Abstract
Purpose
The anatomic and physiologic constraints for pediatric cavopulmonary assist differ markedly from adult Fontan circulations due to smaller vessel sizes and risk of elevated pulmonary resistance. In this study, hemodynamic and hemolysis performance capability of a catheter-based viscous impeller pump (VIP) to power the Fontan circulation is assessed at a pediatric scale (~15 kg) and performance range (0-30 mmHg).
Methods
Computer simulation and mock circulation studies were conducted to assess the hydraulic performance, acute hemodynamic response to different levels VIP support, and the potential for vena cavae collapse. Computational fluid dynamics (CFD) simulations were used to estimate VIP hydraulic performance, shear rates, and potential for hemolysis. Hemolysis was quantified in a mock loop with fresh bovine blood.
Results
A VIP augmented 4-way total cavopulmonary connection flow at pediatric scales and restored systemic pressures and flows to biventricular values, without causing flow obstruction or suction. VIP generated flows up to 4.1 L/min and pressure heads of up to 38 mmHg at 11,000 rpm. Maximal shear rate was 160 Pa, predicting low hemolysis risk. Observed hemolysis was low with plasma free hemoglobin of 11.4 mg/dL/hr.
Conclusions
A VIP will augment Fontan cavopulmonary flow in the proper pressure and flow ranges, with low hemolysis risk under more stringent pediatric scale and physiology compared to adult scale. This technology may be developed to simultaneously reduce systemic venous pressure and improve cardiac output after stage-2 or -3 Fontan repair. It may serve to compress surgical staging, lessening the pathophysiologic burden of repair.
Keywords: Fontan Circulation, cavopulmonary assist device, mock circulation, CFD, pump performance, hemolysis
I. Introduction
Despite advances, palliative repair of functional single ventricle remains an enigmatic challenge [1]. Intractable morbidities in the interim-staged palliative approach include severe hypoxemia, ventricular hypertrophy, sudden hemodynamic instability, and neurocognitive dysfunction. Late sequelae have been linked to prior repair in which a shunt source of pulmonary blood flow was utilized [2]. Furthermore, recent studies question the benefit of interim staging with respect to late Fontan ventricular function and functional status [3]. It would appear that a physiologic cost incurred early in repair is reflected in suboptimal late functional status.
In the setting of preserved systolic ventricular function, modest augmentation of cavopulmonary blood flow would shift univentricular Fontan circulation toward “biventricular Fontan”: single ventricle anatomy with the physiologic attributes of more stable 2-ventricle physiology [4]. Development of a safe and reliable means to apply cavopulmonary assist in younger patients as a bridge to Fontan may permit compression of surgical staging of Fontan conversion [5]. It may ultimately obviate dependence on use of a systemic arterial shunt source of pulmonary blood flow and resolve the associated hallmarks of hypoxemia, ventricular volume overload, unstable parallel circulations, and impaired diastolic coronary perfusion. A clinical device to accomplish this does not currently exist.
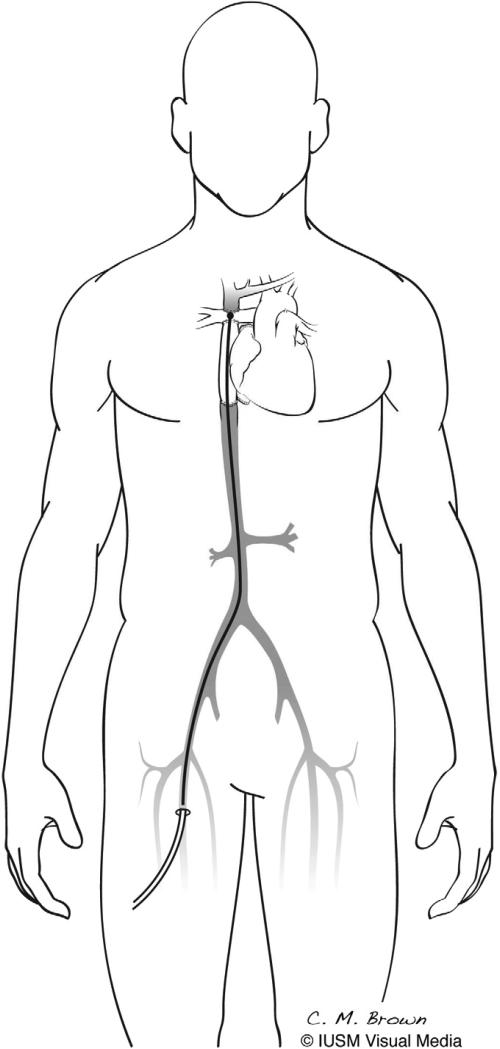
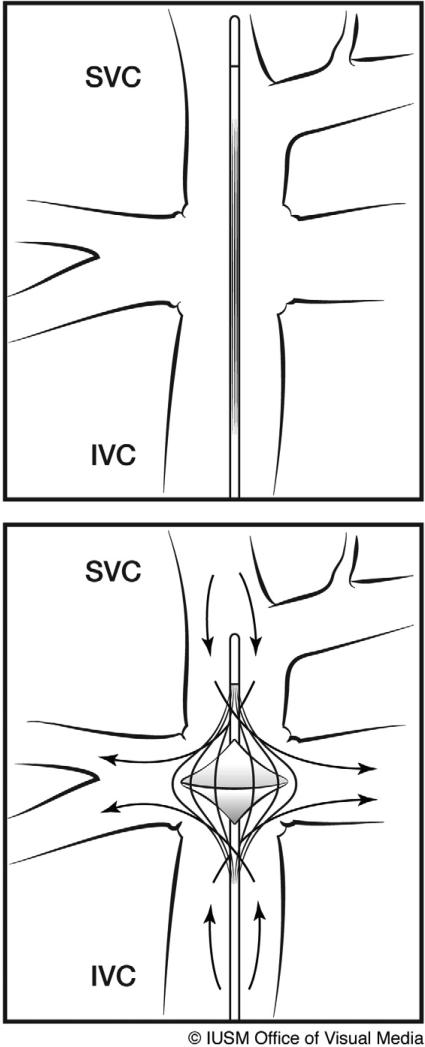
To address these problems, we are developing an expandable viscous impeller pump (VIP) to provide temporary cavopulmonary support [6]. The VIP is a catheter-based, biconical, vaned rotary pump that can be inserted percutaneously using modified Seldinger technique and advanced to the cavopulmonary junction via either superior or inferior vena cava and expanded (Figure 1). The biconical design of the VIP allows for simultaneous pumping of blood from both superior and inferior vena cavae to each of the pulmonary arteries using only a single pump head. The VIP is designed to fit within the cavopulmonary junction of a pediatric patient and is stabilized inside the cavopulmonary junction by an expandable nitinol cage.
Figure 1.
Viscous impeller pump (VIP). A catheter-based, biconical, vaned pump which can be inserted percutaneously and advanced to the cavopulmonary junction, expanded, and rotated.
In pediatric patients, the anatomic and physiologic constraints for cavopulmonary assist are more stringent than for adults with failing Fontan circulations. Chief among these are smaller vessel size and risk of elevated pulmonary vascular resistance. This study was performed to assess performance feasibility of a VIP within the anatomic and physiologic limitations of pediatric Fontan using computer simulation, mock circulatory system, and computational fluid dynamic (CFD) methods.
II. Methods
Computer Simulation Study
A previously reported computer simulation model of the pediatric biventricular cardiovascular system [7] was modified to simulate single ventricle Fontan physiology of a 4-year old (~15 kg). The biventricular computer simulation model has been used in previous studies to develop and test physiologic control algorithms for mechanical circulatory support devices [8-10]. Briefly, the computer model subdivides the Fontan circulatory system into 2 heart valves and 8 blocks, which include common atrium, single ventricle, pulmonary and systemic circulations, vena cava, aorta, and coronary circulation. The volume of blood in each block is described by a differential equation as a function of volume (V), pressure (P), compliance (C), and resistance (R), which is an expression for the macrosopic material balance for the block given by:
where dVn/dt is the rate of change of volume in block n, Fin is the blood flow rate into the block, and Fout is the blood flow rate out of the block.
The heart rate, resistances, and compliances were modified to reproduce hemodynamic pressure and flow waveforms of the univentricular Fontan physiology of a 4 year old based on literature [11-13] and clinical guidance. A model of the VIP was integrated at the cavopulmonary junction. Simulations were conducted to predict acute hemodynamic responses from 0 to 3.5 L/min of VIP support in 0.25 L/min increments. Ventricular, aortic, and cavopulmonary pressures, aortic, coronary, and cavopulmonary flows, and ventricular volume and external work were calculated. These parameters were compared to normal biventricular physiology values.
Mock Circulation Studies
Acute Hemodynamic Performance
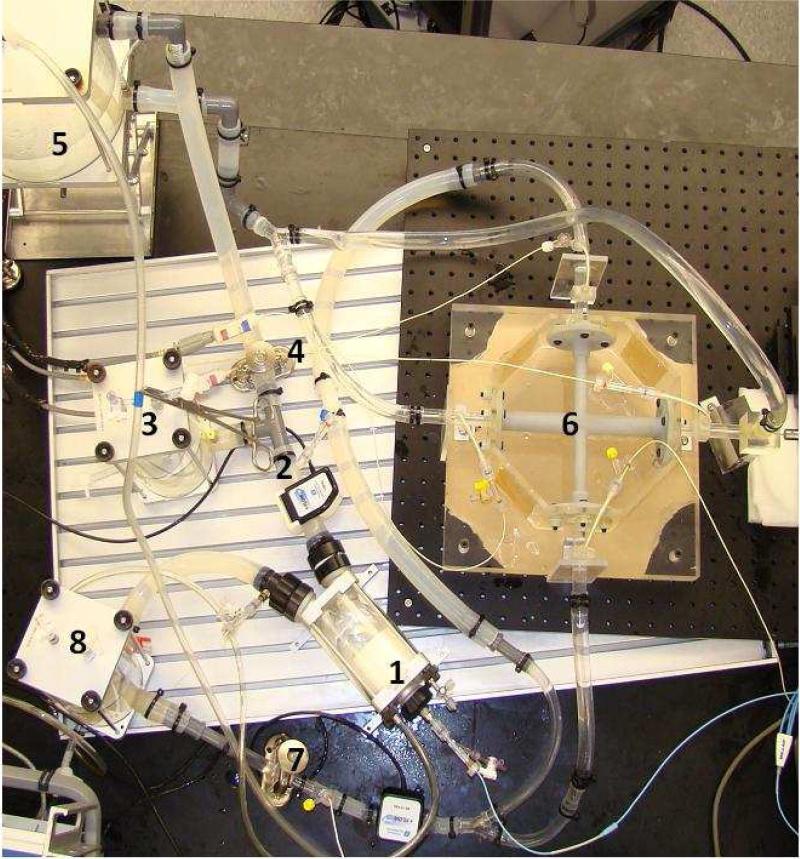
A mock circulation system consisting of a silicone ventricle, aorta, systemic and pulmonic resistances and compliances, and a cavopulmonary junction was used to simulate the univentricular Fontan circulation (Figure 2a). The cavopulmonary junction is rigid with 11 mm diameter SVC and IVC and 9mm diameter pulmonary arteries that are connected to flexible silicone tubing. The prototype pediatric VIP measures 9.75 mm in height and 9.5 mm in diameter (expanded). Ventricular pressure, heart rate, systemic and pulmonary resistances and compliances were adjusted to reproduce hemodynamic waveforms of univentricular Fontan physiology of a 4 year old. Baseline hemodynamic pressure and flow data were collected for the univentricular Fontan circulation (no VIP support). The VIP was introduced at the cavopulmonary junction and data were collected at VIP rotational speeds of 3000 -11000 rpm. The vena cavae were partially clamped (60%) to test for risk of vena caval collapse (negative vena caval pressure) with the VIP operating at its maximum operational speed of 11000 rpm. The potential of the VIP to impede Fontan cavopulmonary flow during pump failure was studied by stopping VIP rotation while leaving the fully-deployed device in place in the midst of the cavopulmonary junction. The hemodynamic effect of off-center alignment of the VIP along the axis of the vena cavae in the cavopulmonary junction was studied by placing the VIP at 20%, 40%, 60%, 80%, and 100% offset conditions along the axis of the IVC. A 20% offset implies that the VIP was placed 20% of the radius of the pulmonary artery away from the optimal location in the TCPC along the axis of the IVC.
Figure 2.
(a) Pediatric Fontan mock circulatory system : (1) Single ventricle, (2) Aorta, (3) Arterial Compliance, (4) Systemic vascular resistance, (5) venous compliance, (6) Fontan junction with cavopulmonary assist device (VIP), (7) Pulmonary resistance, and (8) Pulmonary compliance elements. (b): Schematic of the hemolysis test set-up.
Hydraulic Performance
A mock circulation consisting of a cavopulmonary junction with VIP, and a resistor was used to characterize hydraulic performance of the VIP. The VIP was operated at 3000-11000 rpm and against five different resistances at each pump speed. Steady-state pressure head and flow rates generated by the VIP were recorded for each operational condition and plotted (H-Q curve) to characterize hydraulic performance.
Data Collection and Analysis
Hemodynamic data were collected using a clinically approved GLP – compliant data acquisition system [14,15]. All transducers were pre- and post-calibrated against known standards to ensure measurement accuracy. In hemodynamic studies, pressure and flow waveforms were used to calculate heart rate, stroke volume, cardiac output, mean aortic pressure (AoP), cavopulmonary pressures, left atrial pressure, and aortic and cavopulmonary flows on a beat-to-beat basis by using the HEART program [16] developed in Matlab (MathWorks, Natick, MA) and averaged to obtain a single mean value. In hydraulic performance characterization studies, pressure and flow waveforms were used to calculate average pressure head across the VIP and average flow rates.
Computational Fluid Dynamics (CFD) Study
Fluent (ANSYS Inc., Canonsburg, PA) was used to model and estimate VIP shear stress as a predictor of hemolysis. The laminar solver was applied for no VIP and stationary VIP conditions. The rotating VIP cases were solved using the transient realizable k-ε model. Blood viscosity of 3.5 cP and a density of 1060 kg/m3 were assumed. The grid was generated using Solid Edge to create the solid model and Gambit (preprocessor for Fluent) to generate the mesh (2 million tetrahedral elements). Motion was modeled using the sliding mesh method. CFD analyses were performed for no vena caval offset and 20% vena caval offset conditions. The flow and the pressure head developed by the VIP were calculated to plot the H-Q curve for the device. The maximum scalar stress was calculated as an indicator of blood damage [17].
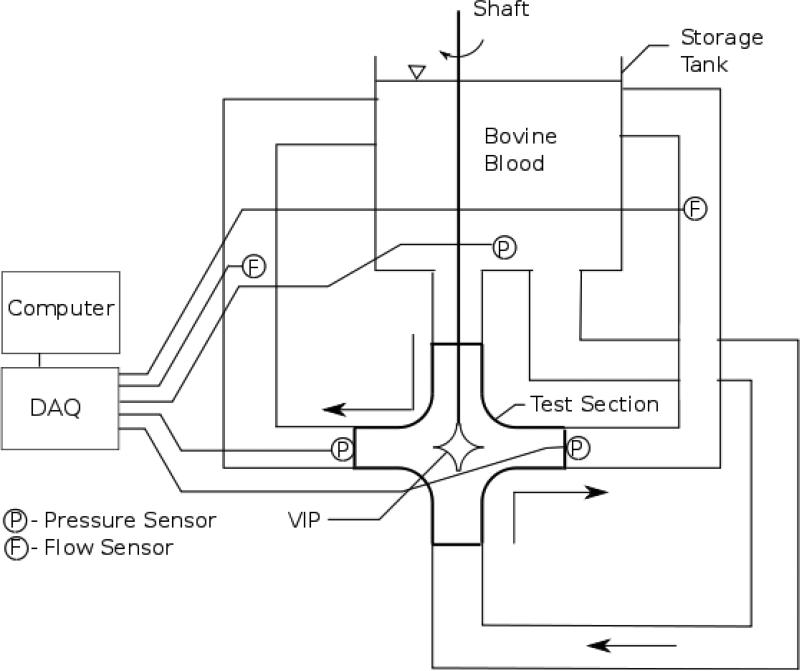
Hemolysis Study
Fresh whole bovine blood (< 48 hrs) was used in an in vitro blood loop to quantify VIP hemolysis (Figure 2b). Blood was heparinized to ACT >300s, and hematocrit adjusted to 28±2% with plasma buffer solution. VIP was operated at 9000 rpm against a pressure head of 15±2 mmHg resulting in a flow rate of 2.2±0.3 L/min. Total blood volume in the in-vitro loop was 1L. In-vitro blood damage was assessed by measuring plasma free hemoglobin (pfHb), hematocrit (Hct), red blood cell (RBC), white blood cell (WBC), and platelet (PLT) counts before device operation (baseline) and every hour thereafter for 6 hours. The pfHb was quantified using a Plasma Photometer (HemoCue, Mission Viejo, CA). Platelet count and hematocrit were measured using CDC Mascot (CDC Technologies, Oxford, CT). Normalized index of hemolysis was calculated using American Society of Testing and Materials (ASTM) standards. The hemolysis study parameters and protocols comply with FDA guidelines for 510k submission and ASTM standards [18,19].
III. Results
Computer Simulation
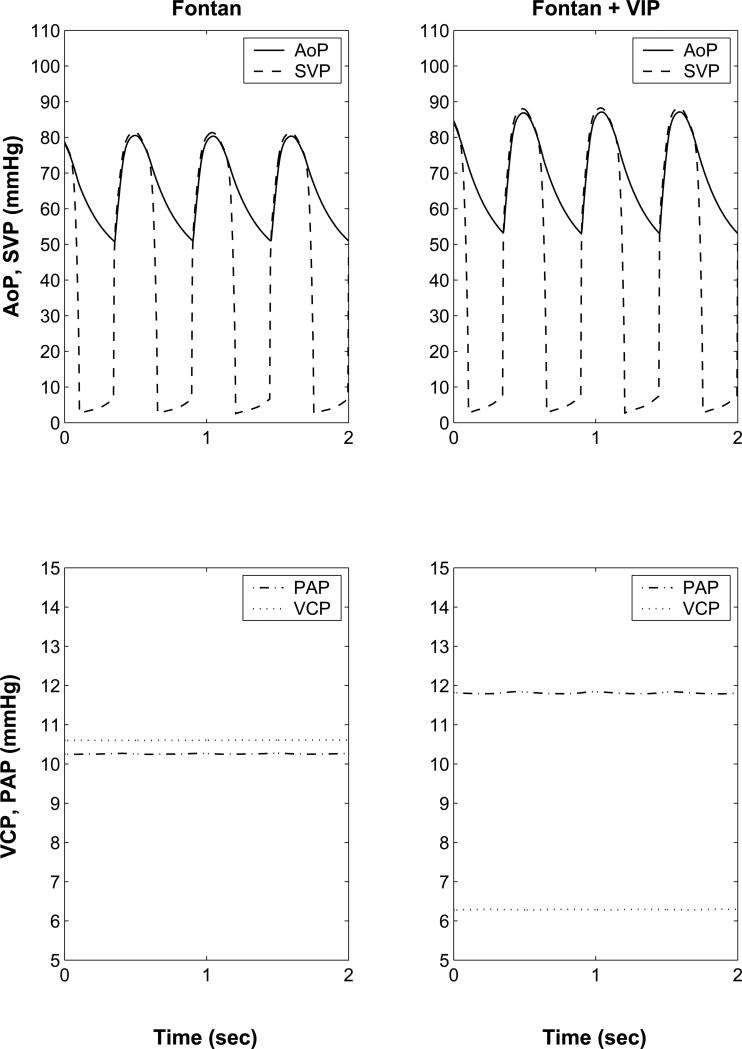
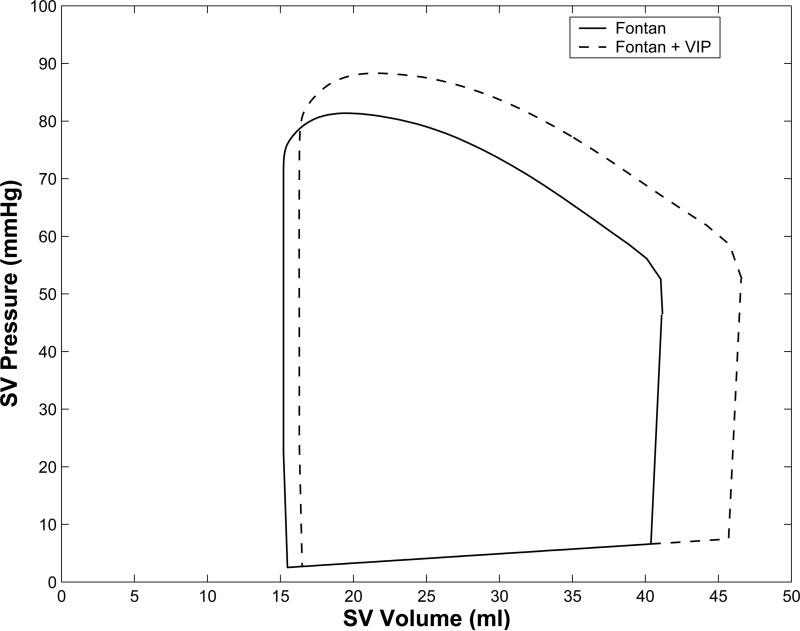
The unsupported Fontan circulation results in diminished cardiac output, and aortic systolic and diastolic pressures compared to normal biventricular circulation (Table 1). VIP support increases cardiac output, ventricular end-diastolic pressures and volumes, and aortic systolic and diastolic pressures in Fontan (Figure 3). 100% VIP support restored cardiac output, ventricular end-diastolic pressures, and aortic systolic and diastolic pressures to normal biventricular circulation values. VIP support increased the ventricular end-systolic and end-diastolic volumes from baseline Fontan values (Figure 3c). Significantly, the restoration of hemodynamic parameters of the Fontan circulation to near normal values was achieved with only modest shift of pressure head (~6 mmHg) in the cavopulmonary junction (CPPH = pressure difference between the vena cavae and proximal pulmonary arteries, Table 1, Figure 3). A simulated VIP flow of 3.5 L/min is higher than the predicted cardiac output of 3.3 L/min due to 0.2 L/min of retrograde flow (recirculation) around the VIP.
Table 1.
Simulation of cavopulmonary assist device flow for normal, Fontan circulation without, and with VIP support. VIP support increases the Fontan circulation cavopulmonary pressure head, cardiac output, and aortic pressures to near normal biventricular values. A simulated VIP flow of 3.5 L/min (full support) is higher than the predicted cardiac output of 3.3 L/min due to retrograde flow (recirculation, ~0.2 L/min) around the VIP.
| Case | HR (bpm) | SV (ml) | CO (l/min) | CO % increase | AoP (mmHg) | CPPH (mmHg) | VIP Flow (L/min) |
|---|---|---|---|---|---|---|---|
| Normal | 120 | 26.2 | 3.26 | 87.7/54.2 | -- | 0 | |
| Fontan | 110 | 26.0 | 2.83 | --- | 80.3/51.0 | 0.3 | 0 |
| Fontan + partial VIP support | 110 | 26.0 | 2.85 | 0.7 | 80.6/51.1 | 0.1 | 1.75 |
| Fontan + partial VIP support | 110 | 26.9 | 2.96 | 4.6 | 82.7/51.7 | -2.6 | 3.0 |
| Fontan + full VIP Support | 110 | 30.3 | 3.30 | 16.6 | 87.1/53.0 | - 5.5 | 3.5 |
HR: heart rate; SV: stroke volume; CO: cardiac output; CO % increase : percent increase from baseline Fontan cardiac output; AoP: aortic pressure; CPPH: cavopulmonary pressure head = vena cava pressure – pulmonary artery pressure.
Figure 3.
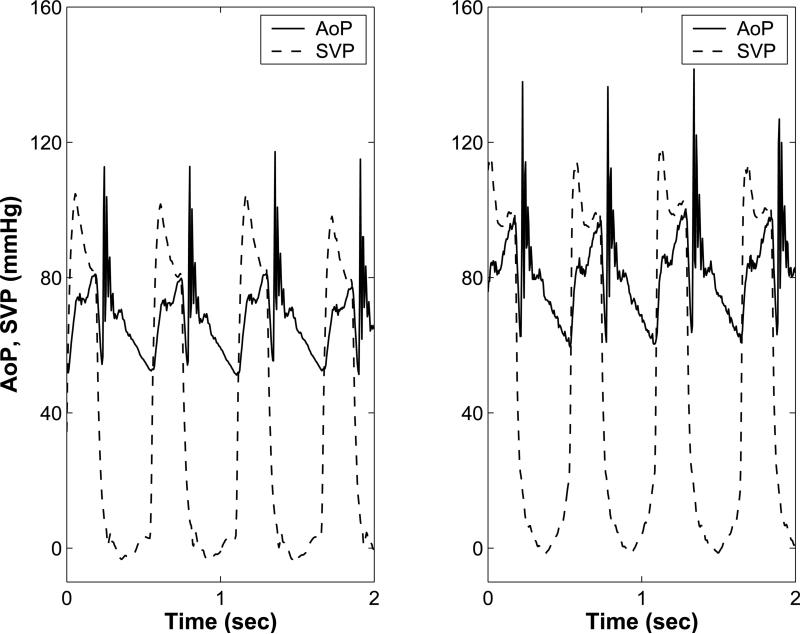
(a): Computer simulation model for a 4 yr old with univentricular Fontan circulation without (left) and with (right) cavopulmonary assist device (VIP) support. Systemic ventricular pressure (SVP, solid), Aortic pressure (AoP, dashed), pulmonary artery pressure (PAP, dash-dot), and vena cava pressure (VCP, dotted) waveforms generated by the (b): Aortic (AoP) and single ventricular pressure waveforms (SVP) obtained from the mock circulation studies with no VIP support (left) and with 100% VIP support (right). VIP support increases ventricular end diastolic pressure and systolic and diastolic pressures. (c): Single ventricular pressure volume loops for Fontan circulation (solid), and Fontan circulation with cavopulmonary assist device (VIP) support (dashed) from the computer simulation show that VIP support increases the stroke volume, peak ventricular pressures, and end systolic and end-diastolic ventricular volumes.
Mock Circulation
Mock circulatory system experiments demonstrate that VIP support augments cardiac output by up to 23%, and mean common atrial pressure by up to 22 mmHg at its maximal operational speeds. Further, VIP support increases pulmonary arterial pressure, aortic systolic and diastolic pressures, and ventricular end-systolic and end-diastolic volumes. Importantly, only modest (8 mmHg) shift of cavopulmonary pressure head in the direction of the single ventricle leads to these significant improvements in hemodynamic parameters of the Fontan circulation. The percentage increase in cardiac output and cavopulmonary pressure head are consistent with computer simulation results (Table 2, Figure 3b).
Table 2.
Mock circulation results for Fontan with various levels of VIP support. The normal operating range of the pediatric VIP is expected to be approximately 5000-7000 rpm. At higher operational speeds, the VIP produces a significant pressure head, which may only be needed in pediatric Fontan patients with relative pulmonary hypertension. Importantly, VIP stoppage does not alter the cardiac output or cavopulmonary pressures from baseline values.
| Case | VIP speed (RPM) | HR (bpm) | SV (mL) | CO (L/min) | CO % increase | VCP (mmHg) | PAP (mmHg) | CAP (mmHg) |
|---|---|---|---|---|---|---|---|---|
| Fontan baseline | -- | 110 | 20.0 | 2.2 | --- | 9 | 8 | 2 |
| Fontan +VIP | 0 | 110 | 20.1 | 2.2 | --- | 9 | 8 | 2 |
| Fontan + VIP | 3000 | 110 | 20.9 | 2.3 | 4.5 | 9 | 12 | 3 |
| Fontan + VIP | 5000 | 110 | 21.8 | 2.4 | 9.1 | 9 | 13 | 4 |
| Fontan + VIP | 7000 | 110 | 24.1 | 2.65 | 20.4 | 8 | 16 | 5 |
| Fontan + VIP | 9000 | 110 | 24.5 | 2.7 | 22.7 | 8 | 25 | 12 |
| Fontan + VIP | 11000 | 110 | 24.7 | 2.72 | 23.6 | 8 | 37 | 24 |
| Fontan + VIP, VC partial clamp | 11000 | 110 | 19.1 | 2.1 | -4.5 | 5 | 24 | 21 |
HR: heart rate; SV: stroke volume; CO: cardiac output; CO % increase: percent increase from baseline Fontan cardiac output; VCP: vena caval pressure; PAP: pulmonary artery pressure; CAP: common atrial pressure.
No cavitation was observed at 11,000 rpm, the maximum rotational speed for the VIP. Partial clamping (50%-60%) of the vena cavae with the VIP at 11,000 rpm reduced mean vena caval pressure from 8 mmHg to 5 mmHg. Substantial clamping (over 80%) of the vena cavae was necessary to generate negative vena caval pressure (indicative of vena caval collapse) with the VIP operating at 11,000 rpm. Simulated VIP failure (non-rotation) reduced cardiac output to baseline Fontan values. Improper alignment of the VIP at the cavopulmonary junction by up to 80% of the radius of the pulmonary arteries, did not significantly impact the overall flow rate. However, substantial reduction (>20%) in flow was observed when the VIP impeller was placed at an offset which exceeded 80% of the radius of the pulmonary arteries.
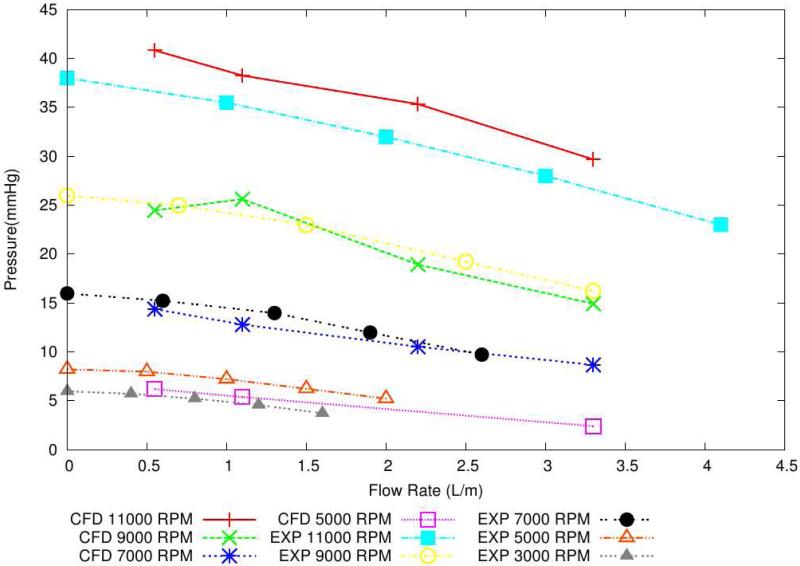
Hydraulic characterization demonstrates that the VIP is capable of pumping up to 4.1 L/min and can pump against a pressure head of up to 38 mmHg (Figure 4a). The H-Q curves show a substantially flat performance profile, ideal for mechanical circulatory support devices.
Figure 4.
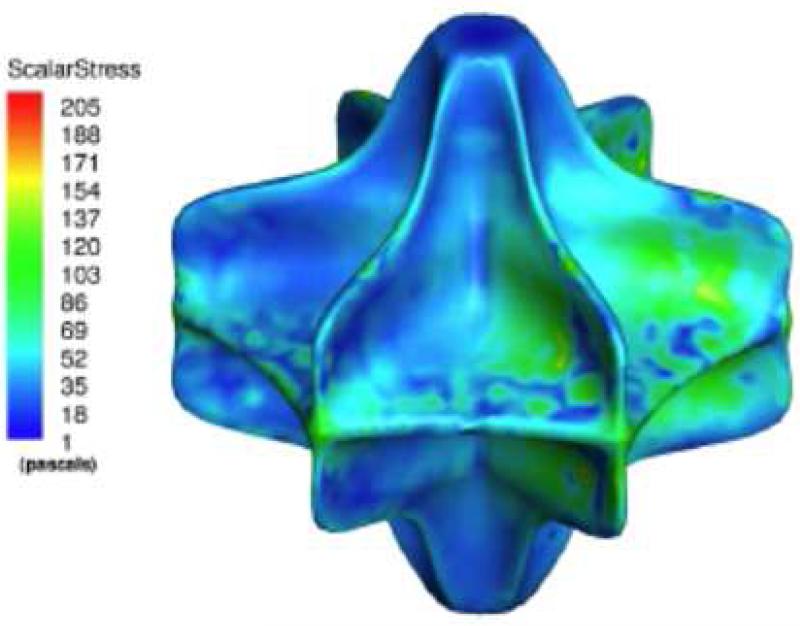
(a): Pump hydraulic performance (H-Q). The VIP is capable of providing flows up to 4 L/min and can pump against a pressure head of up to 40 mmHg. (b): Scalar shear stress contours show maximum shear stress of 160 Pa (clinical threshold > 300 Pa), indicating low risk for hemolysis.
Computational Fluid Dynamics
CFD did not predict any areas of low velocity or low shear stress, suggesting minimal risk of blood stagnation in the VIP. Peak velocities of 7 m/s were observed at the vane edges, but CFD predicted low hemolysis rates with a maximal shear stress of 160 Pascal at 11,000 rpm (clinical threshold ≈ 400 Pascal [17], Figure 4b). A vena caval offset of 20% did not diminish average flow rates compared to the no vena caval offset condition.
Hemolysis Testing
The pfHb at the beginning of the 6 hour test period was 0 mg/dL. At the end of the 6-hour test period was 70 mg/dL (average hemolysis rate = 11.4 mg/dL/hr, peak hemolysis rate = 20 mg/dL/hr). The Normalized Index of Hemolysis, calculated per ASTM standards, was 0.07 g/100L. Hct, RBC, WBC, and platelet counts over the 6-hour period did not vary significantly from baseline.
IV. Discussion
Mechanical cavopulmonary assist within the total cavopulmonary connection (TCPC) presents unique anatomic and physiologic challenges, which are markedly dissimilar to any other mechanical circulatory support application. Flow must be augmented in a highly complex 3- or 4-axis geometry in which incoming and outgoing flows are perpendicular. The pump will provide support in a location where no ventricle will recover to assume function of the pump; thus, it is not a ventricular assist device. The ambient cavopulmonary pressures are low (10-15 mmHg) and the ideal pump should generate a substantial amount of flow while maintaining a low pressure head (6-8 mmHg) to avoid perfusion injury to the lung. However, higher pressure heads (~ 20-30 mmHg) may be required in pediatric Fontan patients with pre-capillary pulmonary hypertension that is unresponsive to pharmacologic therapy. Since there is no volume reservoir for the pump inlet to draw from, vein collapse and cavitation due to inlet suction must be avoided. Additionally, there is no natural barrier (such as a valve) present within the TCPC pathway to prevent recirculation around the pump body. Significant recirculation around the pump would reduce hydraulic efficiency and may increase risk of hemolysis. Finally, it is critical that the cavopulmonary pathways remain unobstructed during pump deployment, weaning, pump shut off or failure, and after the pump is withdrawn.
Implantation of microaxial pumps in the superior and/or inferior vena cavae have been proposed as a means of providing cavopulmonary support [4,5]. Implantation of one microaxial pump in the superior or inferior vena cava alone would lead to undesirable back pressure in the opposing vena cava. Implantation of two microaxial pumps in the superior and inferior vena cavae have significant limitations: (1) need to implant two devices to satisfactorily augment the double inlet, double outlet flow pattern characteristic of the TCPC, increasing the complexity of implantation and explantation, and risk of failure; (2) obstructive to flow, which significantly limits the ability to wean support to no net contribution to Fontan flow, and may be catastrophic in the event of pump failure; (3) have an inherently high risk of inlet suction due to high rotational speed; (4) any imbalance in flows between the pumps will lead to undesirable back pressure; and (5) have a high degree of complexity and very low manufacturing tolerances, which may increase risk of mechanical failure. To avoid these limitations, modification of the existing Fontan junction to a Y-shaped three-way junction has been proposed [20]. While this approach enables Fontan support using a single microaxial pump, it would require an additional major surgical procedure with cardiopulmonary bypass to reconstruct the Fontan cavopulmonary junction. Further, implantation of a microaxial pump in a 3-way junction (+/- barrier to recirculation) would have obstructive potential, which will complicate the ability to wean cavopulmonary support and may be catastrophic in the case of pump failure.
To overcome these limitations, we are developing a cavopulmonary assist device (VIP), which is both expandable and multi-directional and can be implanted percutaneously [20]. The biconical design of the VIP allows for a single impeller to stabilize and augment cavopulmonary flow in 4 axes. No reconstruction of the existing cavopulmonary junction is required. Computer simulation and mock circulation results demonstrate that the VIP may augment cavopulmonary flow and restore cardiac output, ventricular pressures and volumes, and aortic pressures to normal biventricular values with only a modest rise in cavopulmonary pressure head (6-8 mmHg). Importantly, these hemodynamic benefits were observed with the VIP operating at nomimal operational speeds (5000-7000 rpm, Table 2). Operation of the VIP at higher rotational speeds (9000-11000 rpm) in pediatric Fontan patients with normal pulmonary resistance may lead to significant increases in pulmonary artery and atrial pressures (Table 2) without a significant increase in cardiac output and should be avoided. However, at higher rotational speeds (9000-11000 rpm), the VIP produces a higher pressure head and can pump against a significantly higher afterload (30-40 mmHg) to support Fontan patients with pre-capillary pulmonary hypertension. Physiologic control algorithms to provide optimal cavopulmonary support and ensure patient safety are currently under development.
Negative pressure and vena cavae collapse is a concern especially in pediatric patients who have smaller vessel dimensions and since there is no venous reservoir for the pump to draw from in a TCPC. However, negative pressures were not observed, even when the vena cavae were partially clamped (60%) with the VIP operating at the maximum operational speed of 11,000 rpm, indicating low risk for this pump to induce suction collapse of the thin-walled vena cavae. Off-center placement of the VIP at the cavopulmonary junction by up to 80% radius of the pulmonary artery (40% Diameter) resulted in negligible reduction in pump flow. Thus, exact placement of the VIP at the cavopulmonary junction, while ideal, is not essential. The results of this study also indicate that the VIP is effective in augmenting cavopulmonary flow in the presence of moderate offset between the left and right pulmonary arteries or vena caval anastamoses at the cavopulmonary junction. When VIP rotation is stopped, cavopulmonary flows and pressures return to, but are no less than baseline (no VIP in the cavopulmonary junction) values, demonstrating that the VIP is not obstructive to Fontan cavopulmonary flow under any circumstance. Numerical simulations confirm this finding, and demonstrate that the presence of a non-rotating VIP impeller will optimize cavopulmonary flow by beneficially splitting incoming TCPC flow toward the outlets and minimizing energy loss which otherwise occurs in a TCPC with no device present [6,21]. Further, no reduction in cavopulmonary flows were observed with nominal values of vena caval offset, indicating that the VIP may be suitable for most Fontan patients.
CFD and hemolysis testing in the mock demonstrated low peak shear stress values and hemolysis rates for the VIP. A pfHb of 70 mg/dL with the VIP compares favorably to pfHb levels obtained after 6-hour in-vitro hemolysis studies with clinically approved blood pumps (pfHb of 80-250 mg/dL [22-24]). The low hemolysis levels and peak shear stress values indicate that the VIP may be an ideal device for short-term (<30 days) cavopulmonary support.
This study demonstrates that a VIP pump scaled for use in young children is capable of functioning well, from a hydraulic and hemodynamic standpoint, within the more rigorous physiologic environment of smaller vessel size and risk of elevated pulmonary arterial pressures. With VIP support, a patient with single ventricle Fontan construction, at any stage of life, may be managed with near normal oxygenation, balanced QP:QS, normal cerebral perfusion, normal systemic venous pressure; normal preload, and normal cardiac output [5]. Systemic transition to an unsupported univentricular Fontan circulation is feasible within a 2-week time frame [26]. Support can be weaned after perioperative third spacing and fluid mobilization are complete and lung function is optimal. The systemic venous compartment will have the necessary time for interstitial and oncotic adaptation to the pressure required to independently perfuse the lungs and maintain cardiac output. Thus VIP cavopulmonary support may enable a direct transition to univentricular Fontan palliation based on more stable two-ventricle physiology and may limit or eliminate the need for interim staging and enable Fontan conversion in one-stage [5,25,26].
Limitations
Animal models of univentricular Fontan circulation that accurately replicate Fontan hemodynamics do not exist, making it a challenge to test the circulatory response to cavopulmonary assist prior to clinical application. Computer simulation and mock circulation of the Fontan circulation is representative of clinical observations from a purely hemodynamic viewpoint, and is not intended to replace the importance and significance of in vivo models. The biventricular computer simulation and mock circulation model, modified to simulate univentricular Fontan circulation in this study, has been validated and used in the development and testing of several blood pumps [7-10, 27-30]. While incapable of replicating all expected clinical responses, in vitro modeling does provide a controlled environment to test the effects of VIP support and potential failure modes, which is valuable in device development and is not possible in vivo. The ventricular contractility and heart rate were kept constant to reduce experimental variability. Physiologically, heart rate and the contractility will increase with increasing preload in accordance with the Frank-Starling mechanism. By extension, it is reasonable to assume that the increase in cardiac output with VIP support may be greater clinically due to Frank-Starling response. The mock circulation system has mechanical valves, which may create large aortic valve pressure gradients and ringing during valve closure. The length of tubing in the mock circulation may cause added inertial effects. However, the inertial effects represent less than 2% of the total power and an inertance mismatch would not affect the results significantly. The impeller tested was a rigid rather than expanding prototype and the cavopulmonary junction was idealized from patient data. The risk of infection and thrombosis with the VIP is expected to be similar to other catheter-based devices (Impella 2.5, IABP), and may limit the period of VIP implantation to 30 days. While the VIP augments pulmonary and systemic flows, the pulmonary blood flow remains non-pulsatile. Generation of a pulse pressure by modulation of pump operational speed may mitigate any deleterious effects of non-pulsatile pulmonary flows [31]. Despite these limitations, this study demonstrates efficacy of pediatric Fontan cavopulmonary assist using a pediatric-scale VIP with minimal risk of vena caval collapse or hemolysis.
Acknowledgement
This work was supported by an award (R01HL098353) from the National Heart, Lung, and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.deLeval M. The Fontan circulation: what have we learned? What to expect? Pediatric Cardiol. 1998;19:316–20. doi: 10.1007/s002469900315. [DOI] [PubMed] [Google Scholar]
- 2.Ashburn DA, McCrindle BW, Tchervenkov CI, Jacobs ML, Lofland GK, Bove EL, et al. Outcomes after the Norwood operation in neonates with critical aortic valve stenosis or aortic valve atresia. J Thorac Cardiovasc Surg. 2003;125:1070–82. doi: 10.1067/mtc.2003.183. [DOI] [PubMed] [Google Scholar]
- 3.Khambadkone S, Li J, de Leval MR, Cullen S, Deanfield JE, Redington AN. Basal pulmonary vascular resistance and nitric oxide responsiveness late after Fontan-type operation. Circulation. 2003;107:3204–8. doi: 10.1161/01.CIR.0000074210.49434.40. [DOI] [PubMed] [Google Scholar]
- 4.Rodefeld MD, Boyd JH, LaLone BJ, Bezrucko AJ, Potter AW, Brown JW. Cavopulmonary assist: circulatory support for the univentricular Fontan circulation. Ann Thorac Surg. 2003;76:1911–6. doi: 10.1016/s0003-4975(03)01014-2. [DOI] [PubMed] [Google Scholar]
- 5.Rodefeld MD, Boyd JH, Myers CD, Presson RG, Wagner WW, Brown JW. Cavopulmonary assist in the neonate: an alternative strategy for single-ventricle palliation. J Thorac Cardiovasc Surg. 2004;127:705–11. doi: 10.1016/j.jtcvs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Rodefeld MD, Coats B, Fisher T, Giridharan GA, Chen J, Brown JW, Frankel SH. Cavopulmonary assist for the univentricular Fontan circulation: von Kármán Viscous Impeller Pump. Journal of Thoracic and Cardiovascular Surgery. 2010;140:529–536. doi: 10.1016/j.jtcvs.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giridharan GA, Pantalos GM, Koenig SC, Mitchell M, Austin E, Gartner M. A computer model of pediatric circulatory systems for testing pediatric assist devices. ASAIO J. 2007;53:74–81. doi: 10.1097/01.mat.0000247154.02260.30. [DOI] [PubMed] [Google Scholar]
- 8.Giridharan GA, Skliar M, Olsen DB, Pantalos GM. Modeling and control of a brushless DC axial flow ventricular assist device. ASAIO J. 2002;48:272–89. doi: 10.1097/00002480-200205000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Giridharan GA, Ewert DL, Pantalos GM, Gillars KJ, Litwak KN, Gray LA, Koenig SC. Left ventricular and myocardial perfusion responses to volume unloading and afterload reduction in a computer simulation. ASAIO J. 2004;50:512–8. doi: 10.1097/01.mat.0000136513.21369.75. [DOI] [PubMed] [Google Scholar]
- 10.Giridharan GA, Koenig SC, Pantalos GM, Litwak KN, Spence PA. Predicted hemodynamic benefits of counterpulsation therapy using a superficial surgical approach. ASAIO J. 2006;52:39–46. doi: 10.1097/01.mat.0000196522.29376.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senzaki H, Masutani S, Kobayashi J, Kobayashi T, Sasaki N, Asano H, Kyo S, Yokote Y, Ishizawa A. Ventricular Afterload and Ventricular Work in Fontan Circulation: Comparison With Normal Two-Ventricle Circulation and Single-Ventricle Circulation With Blalock-Taussig Shunts. Circulation. 2002;105:2885–92. doi: 10.1161/01.cir.0000018621.96210.72. [DOI] [PubMed] [Google Scholar]
- 12.Sachar GB, Fuhrman BP, Wang Y, Lucas RV, Jr, Lock JE. Rest and exercise hemodynamics after the Fontan procedure. Circulation. 1982;65:1043–8. doi: 10.1161/01.cir.65.6.1043. [DOI] [PubMed] [Google Scholar]
- 13.Mace L, Dervanian P, Bourriez A, Mazmanian GM, Lambert J, Losay, Neveux JY. Changes in venous return parameters associated with univentricular Fontan circulations. Am J Physiol Heart Circ Physiol. 2000;279:H2335–43. doi: 10.1152/ajpheart.2000.279.5.H2335. [DOI] [PubMed] [Google Scholar]
- 14.Drew GA, Koenig SC. Biomedical patient monitoring, data acquisition, and playback with LabVIEW®. In: Olansen JB, Rosow E, editors. Virtual bio-instrumentation: biomedical, clinical, and healthcare applications in LabVIEW®. Prentice Hall; Upper Saddle River, NJ: 2002. pp. 180–6. [Google Scholar]
- 15.Koenig SC, Woolard C, Drew GD, Unger L, Gillars KJ, Ewert DL, Gray LA, Pantalos GM. Integrated data acquisition system for medical device testing and physiology research in compliance with Good Laboratory Practices. Biomed Instrum Technol. 2004;38:229–40. doi: 10.2345/0899-8205(2004)38[229:IDASFM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder MJ, Perrault B, Ewert DL, Koenig SC. HEART: an automated beat-to-beat cardiovascular analysis package using Matlab. Comput Biol Med. 2004;34:371–88. doi: 10.1016/S0010-4825(03)00087-8. [DOI] [PubMed] [Google Scholar]
- 17.Blackshear P, Blackshear GL. Mechanical Hemolysis. In: Skalak R, editor. Handbook of Bioengineering. McGraw-Hill; New York: 1987. pp. 151–9. [Google Scholar]
- 18.ASTM F1841-97: Standard Practice for Assessment of Hemolysis in Continuous Flow Blood Pumps.
- 19.ASTM F1830-97: Standard Practice of for Selection of Blood for In Vitro Evaluation of Blood Pumps.
- 20.Error! Hyperlink reference not valid., Error! Hyperlink reference not valid., Error! Hyperlink reference not valid., Error! Hyperlink reference not valid., Error! Hyperlink reference not valid., Error! Hyperlink reference not valid., Error! Hyperlink reference not valid. An artificial right ventricle for failing fontan: in vitro and computational study. Ann Thorac Surg. 2009;88(1):170–6. doi: 10.1016/j.athoracsur.2009.03.091.
- 21.Soerensen DD, Pekkan K, de Zelicourt D, Sharma S, Kanter K, Fogel M, Yoganathan AP. Introduction of a new optimized total cavopulmonary connection. Ann Thorac Surg. 2007;83:2182–80. doi: 10.1016/j.athoracsur.2006.12.079. [DOI] [PubMed] [Google Scholar]
- 22.Giridharan GA, Sobieski MA, Ising MS, Slaughter MD, Koenig SC. Blood trauma testing for mechanical circulatory support devices. Biomed Instr & Tech. 2011;45:334–339. doi: 10.2345/0899-8205-45.4.334. [DOI] [PubMed] [Google Scholar]
- 23.Svitek RG, Smith DE, Magovern JA. In vitro evaluation of the TandemHeart pediatric centrifugal pump. ASAIO Journal. 2007;53(6):747–753. doi: 10.1097/MAT.0b013e318154ca74. [DOI] [PubMed] [Google Scholar]
- 24.Tamari Y, Lee-Sensiba K, Leonard EF, Parnell V, Tortolani AJ. The effects of pressure and flow on hemolysis caused by Bio-Medicus centrifugal pumps and roller pumps. guidelines for choosing a blood pump. The Journal of Thoracic and Cardiovascular Surgery. 1993;106(6):997–1007. [PubMed] [Google Scholar]
- 25.Myers CD, Ballman K, Riegle LE, Mattix KD, Litwak K, Rodefeld MD. Mechanisms of systemic adaptation to univentricular Fontan conversion. J Thorac Cardiovasc Surg. 2010;140:850–856. doi: 10.1016/j.jtcvs.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodefeld MD, Frankel SH, Giridharan GA. Cavopulmonary Assist: (Em)powering the Univentricular Fontan Circulation. Seminars in Thoracic and Cardiovascular Surgery Pediatric Cardiac Surgery Annual. 2011;14(1):45–54. doi: 10.1053/j.pcsu.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pantalos GM, Koenig SC, Gillars KJ, Giridharan GA, Ewert DL. Characterization of an adult mock circulation for testing cardiac support devices. ASAIO Journal. 2004;50:37–46. doi: 10.1097/01.mat.0000104818.70726.e6. [DOI] [PubMed] [Google Scholar]
- 28.Giridharan GA, Pantalos GM, Koenig SC, Gillars KJ, Skliar M. Physiologic control of rotary blood pumps: An in vitro study. ASAIO Journal. 2004;50:403–409. doi: 10.1097/01.mat.0000136652.78197.58. [DOI] [PubMed] [Google Scholar]
- 29.Litwak KN, Koenig SC, Giridharan GA, Gillars KJ, Pantalos GM. Ascending aorta outflow graft location and pulsatile ventricular assist provide optimal hemodynamic support in an adult mock circulation. Artificial Organs. 2005;29:629–635. doi: 10.1111/j.1525-1594.2005.29100.x. [DOI] [PubMed] [Google Scholar]
- 30.Kaebnick BW, Giridharan GA, Koenig SC. Quantification of pulsatility as a function of vascular input impedance: An in-vitro study. ASAIO Journal. 2007;53(2):115–121. doi: 10.1097/01.mat.0000250265.69542.80. [DOI] [PubMed] [Google Scholar]
- 31.Ising MS, Warren S, Sobieski MA, Slaughter MS, Koenig SC, Giridharan GA. Flow modulation algorithms for a continuous flow ventricular assist device to increase vascular pulsatility: A computer simulation study. Cardiovascular Engineering and Technology. 2011;2(2):90–100. [Google Scholar]