which displays an aromatic unit as a branch is labeled as bifurcated.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Huntington JA, Baglin TP. Trends Pharmacol Sci. 2003;24:589. doi: 10.1016/j.tips.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Hirsh J, Anand SS, Halperin JL, Fuster V. Circulation. 2001;103:2994. doi: 10.1161/01.cir.103.24.2994. [DOI] [PubMed] [Google Scholar]
- 3.Monteiro RQ. An Acad Bras Cienc. 2005;77:275. doi: 10.1590/s0001-37652005000200007. [DOI] [PubMed] [Google Scholar]
- 4.Bock PE, Panizzi P, Verhamme IM. J Thromb Haemost. 2007;5(Suppl 1):81. doi: 10.1111/j.1538-7836.2007.02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Cera E. Mol Aspects Med. 2008;29:203. doi: 10.1016/j.mam.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogg PJ, Jackson CM, Labanowski JK, Bock PE. J Biol Chem. 1996;271:26088. doi: 10.1074/jbc.271.42.26088. [DOI] [PubMed] [Google Scholar]
- 7.Anastasiou G, Gialeraki A, Merkouri E, Politou M, Travlou A. Blood Coagul Fibrinolysis. 2012;23:1. doi: 10.1097/MBC.0b013e32834cb271. [DOI] [PubMed] [Google Scholar]
- 8.Greinacher A, Warkentin TE. Thromb Haemost. 2008;99:819. doi: 10.1160/TH07-11-0693. [DOI] [PubMed] [Google Scholar]
- 9.Liu LW, Rezaie AR, Carson CW, Esmon NL, Esmon CT. J Biol Chem. 1994;269:11807. [PubMed] [Google Scholar]
- 10.Richardson JL, Kroger B, Hoeffken W, Sadler JE, Pereira P, Huber R, Bode W, Fuentes-Prior P. EMBO J. 2000;19:5650. doi: 10.1093/emboj/19.21.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sidhu PS, Liang A, Mehta AY, Abdel Aziz MH, Zhou Q, Desai UR. J Med Chem. 2011;54:5522. doi: 10.1021/jm2005767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel Aziz MH, Sidhu PS, Liang A, Kim JY, Mosier PD, Zhou Q, Farrell DH, Desai UR. J Med Chem. 2012 doi: 10.1021/jm300670q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steindl TM, Schuster D, Wolber G, Laggner C, Langer T. J Comput Aided Mol Des. 2006;20:703. doi: 10.1007/s10822-006-9066-y. [DOI] [PubMed] [Google Scholar]
- 14.Oprea TI, Matter H. Curr Opin Chem Biol. 2004;8:349. doi: 10.1016/j.cbpa.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Lower M, Proschak E. Mol Inf. 2011;30:398. doi: 10.1002/minf.201100007. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Morrow JK, Tran HT, Phatak SS, Du-Cuny L, Zhang S. Curr Pharm Des. 2012;18:1217. doi: 10.2174/138161212799436386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waszkowycz B, Clark DE, Gancia E. Wiley Interdisciplinary Reviews Comput Mol Sci. 2011;1:229. [Google Scholar]
- 18.Musmuca I, Caroli A, Mai A, Kaushik-Basu N, Arora P, Ragno R. J Chem Inf Model. 2010;50:662. doi: 10.1021/ci9004749. [DOI] [PubMed] [Google Scholar]
- 19.Švegelj MB, Turk S, Brus B, Rižner TL, Stojan J, Gobec S. J Chem Inf Mod. 2011;51:1716. doi: 10.1021/ci2001499. [DOI] [PubMed] [Google Scholar]
- 20.Vasanthanathan P, Lastdrager J, Oostenbrink C, Commandeur JNM, Vermeulen NPE, Jørgensen FS, Olsen L. Med Chem Commun. 2011;2:853. [Google Scholar]
- 21.Malvezzi A, Queiroz RF, de Rezende L, Augusto O, do Amaral AT. Mol Inf. 2011;30:605. doi: 10.1002/minf.201100016. [DOI] [PubMed] [Google Scholar]
- 22.Kitchen DB, Decornez H, Furr JR, Bajorath J. Nat Rev Drug Discov. 2004;3:935. doi: 10.1038/nrd1549. [DOI] [PubMed] [Google Scholar]
- 23.Raghuraman A, Mosier PD, Desai UR. ACS Med Chem Lett. 2010;1:281. doi: 10.1021/ml100048y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raghuraman A, Liang A, Krishnasamy C, Lauck T, Gunnarsson GT, Desai UR. Eur J Med Chem. 2009;44:2626. doi: 10.1016/j.ejmech.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klebe G. Drug Discov Today. 2006;11:580. doi: 10.1016/j.drudis.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyne PD. Drug Discov Today. 2002;7:1047. doi: 10.1016/s1359-6446(02)02483-2. [DOI] [PubMed] [Google Scholar]
- 27.Verghese J, Liang A, Sidhu PS, Hindle M, Zhou Q, Desai UR. Bioorg Med Chem Lett. 2009;19:4126. doi: 10.1016/j.bmcl.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raghuraman A, Mosier PD, Desai UR. J Med Chem. 2006;49:3553. doi: 10.1021/jm060092o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raghuraman A, Riaz M, Hindle M, Desai UR. Tetrahedron Lett. 2007;48:6754. doi: 10.1016/j.tetlet.2007.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunnarsson GT, Desai UR. J Med Chem. 2002;45:1233. doi: 10.1021/jm020012q. [DOI] [PubMed] [Google Scholar]
- 31.Al-Horani R, Liang A, Desai UR. J Med Chem. 2011;54:6125. doi: 10.1021/jm2008387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Copeland R, Balasubramaniam A, Tiwari V, Zhang F, Bridges A, Linhardt RJ, Shukla D, Liu J. Biochemistry. 2008;47:5774. doi: 10.1021/bi800205t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tollefsen DM. Adv Exp Med Biol. 1992;313:167. doi: 10.1007/978-1-4899-2444-5_17. [DOI] [PubMed] [Google Scholar]
- 34.Desai UR. Med Res Rev. 2004;24:151. doi: 10.1002/med.10058. [DOI] [PubMed] [Google Scholar]
- 35.Raman R, Sasisekharan V, Sasisekharan R. Chem Biol. 2005;12:267. doi: 10.1016/j.chembiol.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Rouet V, Hamma-Kourbali Y, Petit E, Panagopoulou P, Katsoris P, Barritault D, Caruelle JP, Courty J. J Biol Chem. 2005;280:32792. doi: 10.1074/jbc.M504492200. [DOI] [PubMed] [Google Scholar]
- 37.Ihrcke N, Wrenshall LE, Lindman BJ, Platt JL. Immunol Today. 1993;14:500. doi: 10.1016/0167-5699(93)90265-M. [DOI] [PubMed] [Google Scholar]
- 38.Linhardt RJ, Toida T. Acc Chem Res. 2004;37:431. doi: 10.1021/ar030138x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.