Abstract
The Melanocortin (MC) system is one of the crucial neuropeptidergic systems that modulate energy balance. The roles of endogenous MC and MC-4 receptor (MC4-R) signaling within the hypothalamus in the control of homeostatic aspects of feeding are well established. Additional evidence points to a key role for the central MC system in ethanol consumption. Recently, we have shown that nucleus accumbens (NAc), but not lateral hypothalamic (LH), infusion of a selective MC4-R agonist decreases ethanol consumption. Given that MC signaling might contribute to non-homeostatic aspects of feeding within limbic circuits, we assessed here whether MC4-R signaling within the NAc and the lateral hypothalamus (LH) alters normal ingestive hedonic and/or aversive responses to ethanol in rats as measured by a taste reactivity test. Adult male Sprague-Dawley rats were given NAc- or LH- bilateral infusion of the selective MC4-R agonist cyclo (NH-CH2-CH2-CO-His-D-Phe-Arg-Trp-Glu)-NH2 (0, 0.75 or 1.5 µg/0.5µl/site) and following 30 min, the animals received 1 ml of ethanol solution (6% w/v) intraoral for 1 minute and aversive and hedonic behaviors were recorded. We found that NAc-, but not LH-administration, of a selective MC4-R agonist decreased total duration of hedonic reactions and significantly increased aversive reactions relative to saline-infused animals which support the hypothesis that MC signaling within the NAc may contribute to ethanol consumption by modulating non-homeostatic aspects (palatability) of intake.
Keywords: ethanol, palatability, MC4 receptors, lateral hypothalamus, nucleus accumbens, taste reactivity
The Melanocortin (MC) system is one of the crucial neuropeptidergic systems that modulate energy balance. The roles of endogenous MC and MC-4 receptor (MC4-R) signaling within the hypothalamus in the control of homeostatic aspects of feeding, food selection and body weight are well established [1,2]. More recently, pharmacological and genetic studies have provided additional evidence that MC signaling plays a key role in ethanol consumption. Intracerebroventricular (i.c.v.) infusion of the non-selective MC3-R/MC4-R agonist Melanotan-II (MTII) decreased ethanol consumption in C57BL/6J mice [3] and AA rats [4]; i.c.v. infusion of the non-selective MC3-R/MC4-R antagonist Agouti-related protein (AgRP)-(83–132) disrupted MTII-induced reduction of ethanol consumption [3] and increased voluntary ethanol consumption in C57BL/6J mice [5]. Furthermore, i.c.v. administration of the highly selective MC4-R agonist cyclo-(NH-CH2-CH2-CO-His-D-Phe-Arg-Trp-Glu)-NH2 in C57BL/6J mice significantly decreased voluntary ethanol consumption and feeding [5]. Consistent with pharmacological studies, genetic deletion of AgRP blunted ethanol self-administration and binge-like ethanol drinking [6]. Recent work from our lab has shown that infusion of a selective MC4-R agonist into the ventral tegmental area (VTA) and the nucleus accumbens (NAc), but not into the lateral hypothalamus (LH), reduces ethanol and food consumption [7], which lead us to propose that endogenous α-MSH within limbic regions might regulate non-homeostatic aspects of ethanol consumption through MC4-R. The present study directly addresses this hypothesis by evaluating whether NAc- or LH-infusions of a selective MC4-R agonist alter normal ingestive hedonic and/or aversive responses to ethanol in adult rats. The taste reactivity (TR) test [8] has been successfully employed to analyze hedonic aspects of ingestive behaviors apart from post-ingestive effects. To do that, stereotyped mouth, tongue, and body movements are employed as indicators of the animal’s perceived palatability of the substance [9]. Ingestive responses are associated with fluid consumption and positive hedonic reactions, while aversive responses promotes the expulsion of fluid from the oral cavity and are valid indexes of negative hedonic reactions to the fluid being evaluated [8,10].
Adult male Sprague-Dawley rats (Charles River Laboratories, Spain) weighing 280–300 g at the beginning of the experiment were housed individually in an environmentally controlled room (22 °C temperature on a 12:12h light:dark cycle). Standard rodent chow and water were provided ad libitum throughout the experiments and all the pharmacological manipulations were conducted at the onset of the dark phase. Behavioral procedures and pharmacological techniques were in compliance with the animal care guidelines established by the Spanish Royal Decrees 1025/2005 for reducing animal pain and discomfort and the protocols were approved by the University of Almería Bioethical Animal Care and Use Committee.
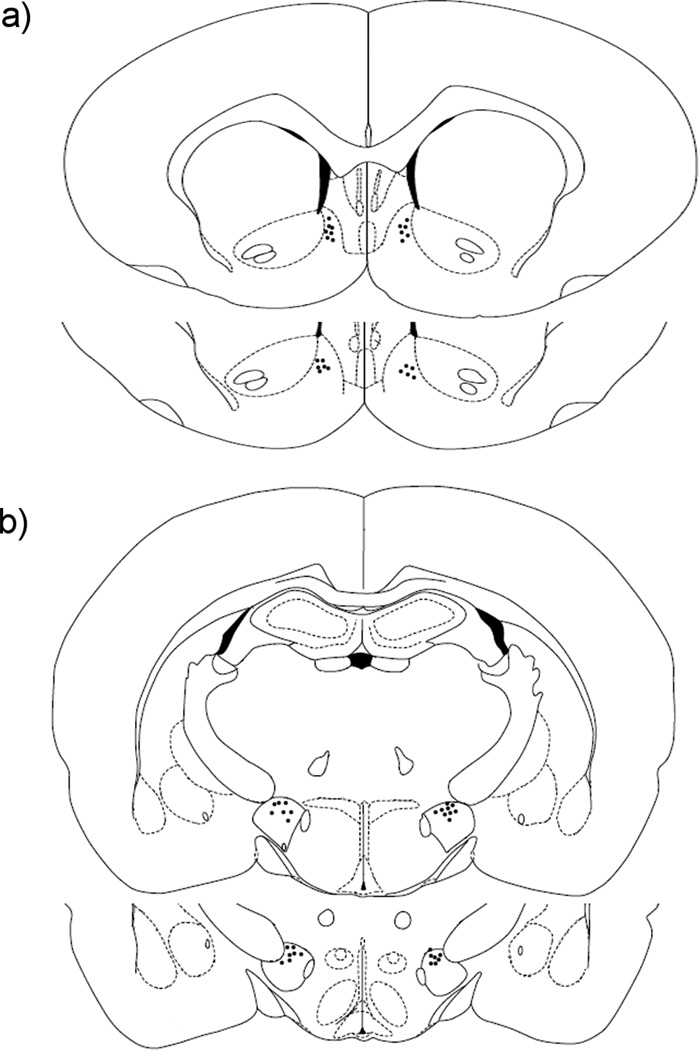
Rats were anesthetized with equitesin (0.3ml/100g) and atropine (0.04 mg / kg) to prevent respiratory distress and then implanted with intraoral and intracerebral (NAc- or LH-directed) cannulas. The intraoral cannula was constructed of PE-100 tubing and was inserted through the cheek caudal to the eye and then subcutaneously to exit near the intrascapular area behind the head [11]. A bilateral 26-G cannula (Plastic One Inc., Germany) aimed at the NAc or LH was implanted using the following stereotaxic coordinates [12]: NAc: 1.7 mm anterior to bregma, 0.8 mm lateral to the midline, and 5.4 mm ventral to the skull surface; LH: 2.12 mm posterior to bregma, 2.10 mm lateral to the midline and 8 mm ventral to the skull surface. At the end of experimental procedures, histological verification of cannula placement was conducted and only animals with correct cannula placement were used for statistical analysis (Figure 1).
Figure 1.
Histological reconstruction showing injection sites (black dots) in the NAc (a) and the LH (b). Sections are labelled according to the distance from Bregma along the rostral–caudal axis. Adapted from Paxinos & Watson (1998).
Rats were given a minimum of 10 days to recover following surgery. During this time, oral cannulas were flushed every other day with distilled water to prevent clogging and body weight was recorded to ensure proper recover from surgeries. Once the animals recovered from surgery, the animals were given 24-h free access to two bottles, one containing plain water and the other containing a solution of ethanol in plain water (6% w/v), for one month to ensure that ethanol was not a novel stimulus at the TR test day. Food and water were available ad libitum. Taste reactivity tests were conducted in a transparent Plexiglas chamber (24 cm × 26 cm) resting on a clear glass plate. A mirror was mounted beneath the chamber at a 45° angle to allow viewing of the ventral surface of the animal. The intraoral infusions were performed at a constant rate of 1 ml/min, via an infusion pump (Harvad apparatus, USA) connected to the intraoral cannula. Subjects were recorded with a digital video camera (Sony, DCR-SR37E) placed focusing on the mirror.
Following one month of continued ethanol access, the animals were weighted and distributed into 3 sub-groups based on baseline ethanol consumption. One hour before the beginning of the dark phase of the light:dark cycle ethanol, water and food were removed from the cages. Then, rats were given bilateral site-directed infusion of the selective MC4-R agonist cyclo(NH-CH2-CH2-CO-His-D-Phe-Arg-Trp-Glu)-NH2 (Phoenix Pharmaceuticals, Inc., Belmont, CA) dissolved in isotonic saline into the NA or LH, at either 1.5 µg/0.5µl/site (NAc n = 8; LH n = 10) or 0.75 µg/0.5µl/site (NAc n = 7; LH n = 10) or an identical volume of isotonic saline, 0.5 µl/site (NAc n = 9; LH n = 10). The doses selected were based on previous studies demonstrating a significant reduction in food and ethanol drinking following central infusion of the compound [7]. Site-directed infusions were given manually over a one minute period with a 1.0 µl Hamilton syringe connected to a 33-G injector cannula (Plastic One Inc., Germany) that protruded 1 mm when aimed to the NAc and 0.2 mm when aimed to the LH. Then, rats were immediately returned to their home cage for 20 min until they were moved to the taste-reactivity test room.
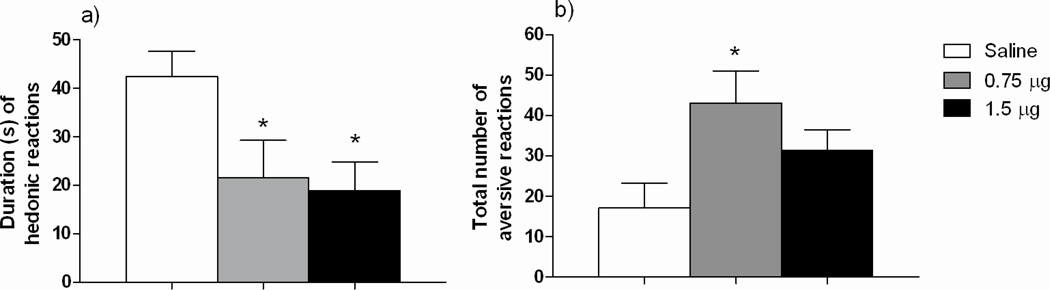
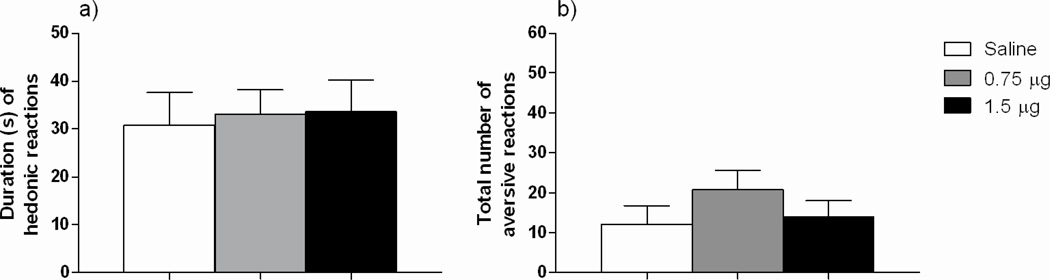
All rats in the study were habituated to the general testing procedure for 3 consecutive days prior to the test day. The animals were placed in the test chamber for 10 min and habituated to the oral presentation of 1 ml of ethanol solution (6% w/v), following similar procedures on the test day. The day of TR testing, subjects were placed in the test chamber for an additional 10 min habituation period (without infusion), followed immediately by the test. During the test day, animals received 1 ml of ethanol solution (6% w/v) for 1 minute in which taste reactivity behaviors were recorded by a camera. The video files were scored, in real time and frame by frame, by two raters blind to the treatment conditions (inter-rater correlations: rs 0.96). Based on previous studies [10, 13], the typical reactions to ethanol flavor were considered and categorized as either hedonically ingestive or aversive. The category of ingestive hedonic reactions included “mouthing”, “tongue protrusions” and “paw licking”. The category of aversive reactions included “snout and facial wiping”, “head shaking”, “forelimb flailing” and “gaping”. Hedonic reactions were measured as number of seconds spent displaying the behaviors and the aversive reactions as number of occurrences. Independent one-way ANOVAs performed on baseline ethanol intake data showed no statistical significant drug main effect, indicating that all rats exhibited similar ethanol intake baseline prior to the TR test: NAc-group: [F (2, 21) = 0.37; p = 0.69], (Mean ± SEM saline group: 2.89 ± 0.29 g/kg/24-h; MC agonist 0.75 µg dose group: 2.57 ± 0.24g/kg/24-h; MC agonist 1.5 µg dose group 2.46 ± 0.52 g/kg/24-h). LH group [F (2, 28) = 0.06; p = 0.93] (Mean ± SEM saline group: 2.43 ± 0.37 g/kg/24-h; MC agonist 0.75 µg dose group: 2.38 ± 0.35 g/kg/24-h and MC agonist 1.5 µg dose group: 2.56 ± 0.38 g/kg/24-h). The ANOVAs performed on taste reactivity data obtained following NAc-infusion of the selective MC4-R agonist (0.75 or 1.5 µg) or saline revealed a statistically significant drug main effect F (2, 21) = 4.65, p = 0.02] (Figure 2). Additional post hoc Newman Keuls (NK) analysis showed that both 0.75 and 1.5 µg doses decreased ingestive reactions (time) relative to saline. The analysis performed for aversive reactions revealed a significant drug main effect [F (2, 21) = 4.14, p = 0.03] and additional NK analysis showed that infusion of 0.75 µg of the MC4-R agonist significantly increased aversive reactions (total number) relative to saline-treated rats. Administration of 1.5 µg of the selective MC4-R agonist triggered a mild increase in aversive reactions, which did not achieve statistical significance compared with saline or the low dose of the agonist employed. On the other hand, separate ANOVAs performed on taste reactivity data following LH-infusion of the selective MC4-R agonist (0.75 or 1.5 µg) or saline clearly show the absence of any statistically significant drug main effect for hedonic ingestive F (2, 27) = 0.56, p = 0.95] or aversive responses [F (2, 27) = 1.04, p = 0.37], indicating that LH-infusion of a selective MC4-R agonist did not significantly alter taste reactivity to ethanol (Figure 3).
Figure 2.
Mean + SEM data showing duration of hedonic reactions (a) and total aversive reactions (b) in response to oral administration of ethanol 6% w/v during a TR test, following NAc infusion of the selective MC4-R agonist cyclo(NH-CH2-CH2-CO-His-D-Phe-Arg-Trp-Glu)-NH2) (0.75 or 1.5 µg) or isotonic saline. Both 0.75 and 1.5 µg doses significantly decreased duration of hedonic reactions while only administration of 0.75 µg increased total aversive reactions relative to saline. *P< 0.05.
Figure 3.
Mean + SEM data showing duration of hedonic reactions (a) and total aversive reactions (b) in response to oral administration of ethanol 6% w/v during a TR test, following NAc infusion of the selective MC4-R agonist cyclo(NH-CH2-CH2-CO-His-D-Phe-Arg-Trp-Glu)-NH2) (0.75 or 1.5 µg) or isotonic saline. LH-infusion of a selective of MC4-R agonist did not significantly alter ethanol palatability.
The present study directly addressed, for the first time, the role of MC4-R signaling within the NAc and the LH in ethanol palatability. The main observations obtained here are: 1) NAc-administration of either 0.75 and 1.5 µg/site of a selective MC4-R agonist decreased total duration of ingestive reactions; 2) NAc-administration of 0.75 µg/site of a selective MC4-R agonist significantly increased aversive reactions relative to saline-infused animals, while the high dose, 1.5 µg/site, elicited a moderate increase which did not attained statistical significance. 3) LH-administration of the selective MC4-R agonist did not significantly modify hedonic ingestive or aversive reactions following intraoral alcohol flushing as measure in the TR test. Because the MC4-R agonist cyclo(NH-CH2-CH2-CO-His-D-Phe-Arg-Trp-Glu)-NH2 is a highly selective agonist for MC4-R, with 90-fold selectivity over MC3-R and a 2000-fold selectivity over MC5-R [14], the present data indicates that endogenous MC signaling within the NAc, but not within the LH, regulates ethanol palatability via the MC4-R.
The TR procedure has been successfully employed to characterize taste palatability of an ample range of tastant stimuli [8, 10, 15], including ethanol solutions [10, 16]. In this regard, it is important to note that our saline-treated rats showed a similar pattern of complex hedonic ingestive and aversive reactions to ethanol that have previously been reported when ethanol is administered and is not a novel stimulus [13, 17]. Thus, consistent with available behavioral and electrophysiological studies, the present observations confirm that, at least in the gustatory dimension, control rats perceive the taste of ethanol as a complex taste with pleasant and unpleasant aspects, coinciding with studies showing that ethanol is perceived by rats as a mixture of sweet and bitter properties [18]. Additionally and according to the ability of the TR test to detect changes in taste palatability as a result of pharmacological manipulation, we found here that stimulation of MC4-R signaling in the NAc, but not in LH, shifted palatability of a 6% w/v ethanol solution to aversive, as evidenced by an increase in aversive reactions and a decrease in ingestive reactions during the taste reactivity test.
Because taste factors must play an important role in the final decision to ingest or reject a fluid [8, 15], one interesting implication of the present data, is that changes in ethanol palatability induced by NAc-infusion of a MC-agonist might negatively influence voluntary ethanol consumption. It is true that, concurrent with the distinction between wanting and liking, consumption and palatability are measures that do not necessarily depend on one another [19, 20]. An increase in the consumption of a tastant may be observed without changes in palatability [21] and viceversa, changes in the palatability of a substance may not necessarily be reflected in intake [17]. Nonetheless, a change in ethanol palatability may be followed by a change in ethanol intake [22]. Therefore, given recent studies providing pharmacological evidence that NA-infusion, but not LH-infusion, of the selective MC4-R agonist cyclo(NH-CH2-CH2-CO-His-D-Phe-Arg-Trp-Glu)-NH2) strongly reduces voluntary ethanol consumption [7], together with the present observations, it is tempting to propose that MC signaling within limbic regions have a key role in the hedonic aspects of ethanol consumption. The specific neural mechanisms involved in the modulatory role of MC signalling within the NAc in ethanol palatability remain unexplored and, based on present data, we cannot make direct conclusions about such mechanisms. However, some tentative ideas pointing to the opiate system are discussed next.
First, there is solid evidence indicating a role for opioid signaling in ethanol palatability [9, 13, 15, 22]. In this regard, human and animal studies have consistently shown that opioid antagonists decrease and agonists increase, both palatability and consumption of ethanol. Thus, rats treated with the opioid antagonist naltrexone showed a reduced palatability (increased aversive responses and reduced ingestive behaviors) [9, 13] and consumption of ethanol [13, 22]. Additionally, rats treated with DAMGO, a specific mu opioid receptors agonist, increased positive hedonic reactions and ethanol consumption [15]. Second, mutually antagonist interactions between MC and opiates have been described in several anatomical sites [23]. Thus, opiate withdrawal is precipitated by MC agonists, opiate tolerance is antagonised by MC antagonists [24] and inhibition of MC signaling alleviates chronic pain [25]. Moreover, administration of opiates to humans suppresses POMC activity and its derived opiates [24], and endogenous α-MSH levels are reduced in opiate dependence [22, 23].
In light of the aforementioned studies showing the existence of important MC-opiate neurobiological interactions, the role of opiates in ethanol palatability and our present data, that matches previous TR profiles emerging in rats treated with opioids antagonists [9, 22], one interesting avenue for future research will be to explore the contribution of MC-opiates interactions within the NAc to the hedonic aspects of ethanol consumption. Further, a greater understanding of the mechanism of such neuropeptides interactions will allow an advancement in potential pharmacological combined treatment approaches for alcoholism.
In conclusion, we report here for the first time direct evidence that MC4-R signaling within the NAc, a limbic region, modulates ethanol palatability while MC4-R signaling within the LH seems to be unrelated to ethanol palatability. Taking together the present data and previous studies [7], we propose that MC signaling within the NAc contributes to ethanol consumption by modulating non-homeostatic aspects (palatability) of intake while in the LH, MC signalling regulates homeostatic aspects (e.g., the caloric properties of ethanol).
HIGHLIGHTS.
We test the role of MC4-R signaling within the NAc and LH in ethanol palatability
Infusion of the MC4R agonist into the NAc reduced ethanol palatability
Infusion of the MC4-R agonist into the NAc increased aversive taste reactions
Infusion of the MC4-R agonist into the LH did not alter ethanol palatability
MC4-R signaling within the NAc modulates non-homeostatic aspects of ethanol intake
Acknowledgements
This work was supported by MEC grants (Spain), SEJ2006-03629, PSI2009-07677 “Programa Salvador Madariaga 2006”, J.A., grant CTS1350, FEDER UALM05-23-006, NIH grants AA013573, AA015148, and the Department of Defense grants W81XWH-06-1-0158 and W81XWH-09-1-0293.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adan RA, Tiesjema B, Hillebrand JJ, la Fleur SE, Kas MJ, de Krom M. The MC4 receptor and control of appetite. Br J Pharmacol. 2006;149:815–827. doi: 10.1038/sj.bjp.0706929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vergoni AV, Bertolini A. Role of melanocortins in the central control of feeding. Eur J Pharmacol. 2000;405:25–32. doi: 10.1016/s0014-2999(00)00538-0. [DOI] [PubMed] [Google Scholar]
- 3.Navarro M, Cubero I, Knapp DJ, Thiele TE. MTII-induced reduction of voluntary ethanol drinking is blocked by pretreatment with AgRP-(83-132) Neuropeptides. 2003;37:338–344. doi: 10.1016/j.npep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Ploj K, Roman E, Kask A, Hyytia P, Schioth HB, Wikberg J, Nylander I. Effects of melanocortin receptor ligands on ethanol intake and opioid levels in alcohol-preferring AA rats. Brain Res Bull. 2002;59(2):97–104. doi: 10.1016/s0361-9230(02)00844-4. [DOI] [PubMed] [Google Scholar]
- 5.Navarro M, Cubero I, Chen AS, Chen HY, Knapp DJ, Breese GR, Marsh DJ, Thiele TE. Effects of melanocortin receptor activation and blockade on ethanol intake: a possible role for the melanocortin-4 receptor. Alcohol Clin Exp Res. 2005;29:949–957. doi: 10.1097/01.ALC.0000167740.19702.8C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navarro M, Cubero I, Ko L, Thiele TE. Deletion of agouti-related protein blunts ethanol self-administration and binge-like drinking in mice. Genes Brain Behav. 2009;8(4):450–458. doi: 10.1111/j.1601-183X.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lerma-Cabrera JM, Carvajal F, de la Torre L, de la Fuente L, Navarro M, E Thiele T, Cubero I. Control of food intake by MC4-R signaling in the lateral hypothalamus, nucleus accumbens shell and ventral tegmental area: Interactions with ethanol. Behav Brain Res. 2012;234:51–60. doi: 10.1016/j.bbr.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143(2):263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 9.Hill KG, Sable HJ, Ferraro FM, Kiefer SW. Chronic naltrexone treatment and ethanol responsivity in outbred rats. Alcohol Clin Exp Res. 2010;34(2):272–279. doi: 10.1111/j.1530-0277.2009.01090.x. [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Cenzano E, Chotro MG. The effect of taste familiarity on intake and taste reactivity in infant rats. Dev Psychobiol. 2010;52(2):109–120. doi: 10.1002/dev.20418. [DOI] [PubMed] [Google Scholar]
- 11.Cubero I, Thiele TE, Bernstein IL. Insular cortex lesions and taste aversion learning: effects of conditioning method and timing of lesion. Brain Res. 1999;839(2):323–330. doi: 10.1016/s0006-8993(99)01745-x. [DOI] [PubMed] [Google Scholar]
- 12.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th. edic. NY: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- 13.Kiefer SW, Hill KG, Coonfield DL, Ferraro FM. Ethanol familiarity and naltrexone treatment affect ethanol responses in rats. Alcohol. 2005;37(3):167–172. doi: 10.1016/j.alcohol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Bednarek MA, MacNeil T, Tang R, Kalyani RN, Van der Ploeg LH, Weinberg DH. Potent and selective peptide agonists of alpha-melanotropin action at human melanocortin receptor 4: their synthesis and biological evaluation in vitro. Biochem Biophys Res Commun. 2001;286:641–645. doi: 10.1006/bbrc.2001.5444. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, Kelley AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacol (Berl) 2002;159:415–423. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]
- 16.Kiefer SW. Alcohol, palatability, and taste reactivity. Neurosci Biobehav Rev. 1995;19:133–141. doi: 10.1016/0149-7634(94)00027-x. [DOI] [PubMed] [Google Scholar]
- 17.Kiefer SW, Bice PJ, Badia-Elder N. Alterations in taste reactivity to alcohol in rats given continuous alcohol access followed by abstinence. Alcohol Clin Exp Res. 1994;18(3):555–559. doi: 10.1111/j.1530-0277.1994.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 18.Kiefer SW, Lawrence GJ. The sweet bitter taste of alcohol-aversion generalization to various sweet quinine mixtures in the rat. Chemical Senses. 1988;13(4):633–641. [Google Scholar]
- 19.Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacol (Berl) 2007;191(3):439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- 20.Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81(2):179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Fudge MA, Kavaliers M, Ossenkopp KP. Allopregnanolone produces hyperphagia by reducing neophobia without altering food palatability. Eur Neuropsychopharmacol. 2006;16(4):272–280. doi: 10.1016/j.euroneuro.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Coonfield DL, Hill KG, Kaczmarek HJ, Ferraro FM, Kiefer SW. Low doses of naltrexone reduce palatability and consumption of ethanol in outbred rats. Alcohol. 2002;26(1):43–47. doi: 10.1016/s0741-8329(01)00180-x. [DOI] [PubMed] [Google Scholar]
- 23.Grossman HC, Hadjimarkou MM, Silva RM, Giraudo SQ, Bodnar RJ. Interrelationships between mu opioid and melanocortin receptors in mediating food intake in rats. Brain Res. 2003;991:240–244. doi: 10.1016/s0006-8993(03)03442-5. [DOI] [PubMed] [Google Scholar]
- 24.Kalange AS, Kokare DM, Singru PS, Upadhya MA, Chopde CT, Subhedar NK. Central administration of selective melanocortin 4 receptor antagonist HS014 prevents morphine tolerance and withdrawal hyperalgesia. Brain Res. 2007;1181:10–20. doi: 10.1016/j.brainres.2007.08.054. [DOI] [PubMed] [Google Scholar]
- 25.Vrinten DH, Adan RA, Groen GJ, Gispen WH. Chronic blockade of melanocortin receptors alleviates allodynia in rats with neuropathic pain. Anesth Analg. 2001;93:1572–1577. doi: 10.1097/00000539-200112000-00052. [DOI] [PubMed] [Google Scholar]