Abstract
Objective
Vein graft failure rates due to adverse graft remodeling remain high with no effective therapy. The mineralocorticoid receptor (MR) plays a role in pathologic arterial remodeling. We have recently demonstrated that MR is upregulated in venous tissues after grafting and hypothesized that MR inhibition would reduce vein graft remodeling.
Methods
Reverse transcription polymerase chain reaction and immunoblotting were used to examine the expression of MR and other components of the renin-angiotensin-aldosterone system in human vein and primary human saphenous vein smooth muscle cells (HSVSMC). Adenoviral reporter gene assays were used to explore MR transcriptional activity in HSVSMC. The effect of MR inhibition on vein graft remodeling in vivo was characterized in a mouse vein graft model.
Results
Messenger RNAs encoding MR, 11-β-hydroxysteroid dehydrogenase 2 (11bHSD2), angiotensin type-1 receptor, and the angiotensin converting enzyme are expressed in whole human vein and in HSVSMC. MR and 11βHSD2 protein expression is confirmed and MR-dependent transcriptional regulation is demonstrated at physiologic aldosterone concentrations in HSVSMC. Treatment of mice with the MR antagonist spironolactone, at doses that do not lower blood pressure (20 mg/kg/day), reduces maximal vein graft intima-media thickness by 68%, with an associated reduction in graft inflammatory cell infiltration and fibrosis.
Conclusions
The MR is expressed in human venous tissue and cells and modulates gene expression in HSVSMC in response to physiologic aldosterone concentrations. In vivo, MR inhibition reduces vein graft thickening and inflammation. These preclinical data support the potential to use MR antagonists as novel treatments to preserve vein graft patency.
INTRODUCTION
Bypass surgery remains an important therapeutic option for patients with arterial occlusive disease; however, vein graft failure rates due to adverse graft remodeling remain high with no effective therapy. Veins placed into the arterial circulation undergo adaptive remodeling with rapid smooth muscle cell (SMC) hyperplasia, thereby reducing wall tension.1 The mechanism involves medial SMC de-differentiation from a contractile phenotype into a synthetic state that proliferates and secretes cytokines and growth factors, which contribute to a robust inflammatory response.2 This process leads to histological changes resembling those seen in arterial atherosclerosis, including the development of inflamed focal lesions that can occlude blood flow or rupture, leading to thrombus formation.3,4 The pathologic changes found in failed vein graft specimens removed from patients undergoing corrective reinterventions have also been demonstrated in mouse models of vein grafting, providing a small animal model to test potential therapies.3,5
The mineralocorticoid receptor (MR) is a hormone-activated transcription factor that modulates gene expression when activated.6 MR is the terminal component of the renin-angiotensin-aldosterone system (RAAS) that is activated in response to hypotension, resulting in production of angiotensin II by the angiotensin converting enzyme (ACE), which promotes adrenal gland aldosterone release via angiotensin type-1 receptors (AT1R). The MR is activated by the steroid hormones aldosterone or cortisol with equal affinity, but the presence of the cortisol-inactivating enzyme 11-β-hydroxysteroid dehydrogenase-2 (11βHSD2) confers aldosterone specificity for MR in tissues where they are co-expressed.6 The MR is most well studied in the kidney where it raises blood pressure when activated by enhancing renal sodium absorption.6 However, our group and others have demonstrated that arterial SMC and endothelial cells (EC) express MR that contributes to arterial remodeling with enhanced vascular inflammation, fibrosis, and SMC hyperplasia.6–8 We recently demonstrated that both MR and 11βHSD2 are upregulated after grafting in a rabbit vein graft model and also in failing saphenous vein grafts explanted from humans, suggesting a potential role for venous MR in vein graft failure.9 Here we further characterize the expression and function of the RAAS in human venous tissue and cells and explore the role of MR antagonism as a potential therapy to prevent adverse remodeling in a mouse model of vein grafting.
METHODS
Reagents and Cell Lines
Aldosterone and spironolactone (Sigma) were resuspended in DMSO, diluted in DMEM (Gibco), and used at the indicated concentrations with corresponding vehicle controls. With approval from the Tufts Institutional Review Board, de-identified samples of discarded human venous tissues that had been endoscopically harvested from patients undergoing indicated coronary artery bypass graft surgery were collected. Primary human VSMC were isolated from saphenous vein specimens as previously described.10 For gene expression studies, primary cells were grown in 10% bovine serum (HyClone) in DMEM and were used between passages 3 and 7.
Quantitative RT-PCR
Total RNA was isolated from cells or tissue, reverse transcribed, and quantitative PCR was performed as described previously11 using primers listed in Supplemental Table 1. Genes with raw Ct values of 35 cycles or greater were considered to be not expressed. The Ct values of expressed genes were normalized to GAPDH. Data are represented as the mean normalized expression (2−△Ct) in RNA isolated from four independent tissue or cell sources each analyzed in duplicate.
Immunoblotting
Cell lysates were prepared as previously described.7 Cell lysates (1 μL control HEK293 cell lysate over-expressing MR or 11βHSD2, 30 μL of denatured human saphenous vein SMC (HSVSMC) supernatant for MR immunoblots, and 50 μL of the resuspended pellet for 11βHSD2 immunoblots) were separated by 10% SDS-PAGE, transferred to 0.45 μm nitrocellulose membranes, immunoblotted with monoclonal antibodies raised against the unique MR N-terminus (generous gift of Celso Gomez-Sanchez)12 or the 11βHSD2 C-terminal catalytic domain (Alpha Diagnostic International), and visualized by standard chemiluminescence techniques as previously described.7
Luciferase Assay
Human saphenous vein SMC were serum-starved in DMEM for 24 hours, then infected with an adenovirus containing a fused mineralocorticoid responsive element (MRE)-firefly luciferase construct9 at a multiplicity of infection of 200, as described.7 After infection, cells were grown in DMEM with 5% charcoal-stripped fetal bovine serum (Atlanta Biologicals) containing vehicle or the indicated hormone(s) for 18 hours. Luciferase activity was determined as described.7 Luciferase units for each sample were normalized to the value obtained for the vehicle-treated sample for a given experiment to generate the reported fold-change in luciferase activity (N=3–4 independent experiments, each in duplicate).
Mouse Inferior Vena Cava to Aorta Vein Graft Model
Animals were handled in accordance with NIH standards and all procedures were approved by the Tufts Medical Center Institutional Animal Care and Use Committee. The inferior vena cava (IVC) from a donor male 12- to 16-week old inbred wild-type C57BL6 mouse (Jackson Laboratories) was grafted into the abdominal aorta of an equivalent recipient mouse as previously described.5 After verifying sufficient blood flow through the graft by in vivo microscopy, recipient mice were randomized to receive spironolactone (20 mg/kg/day)- or placebo-releasing drug pellet (Innovative Research of America, N=8 per treatment). We and others have demonstrated that at this low dose, spironolactone does not change mouse systolic or diastolic tail cuff blood pressure (data not shown and13). Early graft thrombosis (within 24 hours) requiring euthanasia occurred in some mice with a survival rate at 4 weeks that was unchanged between the two treatment groups. After 4 weeks, the grafted and native IVC were harvested (N=4 per group), fixed in 10% neutral-buffered formalin, and embedded in paraffin for subsequent immunohistochemical analyses.10
Immunohistochemistry
Sections of embedded vessels were collected at 200-micron intervals covering the central 1.6 mm of the graft, yielding 8 sections spanning approximately 60% of the total graft length. Serial parallel sections were stained with H&E, Trichrome stain, and smooth muscle-specific alpha actin antibody as described.14 The area of the vessel intima and media was quantified from H&E-stained sections using ImagePro 6.2 software (Media Cybernetics). Vessel extracellular matrix (ECM) content was quantified in trichrome-stained sections and expressed as the absolute intima-media ECM area. For mononuclear (MN) and polymorphonuclear (PMN) cell quantification, nuclei were manually counted in H&E-stained sections. Serial sections stained with an anti-smooth muscle alpha actin antibody were used to count the number of actin-positive cells and actin-positive area within the grafted vessel wall. All histological quantifications were performed by a treatment-blinded investigator and reported for the section with the thickest intima-media area for each mouse.
Statistical Analysis
Values are reported as mean ± standard error of the mean. Statistical comparisons were made by t-test, one- or two-factor ANOVA where appropriate, with Student-Newman-Keuls or Mann-Whitney post test using SigmaPlot 11.0 (Systat Software). P<0.05 was considered significant.
RESULTS
Expression of RAAS Genes in human saphenous vein tissue and SMC
Total RNA was isolated from human saphenous vein samples collected from the operating room and from low passage primary cultured HSVSMC (N=4 of each) and quantitative RT-PCR was performed using primers specific to genes encoding components of the RAAS. Figure 1A demonstrates that MR, glucocorticoid receptor (GR), 11βHSD2, ACE-1, and AT1R mRNAs are indeed expressed in human saphenous vein tissue and HSVSMC from male and female patients with varied cardiac risk factors undergoing cardiac bypass surgery, while renin and aldosterone synthase (AS) mRNA are not detected. MR and 11βHSD2 protein expression was examined in cell lysates from primary cultured HSVSMC. Immunoblot revealed the characteristic 107 kDa MR protein band and the 41 kDa 11βHSD2 protein band, as demonstrated by comparison with overexpressed proteins (positive (+) and negative (−) control lanes on the same immunoblot; Figure 1B). These data support the potential for MR to be activated by aldosterone in HSVSMC.
Figure 1.
Expression of Renin-Angiotensin-Aldosterone System (RAAS) components in human saphenous vein tissue and smooth muscle cells (HSVSMC). (A) Quantitative RT-PCR was conducted to identify RAAS gene mRNA isolated from human saphenous vein (white bars) and HSVSMC (black bars). N=4, ND=not detected. (B) Immunoblots on HSVSMC lysates with antibody specific for MR (top) and 11bHSD2 (bottom) protein. Lysate from HEK293 cells transfected with empty plasmid (−) or with plasmid that expresses MR or 11bHSD2 (+) serve as negative and positive controls, respectively.
Endogenous MR in HSVSMC is transcriptionally activated by physiologic aldosterone concentrations
Steroid hormones modulate cellular physiology by binding to steroid receptors to regulate gene expression. We have previously demonstrated that MR in HSVSMC can modulate gene transcription when activated by pathologic aldosterone concentrations, as in patients with congestive heart failure (10 nM);6 however, most vein graft patients have normal serum aldosterone levels (~1 nM). A sensitive adenoviral reporter of MR-mediated gene expression was used to explore the dose-response relationship of aldosterone activation of HSVSMC MR. Gene expression was activated by aldosterone in a dose-dependent manner beginning at a concentration of 1 nM (Figure 2A), consistent with physiologic aldosterone levels15 and the known Kd of the MR for aldosterone binding.6 Treatment of cells with aldosterone in the presence of the MR antagonist spironolactone inhibited aldosterone-dependent transcriptional activation (Figure 2B) supporting that transcriptional activation by aldosterone is mediated by endogenous MR in HSVSMC. These data support the potential for MR to be transcriptionally activated in saphenous vein SMC by relevant circulating aldosterone levels found in vein graft patients and could therefore play a role in vein graft remodeling in vivo.
Figure 2.
Functional MR in HSVSMC. Primary HSVSMC were infected with an adenovirus containing a mineralocorticoid-inducible response element (MRE)-luciferase transcriptional reporter. (A) Infected cells were treated with increasing doses of aldosterone (Aldo) and Aldo-induced changes in luciferase activity were measured. (B) Cells were treated with 10 nM Aldo and/or the MR antagonist spironolactone (Spiro). *p<0.05 vs. vehicle, #p<0.05 vs 10 nM aldo.
MR inhibition with spironolactone attenuates vein graft remodeling in vivo
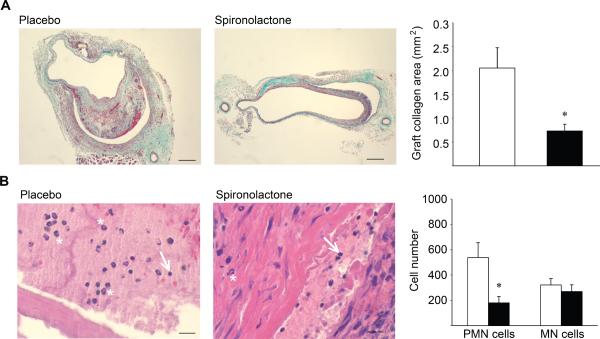
Using a mouse model of IVC-to-aorta interposition grafting we investigated the effects of MR inhibition on vein graft remodeling. At the time of vein grafting, mice were randomized to treatment with placebo or to the clinically available MR antagonist spironolactone (20 mg/kg/day, a dose that does not significantly reduce blood pressure (data not shown and13)). The native (ungrafted) and grafted IVC were harvested and graft remodeling was characterized histologically after 4 weeks, a time point when significant vessel remodeling and inflammation have been observed in multiple animal models of vein grafting5,16 and in humans.17 In both treatment groups, the grafted IVC exhibited dramatic remodeling with pathology similar to that seen in human vein grafts, including substantial vessel thickening (Figure 3) and SMC hyperplasia (Figure 4), when compared to the native IVC. Spironolactone treatment resulted in a 68% reduction in the maximal graft intima-media thickness (Figure 3). The degree of SMC hyperplasia was quantified in sections stained with smooth muscle-specific actin antibody. Despite decreased total graft thickness, there is no difference in the degree of SMC hyperplasia as measured by smooth muscle actin-positive area and the number of smooth muscle cells in vein grafts from spironolactone-treated mice (Figure 4). Vein graft fibrosis was also quantified in trichrome-stained sections from the area of maximal thickness. Spironolactone treatment reduced vessel intima-media collagen area by 53% (Figure 5A). These data support that MR antagonism does not alter the extent of SMC hyperplasia in response to grafting but rather attenuates the degree of fibrosis in the grafted veins. The number of inflammatory cells in the grafted vessel wall was quantified by examining serial sections under high magnification (40×). Inflammatory cells were counted based on nuclear morphology on H&E-stained sections revealing a 3-fold reduction in polymorphonuclear (PMN) inflammatory cells in the grafts of animals treated with spironolactone with no difference in the number of mononuclear inflammatory cells (Figure 5B).
Figure 3.
MR antagonism reduces vein graft thickening in mice. (A) Representative H&E-stained sections from the native inferior vena cava (IVC) and throughout the grafted IVC from placebo- and Spiro-treated mice. Images are arranged from proximal (left) to distal (right) orientation. Scale bar = 0.1 mm. (B) Quantification of the intima-media area of the section with maximal vessel thickness (black boxes in (A)) from placebo- (white bar) and Spiro-treated (black bar) mice. *p<0.05 vs. placebo.
Figure 4.
Vein graft SMC hyperplasia with MR antagonism. Immunohistochemical staining of native and grafted IVC sections using SMC-specific alpha-actin antibody. Actin positive area and nuclei count in SMC actin-positive regions are quantified in vein sections from Spiro-treated animals (black bars) and placebo-treated animals (white bars). Scale bar = 0.1 mm. *p<0.05 vs. placebo.
Figure 5.
Inhibition of MR reduces vein graft fibrosis and inflammation. (A) Quantification of vein graft trichrome-stained collagen area on the thickest sections from placebo- (white bars) and Spiro-treated (black bars) mice. Scale bar = 0.1 mm. (B) Quantification of inflammatory cells in high-power (40×) images of H&E-stained sections placebo- and Spiro-treated animals. Representative mononuclear (MN) cells are indicated by white arrows and polymorphonuclear (PMN) cells are indicated by white asterisks. Scale bar = 0.02 mm. *p<0.05 vs. placebo.
DISCUSSION
In this study, we demonstrate that human venous tissue expresses MR and other components of the RAAS and that MR in human saphenous vein SMC is transcriptionally active at physiologically relevant aldosterone concentrations. Moreover, in a mouse model of vein grafting, MR antagonism with the clinically available drug, spironolactone, prevents vein graft remodeling with significant reductions in maximal graft thickening, total graft fibrosis, and graft inflammation.
The responsiveness of the vasculature to aldosterone has been controversial and relatively unexplored in the venous system. Here we demonstrate that the RAAS components MR, GR, 11βHSD2, ACE, and AT1R, are all expressed in human saphenous vein and cultured human saphenous vein SMC isolated from individuals with diverse cardiac risk factors. The finding that 11βHSD2 mRNA and protein are expressed in HSVSMC supports that human venous SMC, like arterial SMC,7 also have the potential to respond to aldosterone, although the potential for cortisol to activate venous SMC MR under certain circumstances cannot be ruled out. In addition, MR and 11βHSD2 are upregulated in failed human vein grafts, suggesting that aldosterone-mediated MR signaling may be enhanced during graft failure.9 The role of local vascular aldosterone production remains controversial.6 The absence of aldosterone synthase mRNA in venous tissue and cells supports that MR activation in venous cells, as in arterial SMC7, likely is dependent on extravascular sources of aldosterone. We have previously demonstrated that angiotensin II, acting via the AT1R, can also directly activate MR in human arterial SMC and that aldosterone can regulate AT1R expression in saphenous veins.7,9 Thus, the presence of AT1R expression in human venous tissue and SMC supports the potential for bidirectional crosstalk between MR and AT1R in the human vein as well.6
In this study, endogenous MR in human venous SMC was transcriptionally activated by concentrations of aldosterone similar to those found in normal patient populations.15 Epidemiological studies demonstrate that in individuals with vascular disease, higher levels of aldosterone—even within the normal range—are associated with a nearly 2.5-fold increase in cardiovascular ischemia and a 3.5-fold increase in cardiovascular death.18 In arterial SMC and EC, aldosterone and the MR upregulate pathways that promote adverse vascular remodeling in response to injury, including medial hypertrophy and atherosclerosis.6,8 Given that the biologic processes leading to adverse vein graft remodeling are much like those seen in arterial injury,3,8 these findings support that MR activation could play an important role in vein graft remodeling.
Multiple mouse vein graft models have been developed. Here we used the IVC-to-abdominal aorta technique originally described by Salzeberg and coworkers5 because the hemodynamics of the end-to-end anastomoses, ratio of graft to host vessel diameters, lack of foreign external support materials, and remodeling processes are all similar to current human surgical techniques and pathologies.5,19 Using this model, we demonstrate that administration of the MR antagonist spironolactone dramatically reduces focal vein graft thickening. This focal pattern of remodeling is particularly disruptive to hemodynamic flow and is the most frequent cause of vein graft failure.20 The vasculoprotective effects of RAAS blockade are traditionally thought to be due to reduced blood pressures achieved through inhibition of renal MR. However, numerous clinical trials of RAAS antagonists have demonstrated cardiovascular benefits that exceed the modest reduction in blood pressure.6,21 Consistent with previous findings,13 the dose of spironolactone used did not significantly change blood pressure (data not shown), supporting a direct protective effect of MR antagonism on the grafted vein. The decreased vessel thickness observed with spironolactone treatment is accompanied by a concomitant reduction in vessel fibrosis. Aldosterone promotes arterial fibrosis in response to injury6,8 and MR directly regulates a number of profibrotic genes in SMC and arteries, including type 1 and type 3 collagens and connective tissue growth factor.7,11 The role of these vascular MR-regulated fibrosis genes in vein graft remodeling and fibrosis warrants further exploration.
The source and type of cells that account for wall thickening as a result of vein graft remodeling is not clear, although prior studies have focused on SMC hyperplasia as the predominant contributor. Here, we observe no MR-mediated changes in graft SMC hyperplasia or cell number but rather that reduced graft thickness resulting from spironolactone treatment is driven--at least in part—by decreased inflammatory cell infiltration. Clinical and animal studies also support a role for inflammation in early vein graft remodeling. In patients undergoing lower extremity bypass surgery, markers of systemic inflammation (C-reactive protein) correlate with the degree of vein graft remodeling after one month.17 Consistent with this finding, blockade of monocyte chemoattractant factor (MCP1), a proinflammatory molecule upregulated by aldosterone in the setting of vascular disease,6 inhibits vein graft remodeling in a canine model.22 We conclude that MR-mediated vascular inflammation may be an important component of vein-graft remodeling. It has also been shown that AT1R blockade attenuates arterial remodeling by reducing inflammatory-like progenitor cells.23 Thus, inflammatory cell recruitment may be a common mechanism in RAAS-mediated vein graft remodeling.
This study has several important limitations that suggest future studies. While we demonstrate a significant reduction in vein graft thickening, fibrosis and inflammation in spironolactone-treated mice, this study is necessarily descriptive and further studies are needed to explore the molecular mechanisms involved in these processes. The use of a mouse vein graft model with a transplanted donor vein will allow for future studies using genetically altered donor and recipient mice to investigate the mechanisms. Although MR antagonist drugs are generally well tolerated, the overall effort, potential risk, and cost of doing a human randomized controlled trial are not insubstantial and thus additional data would be helpful to justify such a trial. This would include additional preclinical studies of MR antagonism in larger animal models such as the rabbit or pig model as well as testing of a more selective MR antagonist, such as eplerenone, to further support the mechanism. Finally, we demonstrate expression of MR in human saphenous vein specimens from patients with diverse clinical characteristics and cardiac risk factors, however future studies would require a much larger sample size to identify specific clinical characteristics that correlate with venous MR activation and would thus identify the patients most likely to benefit from MR antagonist therapy in a clinical trial.
In summary, vein graft surgery for obstructive coronary or peripheral vascular disease remains an evidenced-based treatment for a large patient population but is limited by a lack of graft durability with no effective therapy.5,24,25 This study implicates the MR in vein graft pathobiology in humans and demonstrates beneficial effects on graft remodeling using the clinically available MR antagonist in a mouse model. This study provides the first preclinical data in support of a clinical trial using an MR antagonist, started at the time of arterial bypass surgery using a venous conduit, to improve graft patency. While further studies are needed to characterize the mechanism of MR-mediated vein graft remodeling and the patient populations most likely to benefit from MR inhibition, the long history of safety and cardiovascular benefit afforded by MR antagonists in patients with cardiovascular disease supports great potential for clinical translation of this study.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Celso-Gomez Sanchez for the generous gift of MR monoclonal antibodies, Pilar Alcaide for assistance with inflammatory cell quantification, Heather Nickerson, Wendy Baur, and Brian Lin for technical assistance, and Greg Imbrie for helpful thoughts and discussions. This work was funded by NIH grant HL095590 to IZJ and AHA grant 11POST5390010 to APM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS Concept and design: AE, APM, MSC, RHK, IZJ
Analysis and interpretation: AE, APM, IZJ
Data collection: APM, MA, CG
Writing the article: AE, APM, IZJ
Critical revision of the article: AE, APM, RHK, IZJ
Final approval of the article: AE, APM, MA, CG, MSC, RHK, IZJ
Statistical analysis: APM, IZJ
Obtained funding: AE, APM, IZJ
Overall responsibility: AE, APM, IZJ
REFERENCES
- (1).Zwolak RM, Adams MC, Clowes AW. Kinetics of vein graft hyperplasia: association with tangential stress. J Vasc Surg. 1987;5(1):126–36. [PubMed] [Google Scholar]
- (2).Parang P, Arora R. Coronary vein graft disease: pathogenesis and prevention. Can J Cardiol. 2009;25(2):e57–e62. doi: 10.1016/s0828-282x(09)70486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Westerband A, Mills JL, Marek JM, Heimark RL, Hunter GC, Williams SK. Immunocytochemical determination of cell type and proliferation rate in human vein graft stenoses. J Vasc Surg. 1997;25(1):64–73. doi: 10.1016/s0741-5214(97)70322-7. [DOI] [PubMed] [Google Scholar]
- (4).Hosono M, Euda M, Suehiro S, Sasaki Y, Shibata T, Hattori K, et al. Neointimal formation at the sites of anastomosis of the internal thoracic artery grafts after coronary artery bybass grafting in human subjects: an immunohistochemical analysis. J Thorac Cardiovasc Surg. 2000;120(2):319–28. doi: 10.1067/mtc.2000.106328. [DOI] [PubMed] [Google Scholar]
- (5).Salzberg SP, Filsoufi F, Anyanwu A, von HK, Karlof E, Carpentier A, et al. Increased neointimal formation after surgical vein grafting in a murine model of type 2 diabetes. Circulation. 2006;114(1:Suppl):I302–7. doi: 10.1161/CIRCULATIONAHA.105.001339. [DOI] [PubMed] [Google Scholar]
- (6).McCurley A, Jaffe IZ. Mineralocorticoid Receptors in Vascular Function and Disease. Mol Cell Endocrinol. 2012;350(2):256–65. doi: 10.1016/j.mce.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ Res. 2005;96(6):643–50. doi: 10.1161/01.RES.0000159937.05502.d1. [DOI] [PubMed] [Google Scholar]
- (8).Jaffe IZ, Newfell BG, Aronovitz M, Mohammad NN, McGraw AP, Perrault RE, et al. Placental growth factor mediates aldosterone-dependent vascular injury in mice. J. Clin. Invest. 2010;120(11):3891–900. doi: 10.1172/JCI40205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Bafford R, Sui XX, Park M, Miyahara T, Newfell BG, Jaffe IZ, et al. Mineralocorticoid receptor expression in human venous smooth muscle cells: a potential role for aldosterone signaling in vein graft arterialization. Am J Phys Heart Circ Phys. 2011;301(1):H41–H47. doi: 10.1152/ajpheart.00637.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Karas RH, Patterson BL, Mendelsohn ME. Human vascular smooth muscle cells contain functional estrogen receptor. Circulation. 1994;89(5):1943–50. doi: 10.1161/01.cir.89.5.1943. [DOI] [PubMed] [Google Scholar]
- (11).Newfell BG, Iyer LK, Mohammad NN, McGraw AP, Ehsan A, Rosano G, et al. Aldosterone regulates vascular gene transcription via oxidative stress-dependent and -independent pathways. Arterioscler Thromb Vasc Biol. 2011;31:1871–80. doi: 10.1161/ATVBAHA.111.229070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, et al. Development of a panel of monoclonal antibodies against a mineralocorticoid receptor. Endocrinology. 2006;147(3):1343–8. doi: 10.1210/en.2005-0860. [DOI] [PubMed] [Google Scholar]
- (13).Cassis LA, Helton MJ, Howatt DA, King VL, Daugherty A. Aldosterone does not mediate angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Brit J Pharmacol. 2005;144(3):443–8. doi: 10.1038/sj.bjp.0706098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Sullivan TR, Jr., Karas RH, Aronovitz M, Faller GT, Ziar JP, Smith JJ, et al. Estrogen inhibits the response-to-injury in a mouse carotid artery model. J Clin Invest. 1995;96(5):2482–8. doi: 10.1172/JCI118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Vasan RS, Evans JC, Larson MG, Wilson PWF, Meigs JB, Rifai N, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Eng J Med. 2004;351:33–41. doi: 10.1056/NEJMoa033263. [DOI] [PubMed] [Google Scholar]
- (16).Angelina GD, Bryan AJ, Williams HM, Soyombo AA, Williams A, Tovey J, et al. Time-course of medial and intimal thickening in pig venous arterial grafts: relationship to endothelial injury and cholesterol accumulation. J Thorac Cardiovasc Surg. 1992;103(6):1093–103. [PubMed] [Google Scholar]
- (17).Owens CD, Rybicki FJ, Wake N, Schanzer A, Mitsouras D, Gerhard-Herman MD, et al. Early remodeling of lower extremity vein grafts: Inflammation influences biomechanical adaptation. J. Vasc. Surg. 2008;47(6):1235–42. doi: 10.1016/j.jvs.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Ivanes F, Susen S, Mouquet F, Pigny P, Cuilleret F, Sautiere K, et al. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. Eur Heart J. 2012;33(2):191–202. doi: 10.1093/eurheartj/ehr176. [DOI] [PubMed] [Google Scholar]
- (19).Yu P, Nguyen BT, Tao M, Campagna C, Ozaki CK. Rationale and practical techniques for mouse models of early vein graft adaptations. J Vasc Surg. 2010;52(2):444–52. doi: 10.1016/j.jvs.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).McGah PM, Leotta DF, Beach KW, Zierler RE, Riley JJ, Aliseda A. Hemodynamic conditions in a failing peripheral artery bypass graft. J Vasc Surg. 2012 doi: 10.1016/j.jvs.2012.01.045. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309–21. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- (22).Tatewaki H, Egashira K, Kimura S, Nishida T, Morita S, Tominaga R. Blockade of monocyte chemoattractant protein-1 by adenoviral gene transfer inhibits experimental vein graft neointimal formation. J Vasc Surg. 2007;45(6):1236–43. doi: 10.1016/j.jvs.2007.01.066. [DOI] [PubMed] [Google Scholar]
- (23).Ohtani K, Egashira K, Ihara Y, Nakano K, Funakoshi K, Zhao G, et al. Angiotensin II type 1 receptor blockade attenuates in-stent restenosis by inhibiting inflammation and progenitor cells. Hypertension. 2006;48(4):664–70. doi: 10.1161/01.HYP.0000237974.74488.30. [DOI] [PubMed] [Google Scholar]
- (24).Ehsan A, Mann MJ, Dell'Acqua G, Dzau VJ. Long-term stabilization of vein graft wall architecture and prolonged resistance to experimental atherosclerosis after E2F decoy oligonucleotide gene therapy. J Thorac Cardiovasc Surg. 2001;121(4):714–22. doi: 10.1067/mtc.2001.111204. [DOI] [PubMed] [Google Scholar]
- (25).Schepers A, de Vries MR, van Leuven CJ, Grimbergen JM, Holers VM, Daha MR, et al. Inhibition of complement component C3 reduces vein graft atherosclerosis in apolipoprotein E3-Leiden transgenic mice. Circulation. 2006;114(25):2831–8. doi: 10.1161/CIRCULATIONAHA.106.619502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.