Abstract
Background
Azathioprine is prescribed as a corticosteroid-sparing agent for many inflammatory conditions, including refractory atopic dermatitis (AD). There is limited prospective data on its appropriate use and monitoring for children with AD.
Objectives
This study was designed to assess clinical response to azathioprine, determine the necessity for repeat measurement of thiopurine methyltransferase (TPMT) activity during treatment, and test the utility of measuring levels of the metabolites 6-thioguanine nucleotide (6-TGN) and 6-methylmercaptopurine (6-MMP).
Methods
Twelve children with severe, recalcitrant AD were treated with oral azathioprine and followed prospectively. Disease severity was determined by the SCORing Atopic Dermatitis Index. Baseline TPMT activity was measured and this was repeated along with 6-TGN and 6-MMP measurement at times of stable improvement, inadequate response, or change in response.
Results
Azathioprine therapy was associated with clinical improvement in all but one subject. There were few adverse effects. Three subjects showed a significant change in TPMT activity during treatment: two had a mild decrease and one demonstrated enzyme inducibility with an increase from the intermediate to the normal activity range. These changes, but not 6-TGN or 6-MMP levels, inversely correlated with the clinical response to therapy.
Limitations
Small sample size
Conclusions
Azathioprine can be of benefit in the treatment of recalcitrant pediatric AD. Repeat assessment of TPMT activity may be helpful for evaluation of non–response or change in response and warrants further study. In contrast, measurement of thiopurine metabolites during treatment was not clinically useful.
Keywords: azathioprine, atopic dermatitis, eczema, pediatric, thiopurine methyltransferase, thiopurine metabolites, monitoring
INTRODUCTION
Severe atopic dermatitis (AD) causes considerable morbidity and may require systemic treatment.1 Evidence and guidance for the appropriate use of immunosuppressive drugs in children with AD are limited.
Azathioprine showed benefit in two randomized, placebo-controlled trials of adult AD treated for 12 weeks and in several retrospective pediatric case series.2–6 It is a 6-mercaptopurine analog that interferes with purine synthesis with cytotoxic properties, selectively inhibiting T-lymphocytes more than B-lymphocytes and perhaps shifting the T-cell profile to one more favorable for atopic patients.7 Thiopurine methyltransferase (TPMT) plays a key role in azathioprine metabolism as the principal catabolic enzyme inactivating the 6-mercaptopurine moiety, although it also produces active methylated metabolites. Individual differences in TPMT activity vary according to common allelic polymorphisms. Assessment of activity prior to beginning azathioprine can help determine appropriate initial dosing as well as decrease the risk of toxic effects.7 However, studies8,9 of azathioprine use for immunobullous and other disorders noted that TPMT levels are not static and induction of activity can occur during treatment, which may affect drug efficacy and disease response.
The purpose of this prospective study was to test for differential TPMT activity levels during treatment of refractory pediatric AD with azathioprine, to establish the clinical utility of measuring levels of the thiopurine metabolites 6-thioguanine nucleotide (6-TGN) and 6-methylmercaptopurine (6-MMP), and to examine the correlation, if any, with clinical response or adverse effects.
METHODS
The study was approved by the University of California, San Diego Institutional Review Board. Subjects were aged 2 to 18 years with chronic, moderate to severe atopic dermatitis (objective SCORing Atopic Dermatitis (SCORAD) index ≥25 and meeting Hanifin and Rajka criteria) attending the pediatric dermatology clinic and Eczema Center at Rady Children’s Hospital. Patients warranting systemic treatment due to repeated failure of topical anti- inflammatory and adjunctive therapies, with significant negative impact on quality of life and whose parents elected to use azathioprine, were prospectively followed. Our standard dosing of azathioprine is based on the initial measurement of red blood cell (RBC) TPMT activity (Mayo Medical Laboratories, Rochester, MN was used whenever possible). Individuals with normal TPMT activity levels (≥15.4 U/mL) started at 2.5mg/kg/day, possible carriers of a mutant allele (11.9–15.3 U/mL) at 2.0 or 2.5mg/kg/day per parental choice after counseling, while definite carriers with intermediate enzyme levels (6.0–11.8 U/mL) began at 1.0mg/kg/day. Individuals with low TPMT levels (≤ 5.9 U/mL) are not prescribed azathioprine in our clinic; pregnant or lactating females and individuals with impaired hepatic, renal, or immune function are also excluded from treatment.
Twelve subjects were treated and assessed from October 2008 to May 2012. Eight had received multiple prior courses of systemic corticosteroids for AD. Subjects 2 and 3 had failed to attain remission with oral cyclosporine and subject 2 additionally failed phototherapy. Six had bridging therapy with oral prednisone (0.5–1.0 mg/kg/day with gradual taper after azathioprine initiation) because it was started by another provider and/or because their disease was too severe to wait 6 to 8 weeks for onset of azathioprine effect.
Clinical evaluation, SCORAD assessments (all by WLT), and laboratory monitoring were performed at 0, 2, 4, 8, and 12 weeks of treatment, and every 4 to 8 weeks thereafter. Azathioprine dosage was adjusted based on response, side effects, and laboratory test results. When needed, dosage was increased by 0.5mg/kg/day increments for those with activity in the normal and possible carrier ranges. Known carriers received additional drug at increments of 0.25mg/kg/day.
TPMT activity levels were re-measured at times of either: 1) stable improvement/plateau, particularly just prior to planned tapering of drug, 2) inadequate response, or 3) change in response. RBC levels of 6-TGN and 6-MMP were also measured at these time-points (Prometheus Laboratories, San Diego, CA). Adverse effects were recorded, with specific questioning at each visit about the presence of gastrointestinal (GI) symptoms, febrile episodes, myalgia, minor or serious infections, and hospitalizations.
RESULTS
Subjects’ baseline severity (all severe with SCORAD >40) and treatment course are presented in Table 1. Age of AD onset ranged from 1 to 24 months and the median age at starting azathioprine therapy was 9.0 years. All but subject 9 had significant improvement, with a decrease in SCORAD of 27.7 +/− 8.7 (mean +/− standard deviation) at the time of stable improvement just prior to starting taper of drug or at response plateau. No subject discontinued treatment due to adverse events. Two experienced minor GI upset for a few weeks that resolved with reinforcing the need to administer with meals and to avoid lying down immediately after. Subject 4 had an absolute neutrophil count of 1292/mm3 at month 4, but subsequent counts were normal and dosing was unaffected. No other child had a significant decrease in white blood cell count during therapy. Subject 6 had mildly elevated serum transaminases three months into treatment, which normalized with dose reduction from 2.5 to 1.75mg/kg/day. He maintained a good response on the lower dose. No additional side effects were noted.
Table 1.
Subjects’ Course and Response to Azathioprine
| Subject No./ Gender |
Age at Starting Azathioprine |
Azathioprine Dose Giving Desired Response |
Time of Assessments* |
Objective SCORAD |
TPMT Level (U/mL RBC) |
6-TGN (pmol/8x10^8 RBC) |
6-MMP (pmol/8x10^8 RBC) |
Outcome of Therapy |
|---|---|---|---|---|---|---|---|---|
| 1/F | 9.4yr (+prednisone) |
2.5 mg/kg/d | Baseline* | 49 | 30.5 EU** (Normal) |
Tapered off at 16.5 months of treatment, remains essentially clear 26 months later |
||
| 11 months (Stable marked improvement, just prior to planned taper) |
6 | 13.5 (Possible carrier) |
111 | Below lower level of quantitation |
||||
| 2/F | 9.4yr | 1.25 mg/kg/d | Baseline | 59 | 11.2 EU** (Intermediate) |
Other parent opted to stop abruptly after 8 months, since able to maintain at mild to moderate disease with topical therapy |
||
| 8 months (Stable improvement, at time of stopping drug) |
24 | 8.8 (Intermediate |
Not done before stopping drug |
Not done before stopping drug |
||||
| 3/M | 5.4yr (+prednisone) |
3.0 mg/kg/d | Baseline | 54 | 18.7 (Normal) |
Improved to very mild disease and tapered off after 13 months of treatment, remains very mild 24 months post-treatment |
||
| 7 months (Change in response – starting to improve) |
38 | 12.4 (Possible carrier) |
122 | 1442 | ||||
| 94 | Below lower level of quantitation |
|||||||
| 4/F | 10.9yr | 3.0 mg/kg/d | Baseline | 54 | 15.6 (Normal) |
Tapering down – currently at 0.3 mg/kg/d with mild disease |
||
| 10 months (Stable marked improvement, just prior to planned taper) |
18 | 15.7 (Normal) |
358 |
5898 |
||||
| 5/F | 11.5yr (+prednisone) |
3.4 mg/kg/d | Baseline | 53 | 15.5 (Normal) |
Given improvement plateaued, switched to mycophenolate after 14 months with further improvement |
||
| 12 months (Stable improvement – plateaued) |
31 | 15.1 (Normal) |
207 | 3897 | ||||
| 6/M | 8.4yr | 2.5 mg/kg/d, lowered to 1.75 mg/kg/d due to mildly elevated AST/ALT (high 50s) |
Baseline | 45 | 15.6 (Normal) |
Tapered off after 15 months of treatment, remains well with very mild residual disease 12 months later |
||
| 10 months (Stable marked improvement, just prior to planned taper) |
14 | 17 (Normal) |
81 | Below lower level of quantitation |
||||
| 7/F | 14.9yr (+prednisone |
2.5 mg/kg/d | Baseline | 44 | 15.2 (Possible Carrier) |
Tapered off after 16 months of treatment, remains essentially clear 15 months later |
||
| 10 months (Stable marked improvement, just prior to planned taper) |
15 | 14.1 (Possible Carrier) |
259 | 967 | ||||
| 8/M | 18.1yr (+prednisone) |
2.8 mg/kg/d | Baseline | 51 | 15.4 (Normal) |
Tapering down – currently at 1.5 mg/kg/d |
||
| 10 months (Stable improvement, just prior to planned taper) |
24 | 22 (Normal) |
262 | 1629 | ||||
| 9/F | 6.7yr (+prednisone |
Partial response but inconsistent control even at 3.0 mg/kg/d |
Baseline | 52 | 11.1 (Intermediate) |
Recently switched to mycophenolate after 15 months of therapy given continued flares |
||
| 7 and 11 months (Inadequate response) |
45 | 16.9 (Normal) |
91 | Below lower level of quantitation |
||||
| 51 | 16 (Normal) |
50 | Below lower level of quantitation |
|||||
| 10/F | 8.6yr | 3.0 mg/kg/d | Baseline | 48 | 14 (Possible Carrier) |
Tapering down – currently at 1.25 mg/kg/d |
||
| 10 months (Stable improvement, just prior to planned taper) |
19 | 15 (Possible Carrier) |
45 | Below lower level of quantitation |
||||
| 11/F | 8.3yr | 3.0 mg/kg/d | Baseline | 46 | 13.7 (Possible carrier) |
Continue for several more months given plaques thick, then plan to taper |
||
| 7 months (Stable Improvement) |
32 | 14.1 (Possible carrier) |
125 | Below lower level of quantitation |
||||
| 12/M | 2.6yr | 2.5 mg/kg/d | Baseline | 48 | 55.4 EU** (Normal) |
Tapering down – currently at 1 mg/kg/d |
||
| 9 months (Stable improvement, just prior to planned taper) |
25 | 63.5 EU** (Normal) |
54 | 2006 | ||||
Abbreviations: AD, atopic dermatitis; SCORAD, SCORing Atopic Dermatitis index; TPMT, thiopurine methyltransferase; 6-TGN, 6-thioguanine nucleotide; 6-MMP, 6-methylmercaptopurine; F, female; M, male; yr, year; mg/kg/d, milligrams/kilogram/day; RBC, red blood cells
Baseline objective SCORAD values are at the start of azathioprine treatment and include the effect of prednisone when given (thus lower than the subject’s actual disease).
Enzyme Units (Prometheus Laboratories, San Diego, CA)
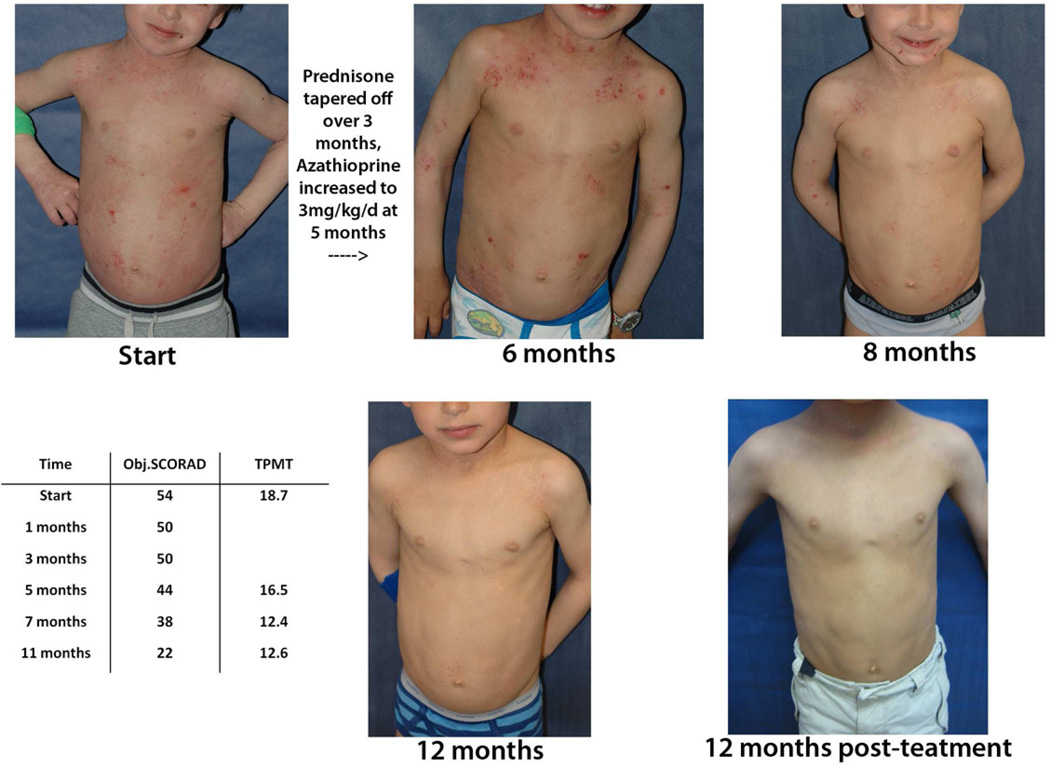
Three children had a change in TPMT level during therapy that modified categorization as normal activity, possible carrier, or intermediate activity/definite carrier. The first was a 9 year-old girl who was transitioned from prednisone to azathioprine, with rapid improvement over 4 months. Her TPMT level shifted from normal activity to possible carrier levels, perhaps contributing to her marked response. Azathioprine was tapered off as planned. Subject 3 had only mild initial response to azathioprine despite increasing the dose to 3mg/kg/day. At about the seventh month, his AD began to improve rapidly and notably corresponded to a decrease in TPMT level to the possible carrier range (Fig 1). Given no adverse effects, the same dose was continued for several months, after which the parents advocated for a rapid taper. Both subjects had minimal disease for 2 years post-therapy.
Figure 1.
Atopic dermatitis in Subject 3 had a delayed response to therapy, with improvement that correlated with a decline in TPMT activity.
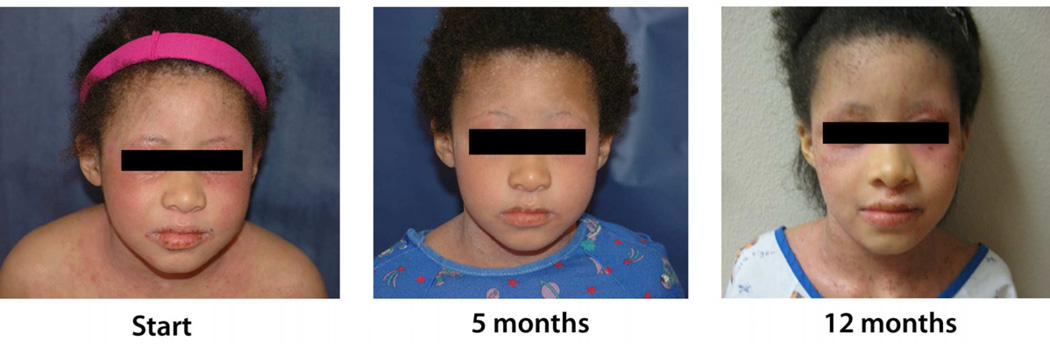
Subject 9 had intermediate TPMT activity at baseline. After mild improvement at 5 months of treatment, she experienced worsening with severe, persistent disease (Fig 2). Two follow-up measurements showed TPMT activity in the normal range. Genotyping did not detect any of the four common enzyme deficiency variants (TPMT*2, TPMT*3A, TPMT*3B, and TPMT*3C). Despite titrating the dose up to 3mg/kg/day, response was insufficient and azathioprine was discontinued after 15 months of treatment.
Figure 2.
Atopic dermatitis in Subject 9 showed an initial mild improvement with systemic treatment, but then worsened and remained severe despite an increased dose of azathioprine.
For the 11 responders, 6-TGN levels ranged from 45 to 358 pmol/8x10^8 RBC at the time their improvement stabilized/plateaued (Table 1). 6-MMP levels at this time-point were quite varied and many had levels below the lower limit of detection.
DISCUSSION
This study provides prospective, long-term data in 12 children to support the utility of azathioprine for recalcitrant AD and assessment of various parameters of drug monitoring during therapy.
TPMT is the best-studied enzyme involved in thiopurine drug metabolism and illustrates the use of pharmacogenomics in clinical practice. Previously, concern for myelosuppression led to initiation of treatment at very low doses, with slow upward titration and sometimes limited effect.7 The availability of a RBC functional assay that reflects hepatic as well as lymphocyte, leukemic blast, and renal cell enzyme activity improved the administration of these drugs. Single nucleotide polymorphisms in the TPMT gene (6p22.3) affect the enzyme's rate kinetics, with 29 different alleles described to date.10,11 Approximately 90% of individuals have normal to high TPMT activity, 10% have intermediate activity, and 1 in 220 to 300 individuals is homozygous for mutant alleles with low to almost undetectable enzymatic activity.10,12 Utilizing the phenotypic classification when initiating therapy has allowed both individuals with normal and intermediate activity to be treated with fewer instances of hematopoetic toxicity.7,13 It particularly helps identify and precludes administration to patients with significant enzyme deficiency who would be at risk for excessive accumulation of active metabolites. We found our TPMT-based dosing scheme effective for nearly all subjects and at doses (see Table) similar to those reported in prior pediatric case series.4–6,13
While TPMT activity is generally measured only once before starting therapy, some have reported increased activity on drug exposure. el-Azhary et al. measured levels on a 1-to-3-month basis and observed fluctuations during the treatment of immunobullous diseases, particularly in those remaining recalcitrant to therapy.8 Similar induction has been reported with azathioprine use to prevent renal transplant rejection, but was not seen in the treatment of inflammatory bowel disease (IBD).9,14,15 Increased enzymatic activity would be expected to result in more rapid inactivation of the 6-mercaptopurine moiety and could potentially give suboptimal effect. If TPMT activity is indeed inducible, continued measurement would be important.
We therefore assessed activity at key time-points during treatment. Only one subject showed a significant increase (intermediate→ normal) and this correlated with a poor therapeutic response. el-Azhary et al. had 3 of 27 patients with similar categorization change and suggested considering alternate therapy when enzyme induction is noted.8 This may also be the case for AD, although one subject limits generalizability. Genotyping did not find this child to carry any of the four TPMT alleles that account for >95% of intermediate and low enzyme activity.10 It is possible that the baseline measurement was incorrect, but we consider this unlikely and her initial response to 1mg/kg/day therapy suggests otherwise. Additional genotyping would be needed to determine if she carries a rare or novel TPMT variant leading to intermediate but further inducible activity. The effects of many TPMT alleles still remain uncharacterized. One patient with a known low activity allele (TPMT*3A) and a novel allele (TPMT*28) had very low measured enzyme activity, yet he tolerated azathioprine at full doses without untoward effects.11
Interestingly, two subjects showed a small decline in TPMT activity during treatment (normal→ possible carrier) and had some of the best results. Subject 3 in particular displayed little initial response, followed by rapid improvement that tracked the decrease in TPMT level. Overall, most of our subjects had unchanged enzyme activity, but in those with a change, the direction correlated inversely with clinical response. Repeat measurement appeared most relevant for those with a lack of improvement or an alteration from prior response.
TPMT levels did not correlate with GI or other side effects, which were overall mild. A single low neutrophil count appeared to be a spurious finding. Nevertheless, monitoring remains important, as cases of neutropenia have been reported with TPMT-based dosing, including individuals with normal enzyme levels.16,17 Only one subject had an adverse event affecting drug dosage, but his improvement remained durable after adjustment.
This study also looked at the clinical utility of measuring 6-TGN and 6-MMP levels during treatment. 6-TGN is the principle active metabolite of azathioprine, while 6-MMP is the inactive metabolite produced by TPMT action on 6-mercaptopurine. These have not traditionally been measured when using azathioprine for dermatologic diseases, but some have proposed their assessment to assist achieving the desired efficacy. Positive response was more frequent with 6-TGN levels above 235 pmol/8x10^8 RBC in subjects with IBD.18 el-Azhary et al. reported optimal 6-TGN levels for immunobullous disease remission to be 150 to 300 pmol/8x10^8 RBC, but found lower requirements for limited (48 to 321 pmol/8x10^8 RBCs) as compared to diffuse disease (83 to 457 pmol/8x10^8 RBCs).8 In our AD patients, levels as low as 45 and 54 pmol/8x10^8 RBCs were effective for severe, diffuse disease, with no difference in response compared to 207 to 358 pmol/8x10^8 RBCs. It is possible higher 6-TGN levels could be noted in these individuals had additional time-points been assessed, but we limited measurement to when they would likely be most useful and did not find the values to be indicative of response. Similar variability in 6-TGN levels has been reported in systemic lupus patients responding and not responding to azathioprine.19 It may be that active metabolites other than 6-TGN play a role, such as the methylated products produced by TPMT which have some cytotoxic and anti-inflammatory effects.7
el-Azhary et al. suggested that low levels of both 6-TGN and 6-MMP indicate problems with medication adherence, whereas low 6-TGN with high 6-MMP levels (deemed >5700 pmol/8x10^8 RBCs) suggest induction of TPMT.8 6-MMP was below detectable in subject 9 who had a poor response and induced TPMT activity. While medication non-compliance could be considered, her 6-TGN levels of 50 and 90 combined with a below-detectable 6-MMP did not differ from levels measured in subjects 3, 6, and 10 who responded to treatment. Thus, measuring metabolite levels did not allow determination of adherence or non-adherence to therapy. High 6-MMP levels have also been associated with hepatotoxicity,18 but our single patient with mild transaminitis had a level below quantitation (<743 pmol/8x10^8 RBCs).
A limitation of this study is the small number of subjects, but children who receive systemic therapy for AD are limited to the most refractory cases.
In conclusion, we found a positive response to azathioprine in 11 of 12 children. Our results suggest that TPMT activity can alter in either direction during treatment. Repeat measurement might be warranted, particularly in cases of non-response to treatment, in order to optimize dosing or to consider alternate therapy. In contrast, 6-TGN and 6-MMP levels did not correlate with clinical response and their measurement does not appear to be helpful in AD treatment monitoring.
Capsule Summary.
There is little prospective data on the use and monitoring of azathioprine for severe pediatric atopic dermatitis.
We found a notable improvement in 11 of 12 subjects, with few adverse effects. Alterations in thiopurine methyltransferase (TPMT) activity during therapy inversely correlated with response. Thiopurine metabolite levels were not significantly different in responders compared to the non-responder.
Repeat assessment of TPMT activity in cases of non–response or change in response to azathioprine warrants additional study.
Acknowledgments
Funding: Dr. Tom is supported by a National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH/NIAMS) research career development grant (K23AR060274). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases or the National Institutes of Health.
Abbreviations
- AD
atopic dermatitis
- TPMT
thiopurine methyltransferase
- 6-TGN
6-thioguanine nucleotide
- 6-MMP
6-methylmercaptopurine
- SCORAD
SCORing Atopic Dermatitis index
- IBD
inflammatory bowel disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no financial disclosures, commercial associations or other conflicts of interest to report relating to the content of this article.
This work has not been previously published nor is it under submission elsewhere. The data was presented in part in poster format at the Society for Pediatric Dermatology 2011 Annual Meeting.
No “ghost” or other third-party writers were involved in manuscript development or production.
REFERENCES
- 1.Simpson EL, Hanifin JM. Atopic dermatitis. Med Clin North Am. 2006 Jan;90:149–167. doi: 10.1016/j.mcna.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Meggitt SJ, Gray JC, Reynolds NJ. Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: a double-blind, randomised controlled trial. Lancet. 2006;367:839–846. doi: 10.1016/S0140-6736(06)68340-2. [DOI] [PubMed] [Google Scholar]
- 3.Berth-Jones J, Takwale A, Tan E, Barclay G, Agarwal S, Ahmed I, et al. Azathioprine in severe adult atopic dermatitis: a double-blind, placebo-controlled, crossover trial. Br J Dermatol. 2002;147:324–330. doi: 10.1046/j.1365-2133.2002.04989.x. [DOI] [PubMed] [Google Scholar]
- 4.Murphy LA, Atherton D. A retrospective evaluation of azathioprine in severe childhood atopic eczema, using thiopurine methyltransferase levels to exclude patients at high risk of myelosuppression. Br J Dermatol. 2002;147:308–315. doi: 10.1046/j.1365-2133.2002.04922.x. [DOI] [PubMed] [Google Scholar]
- 5.Hon KL, Ching GK, Leung TF, Chow CM, Lee KK, Ng PC. Efficacy and tolerability at 3 and 6 months following use of azathioprine for recalcitrant atopic dermatitis in children and young adults. J Dermatolog Treat. 2009;20:141–145. doi: 10.1080/09546630802512646. [DOI] [PubMed] [Google Scholar]
- 6.Waxweiler WT, Agans R, Morrell DS. Systemic treatment of pediatric atopic dermatitis with azathioprine and mycophenolate mofetil. Pediatr Dermatol. 2011;28:689–694. doi: 10.1111/j.1525-1470.2011.01488.x. [DOI] [PubMed] [Google Scholar]
- 7.Patel AA, Swerlick RA, McCall CO. Azathioprine in dermatology: the past, the present, and the future. J Am Acad Dermatol. 2006;55:369–389. doi: 10.1016/j.jaad.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 8.el-Azhary RA, Farmer SA, Drage LA, Rogers RS, 3rd, McEvoy MT, Davis MD, et al. Thioguanine nucleotides and thiopurine methyltransferase in immunobullous diseases: optimal levels as adjunctive tools for azathioprine monitoring. Arch Dermatol. 2009;145:644–652. doi: 10.1001/archdermatol.2009.81. [DOI] [PubMed] [Google Scholar]
- 9.Mircheva J, Legendre C, Soria-Royer C, Thervet E, Beaune P, Kreis H. Monitoring of azathioprine-induced immunosuppression with thiopurine methyltransferase activity in kidney transplant recipients. Transplantation. 1995;60:639–642. doi: 10.1097/00007890-199510150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Appell ML, Wennerstrand P, Peterson C, Hertervig E, Mårtensson LG. Characterization of a novel sequence variant, TPMT*28, in the human thiopurine methyltransferase gene. Pharmacogenet Genomics. 2010;20:700–707. doi: 10.1097/FPC.0b013e3283402ee4. [DOI] [PubMed] [Google Scholar]
- 11.Landy J, Bhuva N, Marinaki A, Mawdsley J. Novel thiopurine methyltransferase variant TPMT*28 results in a misdiagnosis of TPMT deficiency. Inflamm Bowel Dis. 2011;17:1441–1442. doi: 10.1002/ibd.21505. [DOI] [PubMed] [Google Scholar]
- 12.Firooz A, Ghandi N, Hallaji Z, Chams-Davatchi C, Valikhani M, Karbakhsh Davari M. Role of thiopurine methyltransferase activity in the safety and efficacy of azathioprine in the treatment of pemphigus vulgaris. Arch Dermatol. 2008;144:1143–1147. doi: 10.1001/archderm.144.9.1143. [DOI] [PubMed] [Google Scholar]
- 13.Murphy LA, Atherton DJ. Azathioprine as a treatment for severe atopic eczema in children with a partial thiopurine methyl transferase (TPMT) deficiency. Pediatr Dermatol. 2003;20:531–534. doi: 10.1111/j.1525-1470.2003.20617.x. [DOI] [PubMed] [Google Scholar]
- 14.Lindqvist M, Hindorf U, Almer S, Söderkvist P, Ström M, Hjortswang H, et al. No induction of thiopurine methyltransferase during thiopurine treatment in inflammatory bowel disease. Nucleosides Nucleotides Nucleic Acids. 2006;25:1033–1037. doi: 10.1080/15257770600890814. [DOI] [PubMed] [Google Scholar]
- 15.Dilger K, Schaeffeler E, Lukas M, Strauch U, Herfarth H, Müller R, et al. Monitoring of thiopurine methyltransferase activity in postsurgical patients with Crohn's disease during 1 year of treatment with azathioprine or mesalazine. Ther Drug Monit. 2007;29:1–5. doi: 10.1097/FTD.0b013e3180312b9a. [DOI] [PubMed] [Google Scholar]
- 16.Gisbert JP, Chaparro M, Gomollón F. Common misconceptions about 5-aminosalicylates and thiopurines in inflammatory bowel disease. World J Gastroenterol. 2011;17:3467–3478. doi: 10.3748/wjg.v17.i30.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meggitt SJ, Anstey AV, Mohd Mustapa MF, Reynolds NJ, Wakelin S. British Association of Dermatologists' guidelines for the safe and effective prescribing of azathioprine 2011. Br J Dermatol. 2011;165:711–734. doi: 10.1111/j.1365-2133.2011.10575.x. [DOI] [PubMed] [Google Scholar]
- 18.Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Théorêt Y, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705–713. doi: 10.1016/s0016-5085(00)70140-5. [DOI] [PubMed] [Google Scholar]
- 19.Askanase AD, Wallace DJ, Weisman MH, Tseng CE, Bernstein L, Belmont HM, et al. Use of pharmacogenetics, enzymatic phenotyping, and metabolite monitoring to guide treatment with azathioprine in patients with systemic lupus erythematosus. J Rheumatol. 2009;36:89–95. doi: 10.3899/jrheum.070968. [DOI] [PubMed] [Google Scholar]