Abstract
Purpose
Adoptive cell therapy (ACT) using autologous tumor-infiltrating lymphocytes (TIL) is a promising treatment for metastatic melanoma unresponsive to conventional therapies. We report here on the results of an ongoing Phase II clinical trial testing the efficacy of ACT using TIL in metastatic melanoma patients and the association of specific patient clinical characteristics and the phenotypic attributes of the infused TIL with clinical response.
Experimental Design
Altogether, 31 transiently lymphodepleted patients were treated with their expanded TIL followed by two cycles of high-dose (HD) IL-2 therapy. The effects of patient clinical features and the phenotypes of the T-cells infused on clinical response were determined.
Results
Overall, 15/31 (48.4%) patients had an objective clinical response using immune-related response criteria (irRC), with two patients (6.5%) having a complete response. Progression-free survival of >12 months was observed for 9/15 (60%) of the responding patients. Factors significantly associated with objective tumor regression included a higher number of TIL infused, a higher proportion of CD8+ T-cells in the infusion product, a more differentiated effector phenotype of the CD8+ population and a higher frequency of CD8+ T-cells co-expressing the negative costimulation molecule “B- and T-lymphocyte attenuator” (BTLA). No significant difference in telomere lengths of TIL between responders and non-responders was identified.
Conclusion
These results indicate that immunotherapy with expanded autologous TIL is capable of achieving durable clinical responses in metastatic melanoma patients and that CD8+ T-cells in the infused TIL, particularly differentiated effectors cells and cells expressing BTLA, are associated with tumor regression.
Keywords: melanoma, tumor-infiltrating lymphocytes, adoptive cell therapy
Introduction
Metastatic melanoma is an aggressive form of cancer highly resistant to traditional forms of therapy, such as chemotherapy and radiation therapy (1). Response rates and survival for patients with advanced stages (IIIc and IV) in response to chemotherapy, such as dacarbazine and temozolomide, have been relatively poor (2). Drugs targeting activated oncogenes of the mitogen-activated protein kinase (MAPK) pathway (3), such as B-RAFV600E has also been actively pursued (4). Recently, a B-RAFV600E inhibitor has been approved by the FDA recently (5). However, although this drug was shown to induce objective tumor regression in a high percentage of patients, these responses are turning out to be of limited duration (6). Originally, due to its relative refractoriness to chemotherapy and radiation therapy, melanoma has been studied as a target for immunotherapy more than many other forms of cancer (7). Most melanoma metastases contain lymphocytic infiltrates, including T cells that recognize melanoma antigens such as Melan-A/MART-1 and NK cells (8). A monoclonal antibody blocking CTLA-4, called ipilimumab, has resulted in durable clinical responses in a subset of patients and significantly increased median overall survival in Stage IV melanoma resulting in its FDA approval recently (9). Thus, enhancing immunological mechanisms are proving successful in the care of metastatic melanoma.
Another promising form of immunotherapy for metastatic melanoma is adoptive cell therapy (ACT) using the infusion of autologous tumor-infiltrating lymphocytes (TIL) expanded ex vivo combined with HD IL-2 therapy (10, 11). ACT involves the isolation of viable tumor tissue and the expansion of TIL with IL-2 over 4–5 weeks from tumor fragments placed in culture (12). The TIL are then further expanded in larger-scale using anti-CD3 activation and exogenous IL-2 in the presence of autologous or allogeneic irradiated feeder cells (12). This protocol has become known as the “rapid expansion protocol” (REP) and can yield as much as 100–150 billion cells for infusion (8, 12). Durable responses to TIL therapy have been improved by the addition of a preparative lymphodepleting regimen using a combination of cyclophosphamide and fludarabine (10, 13) which leads to an increase in persistence of the transferred cells (13). Further improvements for this approach will be dependent on an increased understanding of the mechanism of TIL antitumor activity, such as determining which lymphocyte subtypes within the heterogeneous bulk population of cells are responsible for tumor regression. Overall, a better understanding of the nature of the T cells mediating objective anti-tumor responses during ACT will allow us to select the most active cell subsets to transfer into patients, or tailor TIL expansion procedures to preferentially expand these active T-cell populations for therapy to improve clinical response rates.
We have undertaken a Phase II ACT clinical trial for metastatic melanoma using expanded TIL followed by HD IL-2 in patients pre-treated with a cyclophosphamide and fludarabine lymphodepleting regimen (10, 13). In this paper, we report results on the clinical response rates of first 31 TIL-treated patients and an analysis of possible predictive biomarkers of therapeutic effectiveness, including phenotypic markers and telomere length in the infused T-cells.
Materials and Methods
Patient population, overall TIL expansion process, and therapy
Patient enrollment, TIL expansion and infusion, and HD IL-2 therapy were carried out under a protocol (2004–0069) approved by the Institutional Review Board of the MD Anderson Cancer Center and an FDA-approved IND (NCT00338377). This is an ongoing Phase II study which contains a randomized component and a non-randomized component. Data presented in this paper are from the non-randomized component whose objective is to determine clinical response rates and predictive biomarkers associated with clinical response in 50 patients. The analysis in this paper is on the first 31 treated patients. Both male and female patients with stage IV melanoma, Stage III in-transit disease, or recurrent regional nodal disease over the age of 18 were enrolled following informed consent. One patient under the age of 18 (a 15-year old female; Patient #2247) was enrolled after a compassionate exemption was approved by the FDA. All types of prior therapy were allowed, including chemotherapy, biochemotherapy, targeted therapy with tyrosine kinase inhibitors and anti-angiogenic agents, and immunotherapy. Patients with brain metastases ≤1 cm were eligible. Please refer to clinical trial NCT00338377 in the NCI website (http://www.cancer.gov/clinicaltrials) for further details on patient inclusion and exclusion criteria. Table S1 (Supplementary Data on-line) and Table 1 also provide further information on the accrued patient clinical and demographic characteristics for this study. All patients were HLA typed at the HLA-A locus in the MD Anderson HLA Typing Laboratory. Expanded TIL were used for functional and phenotypic analysis under an IRB-approved protocol (LAB06-0755) approved by the MD Anderson Cancer Center IRB.
Table 1.
Patient number, disease stage, clinical outcome, number of TIL infused, and sites of disease
| Patient number | Stage | Clinical responsea | PFSb (months) | OSc (months) | Infused cells (x109) | Sites of disease | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| irRC | RECIST | SCd | LN | Lung | Liver | Other visceral | Bone | Brain | |||||
| 2054/2256 | IV M1c | PR | PR | 22+ | 27+ | 80.6 | X | X | |||||
| 2124 | IV M1a | PR | PR | 31+ | 35+ | 104.9 | X | X | |||||
| 2131 | IV M1b | PR | PR | 22 | 37+ | 89 | X | X | |||||
| 2150/2153 | IV M1c | PR | PR | 22+ | 29+ | 89 | X | X | X | X | |||
| 2173 | IV M1c | PR | PR | 8 | 15 | 115 | X | X | X | ||||
| 2180 | IIIc | PR | PR | 9 | 27+ | 58 | X | ||||||
| 2215 | IV M1c | PR | PR | 28+ | 32+ | 130 | X | X | X | X | |||
| 2258 | IV M1c | PR | PR | 25+ | 27+ | 74 | X | X | X | ||||
| 2261 | IIIc | CR | CR | 18+ | 21+ | 150 | X | X | |||||
| 2262 | IV M1c | PR | PDe | 22+ | 27+ | 99 | X | X | X | ||||
| 2267 | IV M1b | PR | PDe | 2+ | 22+ | 57 | X | X | X | ||||
| 2340 | IV M1c | PR | PR | 11 | 19+ | 46 | X | X | X | X | X | ||
| 2350 | IV M1c | PR | PR | 10 | 17+ | 105 | X | X | X | X | |||
| 2357 | IV M1c | PR | PR | 13+ | 18+ | 109 | X | X | X | X | X | ||
| 2379 | IV M1b | CR | CR | 11+ | 14+ | 100 | X | X | |||||
| 2044 | IV M1c | SD | SD | 3 | 4 | 19.9 | X | X | X | ||||
| 2104 | IV M1c | SD | PD | 2 | 5 | 85 | X | X | |||||
| 2114 | IV M1b | SD | SD | 4 | 25 | 8 | X | X | X | ||||
| 2125 | IV M1c | SD | SD | 4 | 6 | 68 | X | X | X | X | |||
| 2132 | IV M1c | SD | SD | 4 | 5 | 35 | X | X | X | ||||
| 2136 | IV M1c | SD | PD | 2 | 7 | 79.8 | X | X | X | X | |||
| 2144 | IV M1b | PD | PD | 3 | 18 | 54.6 | X | X | X | ||||
| 2146 | IV M1c | SD | PD | 6 | 8 | 8 | X | X | X | X | X | ||
| 2175 | IV M1b | SD | SD | 29+ | 33+ | 55.4 | X | X | |||||
| 2245 | IV M1c | PD | PD | 2 | 4 | 38 | X | X | |||||
| 2247 | IV M1c | SD | PD | 1 | 5 | 60 | X | X | X | X | X | X | |
| 2281 | IV M1c | PD | PD | 2 | 6 | 62.5 | X | X | X | ||||
| 2284 | IV M1b | PD | PD | 3 | 19+ | 86 | X | X | |||||
| 2299 | IV M1b | SD | SD | 6 | 9+ | 53.8 | X | X | |||||
| 2338 | IV M1c | SD | PD | 6 | 14+ | 35 | X | X | X | ||||
| 2373 | IV M1b | SD | PD | 4 | 5 | 59.2 | X | X | X | ||||
Best overall response measured by irRC criteria or RECIST.
Based on irRC (as of October 17, 2011).
As of October 17, 2011.
Abbreviations: CR, complete response; PR, partial response; SD, stabilization of disease; PD, progressive disease; PFS, progression-free survival; OS, overall survival; SC, subcutaneous; LN, lymph node.
These patients developed small new brain metastases after receiving TIL (hence the designation as PD according to RECIST) that were effectively treated with stereotactic radiosurgery (#2262) or whole brain radiation (#2267).
TIL expansion for therapy and measurement of anti-tumor cell reactivity
Fig. S1A (Supplementary Data on-line) shows the overall scheme for TIL expansion first from surgical tumor harvest followed by the “rapid expansion protocol” (REP), and TIL infusion together with HD IL-2 therapy. TIL were obtained from resected tumors and expanded under current Good Manufacturing Practices (cGMP) conditions in the GMP Cell Processing Facility at MD Anderson Cancer Center. In most cases, one solitary tumor nodule was selected for TIL expansion, while in some cases two to three smaller nodules were used. In previous studies we and others have found no association between the success of TIL expansion and the site of tumor resection (14). TIL were expanded from 3–5 mm3 cut tumor fragments according to previously published methods (11, 13) after removing extraneous connective, necrotic, and non-tumor tissue using manual dissection. The TIL expanded from the tumor fragments (pre-REP TIL) to minimum of a total of 48 × 106 cells were cryopreserved and kept for further expansion (Fig. S1A) for therapy according to the criteria in the clinical protocol. TIL that did not expand to these minimal numbers were not further expanded and these patients did not go on to receive treatment. Cryopreserved pre-REP TIL from patients to be treated were thawed and further expanded using the rapid expansion protocol (REP) to generate the final TIL infusion product (post-REP TIL) (12). A small sample of the pre-REP TIL before cryopreservation was analyzed for anti-tumor reactivity as a possible predictive biomarker using an autologous melanoma cell line generated from the patient (when possible), or a semi-allogeneic melanoma cell line that had at least one matched HLA-A allele, as targets. Details on the methodology used to expand TIL for therapy and the determination of pre-REP TIL anti-tumor reactivity can be found in Supplementary Methods on-line.
TIL therapy and blood sampling
Each patient received a course of lymphodepleting chemotherapy before TIL infusion (day 0) with cyclophosphamide (60 mg/kg) on day -7 and -6 and fludarabine (25 mg/m2) given from day -5 to day -1 (Fig. S1B in Supplementary Data on-line). The harvested autologous TIL (in approximately 500 ml cell suspension in saline) were intravenously infused into each patient over a 20 min period by gravity using a regular infusion line with an in-line 100 μm mesh to remove any large aggregates or debris. The following morning, the patients received bolus HD IL-2 (720,000 IU/kg) every 8 h to tolerance (15). A second course of HD IL-2 therapy was given approximately 21 days after TIL infusion in a similar manner (Fig. S1B on-line). Hematologic and biochemical parameters were monitored daily during IL-2 administration. Intravenous blood samples (10 ml) were collected from patients before and after lymphodepletion on day -7 and day 0 before chemotherapy and before TIL infusion, respectively. Subsequent blood samples (50 ml) were collected on days 7, 14, 21, 35, and 70 after TIL infusion. The samples were analyzed for total white blood cell (WBC) count, absolute lymphocyte count (ALC), and absolute neutrophil count (ANC) in the Division of Pathology and Laboratory Medicine at MD Anderson Cancer Center.
Measurement of clinical responses by irRC and RECIST
Tumor response to therapy was done using the immune-related response criteria (irRC), a modified version of the WHO criteria (16). Response evaluation criteria in solid tumors (RECIST 1.1) was also used to assess clinical response (17) to compare to the irRC response rates found. Throughout the study, irRC was used to determine official response rates and progression-free survival times. For the purposes of this paper, we define responders as complete and partial responders and non-responders as patients with progressive or stable disease by determining the the best overall response (BOR) using irRC (irBOR). Details how and when tumor burden measurements were taken and irRC applied to determining the type of clinical response and whether any progression had occurred can be found in Supplementary Methods and Table S2 on-line.
Flow cytometry analysis of infused TIL
Antibodies to human CD4, CD8, CD27, CD28, CD62L, CD45RA and CD272 (BTLA, clone J168-540.90.22) were from BD Biosciences (San Jose, CA). Antibodies to human CD279 (PD-1, clone EH12.2H7) and Perforin (clone dG9) were obtained from BioLegend (San Diego, CA). Anti-human TIM3 (clone F38-2E2) was from eBiosciences (San Diego, CA), anti-human LAG-3 (clone 17B4) was obtained from Enzo Life Sciences (Plymouth meeting, PA) and anti-human CD270 (HVEM, clone ANC3B7) was purchased from Ancell (Bayport, MN). Staining of all TIL infusion products was done on samples cryopreserved immediately following harvesting of the clinical TIL products and washing and concentrating using the Cobe 2991 machines. Details on the flow cytometry antibody staining techniques are available in Supplementary Methods on-line. Acquisition was done the next day after staining and fixing the samples. The samples were read on a FACSCanto II instrument (BD Biosciences) and data analysis was performed using FlowJo software (Tree Star, Inc., Ashland, OR). Live cells were first gated using FSC and SSC parameters; dead cell exclusion was then performed by gating out the Aqua positive cells before gating on any antibody positive populations. For CD8+ differentiation status determination, the Aqua negative cells were gated on CD8+CD4− cells. This CD8+ population was then further selected for CD45RA− (which constitute the overwhelming majority of TIL) and the resulting population was analyzed on a plot of CD62L versus CD27, where CD27+CD62L+ cells were designated TCM (central memory), CD27+CD62L− TEM (effector-memory), and CD27−CD62L−TEFF (effector) (18–20).
Tracking of TCR Vβ clonotypes post-infusion by gene cloning and sequencing
Total RNA was isolated from TIL or patients' PBMC after adoptive transfer using Qiagen RNeasy Kit (74104). RNA quality was monitored by agarose gel electrophoresis to assess RNA degradation. TCR Vβ specific cDNA was synthesized using Clonetech 5' RACE Smarter kit. The primer in cDNA synthesis specifically binds to the Vβ constant region and can recognize both C1 and C2 (21). During cDNA synthesis, a 5' end adapter was added to each cDNA. Nested primers for adapter and Vβ constant regions were used for PCR amplification. The resulting Vβ specific PCR products were purified from an agarose gel and ligated into a TA cloning vector (Invitrogen). For TCR Vβ gene analysis, the CDR3 region of 96 TCR Vβ positive DNA samples were prepared and confirmed, and then sequenced. To characterize each individual TCR Vβ clonotype, sequence data from each sample was further analyzed using the international ImMunoGeneTics information system (IMGT) program. The frequency of each dominant Vβ clonotype was calculated by determining the percentage of each specific CDR3 sequence found within the 96 clones picked and sequenced.
Detection and measurement of telomere length in TIL by Southern blotting
Telomere length analysis of a sample of the TIL infusion product was performed using Southern blotting technique (22, 23) on isolated genomic DNA with a TeloTAGGG Telomere Length Assay kit (Roche Diagnostics, Indianapolis, IN). The telomere lengths were then determined using the chmoluminescent telomeric repeat probes supplied with the kit followed by exposure to BioMax Light chemoluminescent film (Sigma-Aldrich, St. Louis, MO). The telomere lengths were determined using the ImageJ analysis program downloaded from the National Institutes of Health (NIH, Bethesda, MD) website (rsbweb.nih.gov/ij/) using a standard curve of migration distance from the first and longest telomere standard (21.4 Kb) to the last and shortest telomere standard (1.9 Kb). Details on the methodology used with the kit to blot for telomeres and the determination of telomere lengths from the exposed blots can be found in Supplementary Methods on-line.
Statistical analysis and correction for multiple variable testing
Fisher's exact tests (24) were used to assess association between categorical variables and tumor response, and Wilcoxon rank-sum tests (25) were used to compare continuous variables by tumor response or type of T-cell. Simple linear regression was performed (Fig. 4D) to assess the association between a continuous predictor and percentage change in tumor burden. Box and whisker plots have been provided for several variables of interest. Due to the large number of comparisons, to account for multiple testing, we set our Type 1 error rate to 0.001. All statistical analyses were performed using SAS 9.2 for Windows (Copyright © 2011 by SAS Institute Inc., Cary, NC) or Graph Pad Prism 5 software.
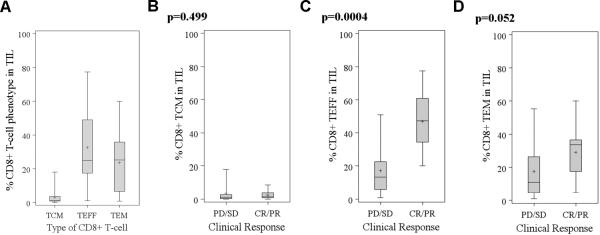
Fig. 4. Comparison of CD8+ T-cell memory phenotype in responders and non-responders.
The percentage of TCM, TEM, and TEFF in the CD8+ TIL subset in all treated patients (A). Wilcoxon rank-sum tests showed significant differences at α=0.001 between the percentage of %CD8+ TIL between TCM and TEFF (p<0.0001) and TCM and TEM (p<0.0001). In the subsequent panels, the percentage of CD8+ T cells with a TCM (B), TEM (C), and TEFF (D) in the infused TIL was compared between responders and non-responders.
Results
Patient population and TIL therapy
Patients of any HLA subtype with stage IIIc–IV (M1a–M1c) disease were recruited into the study. TIL were first expanded from tumor fragments with IL-2 for 5 weeks and cryopreserved for further expansion in the REP if a minimum of 48 × 106 cells was reached. This was required so that at least 40 × 106 cells were available for the REP after samples for quality control and anti-tumor analysis (8 × 106) were removed. Previous data from our group has found that the success rate in meeting this threshold for pre-REP TIL expansion is about 62% regardless of the type of prior therapy before the tumor harvest (14). Patients with cryopreserved pre-REP TIL having progressive disease and who met eligibility criteria were treated after further expansion of their thawed TIL using the REP. Only patients treated with their final post-REP TIL product were included in this study. Table S1 (Supplementary Data) summarizes the demographic and clinical characteristics of the treated patients (n=31). Most patients were stage IV at the time of TIL infusion and had received a variety of different prior therapies within 2 months of therapy, including biochemotherapy, radiotherapy, targeted therapy, and immunotherapy with IL-2, IFN-α, or GM-CSF. Most patients received at least 2 prior therapies for metastatic disease. Only one patient was treated with a selective mutant B-RAF inhibitor (GSK-2118436) (26), and none of the patients had prior ipilimumab therapy. The median age was 46 years with 65% males. Fig. 1B shows the overall scheme for patient therapy, indicating the time points for the starting the lymphodepleting preparative regimen (Day -7), TIL infusion (Day 0), followed by the two cycles of HD IL-2 therapy. The total TIL infused ranged from 8–150 × 109 cells (Table 1).
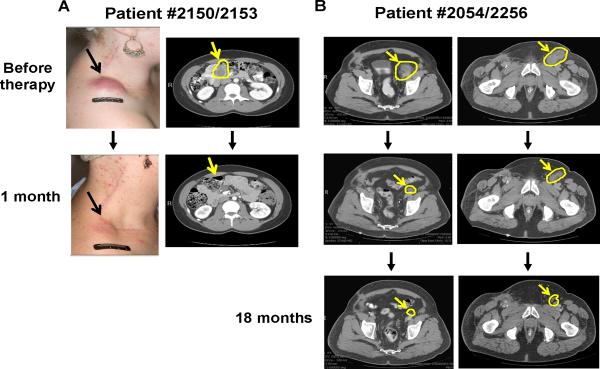
Fig. 1. Objective tumor regression in patients receiving autologous TIL therapy.
CT scans and photos of two representative patients (#2150/2153 and #2054/2256) before and after TIL therapy at different time points. Changes in a large subcutaneous mass in the right shoulder and of a large inguinal lymph node lesion after 1 month are shown for Patient #2150/2153 (A). Changes are shown in abdominal tumors after 1 month and 18 months post TIL infusion in Patient #2054/2256 (B).
Treatment-related toxicity and measurement of hematopoietic parameters
Patients experienced only transient, reversible adverse reactions (e.g., fever, chills, shortness of breath, increased heart rate) in the few hours following TIL transfer. No Grade 3 or 4 toxicities according to the National Cancer Institute Common Toxicity Criteria (NCICTC) were noted after TIL infusion before HD IL-2 therapy (27). Hematologic toxicities due to the preparative chemotherapy were anticipated and transient. Neutropenia and lymphopenia were observed in all patients as expected. All patients were also treated with platelet transfusions and red blood cell transfusion as needed and their counts eventually increased back to normal levels.
Fig. S2 (Supplementary Data on-line) shows the drop of total white blood cells (WBC), absolute neutrophil count (ANC), and absolute lymphocyte count (ALC) after the preparative chemotherapy and also shows the recovery of these populations following TIL infusion over a 70-day period in all patients, comparing the responders and non-responders. There was no statistically significant difference in the percentage change from day-7 to day-0 between responders and non-responders for WBC, ALC, or ANC (Fig. S2). The usual Grade 3 and 4 toxicities according to the NCICTC were found with bolus HD IL-2 infusion (pulmonary, renal, and liver dysfunction, and mental confusion). However, all of these were transient in nature and responded to standard interventions and/or resolved after IL-2 therapy. Most patients (28/31) received two cycles of IL-2 therapy.
Tumor response and association with patient clinical parameters
Table 1 provides a summary of major patient clinical parameters and clinical responses following TIL therapy. Clinical response rates according to the new irRC criteria established by Wolchok et al. (16) that more closely reflects the dynamics of a response one would expect with immunotherapies as opposed to chemotherapies. We noted objective tumor regression of tumors in multiple sites in responding patients, including sub-cutaneous, lung, liver, lymph node, and of tumors growing attached to major organs such as the spleen and heart. Fig. 1 shows examples of objective tumor regressions at visceral and non-visceral sites following TIL infusion in two representative patients by CT scans and photographs. We also noted in some cases that a mass underwent a durable partial regression by CT, and the remnant lesion was no longer 2-fluorodeoxyglucose (FDG) avid by positron emission tomography (PET). An example of this is shown in Patient #2262 (Fig. S3 on-line) in which a large tumor mass attached to the aorta and the heart shrunk by >60% in volume at 18 months after TIL infusion and the remnant lesion persisting did not show any activity by PET scanning.
No association was found between a number of other variables, such as patient gender and age, tumor stage, number and type of prior therapy for metastatic disease, initial tumor burden, serum LDH levels at time of TIL transfer, and number of IL-2 doses administered after TIL transfer) and clinical response (Tables S3, S4 on-line). Using irRC, the BOR (PR and CR) was 48.4% (15/31). Two patients (6.5%) experienced a CR. One patient (#2357) had a complete regression of all measureable lesions by CT scan, but had non-measurable lesions in the bone that remained stable. Many of the clinical responses have been quite extensive, with 12/15 responders having >70% reduction and 4/15 having a 100% reduction in their measureable tumor burden, as shown in a waterfall plot analysis (Fig. 2).
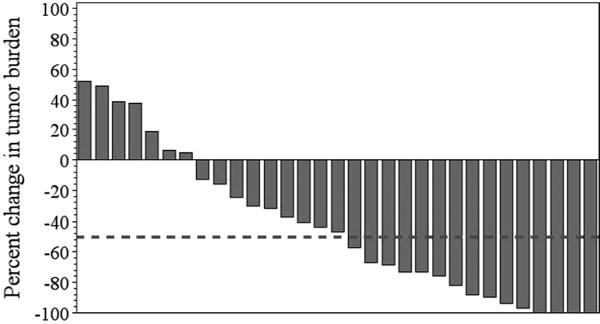
Fig. 2. Waterfall plot of percent change in measureable tumor burden in all treated patients (n=31).
The patients were treated between August 23, 2007 and October 6, 2010. Clinical responses were evaluated using the irRC criteria from whole body CT scans, as described in the Materials and Methods section. The percent change in tumor burden using the best overall irRC response after TIL infusion is shown for all patients. The dotted line indicates the −50% tumor burden reduction point needed to reach an objective clinical response according to irRC. Of the 15 patients who responded, 12 had ≥70% reduction in their tumor burden and 4 patients had a 100% reduction in measureable tumor burden. Two patients had non-measureable bony lesions that remained stable throughout the study period. This was not accounted for in determining the change in tumor burden shown.
We also measured responses using RECIST 1.1 (17) and found that the BOR was 41.9% (13/31). Overall, two patients scored as responders with irRC (16), but were non-responders (PD) using RECIST due to the development of new lesions detected after TIL infusion. One of these patients (#2262) developed a small brain lesion 2 months after TIL ACT that was surgically resected, but was still considered as progression-free according to irRC. At follow-up, this patient was progression-free by irRC after surgery for >22 months and the initial sites of disease continue to respond. The other patient (#2267) had a PR by irRC but had a developing new small brain lesion by 2 months after TIL treatment. This patient was treated by whole brain radiation and was alive >22 months after TIL therapy.
Overall survival (OS) and progression-free survival (PFS) were estimated from the treatment start date for all 31 patients (Fig. S4 on-line). The median OS time was not reached by the end study; the median PFS time was 7.6 months (95% CI: 4.1122.2 months). The 6-month OS and PFS rates were 81% (95% CI: 62–91%) and 57% (95% CI: 38–73%), respectively. In order to compare unbiased differences in OS and PFS between responders and non-responders, we performed a landmark survival analysis (28) starting at the 3-month time point after TIL infusion (Fig. S5 on-line). The landmark 6-month PFS rates for irRC responders and non-responders were 88% (95% CI: 39–98%) and 47% (95% CI: 21–69%), respectively. The landmark 18-month OS rates for responders and non-responders at the landmark were 87% (95% CI: 36–98%) and 48% (95% CI: 26–67%), respectively. The landmark median OS time for non-responders was 463 days (95% CI: 76 - . days) but could not be estimated for responders. The landmark median PFS times for non-responders and responders were 105 days (95% CI 33- . days) and 577 days (95% CI 138- . days). Change in tumor burden over time in all treated patients over the first 20–22 months after TIL infusion can be found in Fig. S6 on-line. Most of the responders showed tumor regression of 50% or more by 4 months, while some responders showed a more protracted decrease in tumor burden. Interestingly, Patient #2175 (non-responder) exhibited a prolonged SD with >29 months PFS by irRC (Fig. S6). Five responding patients, although achieving a response by irRC, were treated with additional therapies for renewed progression within the study period; these patients are listed in Fig. S6 (panel C) together with the time point and type of therapy they received after TIL.
Pre-REP anti-tumor reactivity and clinical response
We determined the anti-tumor reactivity of the pre-REP TIL as a possible predictive biomarker for irRC clinical response. Pre-REP TIL were cultured in IL-2 from tumor fragments for 5 weeks and tested with either autologous melanoma tumor cell lines (if available) or semi-allogeneic cell lines matched minimally at one HLA-A allele. We have found that IL-2-expanded TIL were functional and responded to polyclonal TCR using anti-CD3 stimulation in both IFN-γ and CTL assays (data not shown). The percentage of treated patients having TIL exhibiting significant anti-tumor-specific IFN-γ responses (≥100 pg/ml IFN-γ after subtraction of controls) either against an autologous or at least one allogeneic matched melanoma cell line was 71% (22/31). We were able to generate an autologous melanoma cell line for testing the pre-REP TIL for 17/31 (55%) of the patients and among these all but two TIL had specific anti-tumor IFN-γ responses. No significant association was found between positive anti-tumor reactivity and clinical response (p= 0.46; Table S3).
Immunophenotyping of infused TIL using flow cytometry
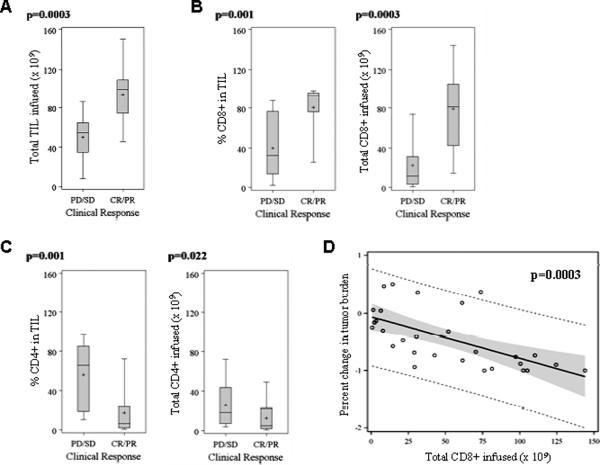
As shown in Table 1, the total amount of infused TIL varied across the patient population. Responding patients were infused with significantly more TIL (median 99 × 109 cells) than non-responders (median 55 × 109) (p= 0.0003; Table S4, Fig 3A). We further analyzed the different subsets of T cells using multi-color flow cytometry for the content of CD3+CD8+, CD3+CD4+ T cells, which revealed that both the percentage and total number of CD8+ T cells infused were significantly associated with clinical response (p= 0.001, 0.0003 respectively; Table S4, Fig. 3B). Non-responders had significantly higher percentages of CD4+ TIL (p=0.001; Table S4, Fig. 3C). We further analyzed the role of total CD8+ TIL as a continuous predictor of percent change in tumor burden (p=0.0003, Fig. 3D).
Fig. 3. Comparison of total cells infused and major T-cell subsets in the infused TIL product between responders and non-responders.
Comparison of total TIL infused (A), the percentage and total number of infused CD8+ T cells (B), and the percentage and total number of CD4+ T cells (C) between responders and non-responders. Linear regression showing the relationship between total CD8+ infused and percentage change in tumor burden (p=0.0003), with the solid line in showing the best fit, the broken line representing the 95% prediction limits, and the grey area indicating the 95% confidence limits (D).
We analyzed the state of differentiation or memory status of the CD8+ subset in the infused TIL. We stained the CD8+ TIL for cell surface markers associated with naïve, effector-memory (TEM), central memory (TCM), and more differentiated effector (TEFF) T cells, using the flow cytometry markers described in the Materials and Methods (19, 29). A viability dye was used to exclude any dead cells from the analysis. Most of the infused CD8+ TIL infused were composed of cells with a TEM (CD8+CD45RA−CD62L−CD27+) and TEFF (CD8+CD45RA−CD62L−CD27−) phenotype with few TCM phenotype (CD8+CD45RA−CD62L+CD27+) cells (Fig. 4A). Responding patients had a significantly higher percentage of TEFF cells than non-responders (p= 0.0004; Fig. 4D).
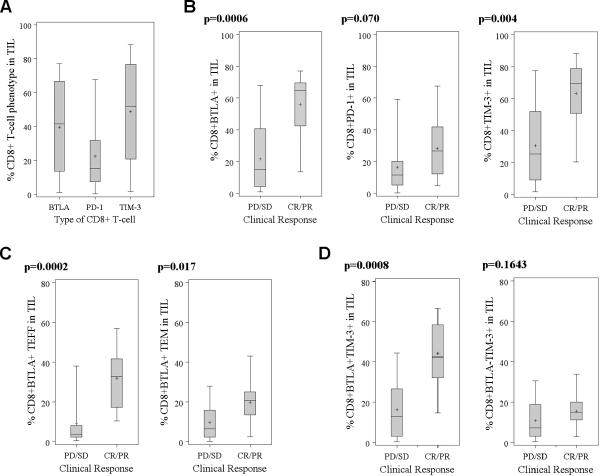
We also explored additional markers associated with CD8+ cytotoxic T-cell activation and differentiation, including PD-1, BTLA, and TIM-3, all of which can function as inhibitory co-receptors on CD8+ T cells (30–32). As shown in Fig. 5A, the percentage of CD8+ T cells expressing PD-1, BTLA, and TIM-3 varied widely across the entire treated population. Responding patients had a significantly higher percentages of CD8+BTLA+ T cells (p= 0.0006; Fig. 5B) and CD8+BTLA+ with a TEFF phenotype (CD45RA−CD62L−CD27−BTLA+) (p=0.0002; Fig. 5C). The percentage of CD8+PD-1+ TIL was not significantly associated with clinical response (p= 0.07; Fig. 5B). There was a trend towards responders receiving a higher percentage of CD8+TIM-3+ T cells in their TIL (Fig. 5B). However, analysis of BTLA co-expression with TIM-3 revealed that only CD8+TIM-3+ T cells co-expressing BTLA were with associated clinical response, while those lacking BTLA co-expression were not (Fig. 5D).
Fig. 5. Comparison of PD-1, BTLA, and TIM-3 on CD8+ TIL in responders and non-responders.
The percentage of cells in the total CD8+ TIL population expressing PD-1, BTLA, and TIM-3 in all treated patients (A). Wilcoxon rank-sum tests showed significant differences at α=0.001 between the percentage of CD8+ TIL between PD-1 and TIM-3 (p=0.0006). The percentage of CD8+PD-1+, CD8+BTLA+, and CD8+TIM-3+ expression in the infused TIL was compared between responders and non-responders (B). The percentage of CD8+BTLA+ T cells in the infused TIL with a TEFF or TEM phenotype was compared between responders and non-responders (C). The percentage of CD8+TIM-3+ T cells in the infused TIL with or without BTLA co-expression was compared between responders and non-responders (D).
Analysis of TIL Vβ clonotype persistence in vivo
Persistence of infused TIL for at least 1 month following adoptive transfer into lymphoablated melanoma patients has been previously found to be associated with objective clinical response to therapy (21). We used a TCR Vβ cloning and CDR3 sequencing approach used previously to track the changes in dominant TCR Vβ clonotypes in PBMC in five responding patients up to 22 months post TIL infusion (patients #2131, #2150/2153, #2258, #2124, and #2180). As shown in Table S5 (on-line), some dominant Vβ clonotypes in the original TIL persisted long-term over the entire 22-month period (e.g., Vβ24–1 and Vβ12–3 in Patient #2150/2153, and Vβ4–1 and Vβ29 in Patient# 2131). However, a significant number of dominant TIL Vβ clonotypes also became undetectable after 1 month, while other previously undetectable Vβ clonotypes emerged and expanded over the following months (Table S5). Similar results were found in other responding patients.
TIL from responders and non-responders did not significantly differ in telomere length
The telomere length of infused TIL products was retrospectively evaluated from cryopreserved samples. A Southern blot method using complementary telomere repeat probes on restriction enzyme-digested genomic DNA was performed to determine not only the average telomere length found in each TIL sample, but also range of telomere lengths that occur (23, 33). Southern blotting has been the most used approach to determine both of these parameters (22, 23). This analysis revealed that each TIL sample did not have a single fixed telomere length for all the cells, but had a range of telomere lengths between 11 to 3 Kb indicating a heterogeneous population of cells of different ages that have undergone different numbers of cell divisions. We determined telomere length corresponding to the peak signal intensity in each lane (patient) representing the telomere length of highest frequency in the cell population. Using this measurement as the overall telomere length in the TIL population, there was no statistically significant differences in telomere length between responders and non-responders (p= 0.3671; Fig. S7 online) or by age (p>0.05).
Discussion
In this study, we demonstrate that adoptive transfer of highly expanded melanoma TIL into patients with metastatic melanoma who had prior lymphoablation can mediate partial and even complete regression of disease at multiple organ sites. Our reported clinical response rates using irRC were 48.4% (15/31 responders) and are comparable to previously reported TIL therapy trials using RECIST (10, 11). A large proportion of our responders (12/15) had >70% tumor regression with four responders achieving a complete reduction in their measureable tumor burden. Only two of these patients were scored as CR since they continued to have non-measureable, yet stable, lesions in the bone. Our landmark analysis revealed a significant increase in OS of TIL responders over non-responders (p=0.033). These results suggest that an initial response to TIL therapy is of significant clinical benefit in these patients.
The total number of infused TIL was a critical parameter associated with clinical response, with responders being infused on average with almost twice the number of TIL as non-responders. These results suggest that ACT using TIL should aim to infuse as many T cells as possible to ensure more consistent clinical benefit. We also tested whether analysis of TIL anti-tumor reactivity at the pre-REP stage can be a predictive biomarker of clinical response. This would be beneficial in selecting which TIL to further expand in large-scale for therapy. However, our data was inconclusive and suggests that positive IFN-γ secretion against melanoma cells at this pre-REP stage may not be predictive and that other biomarkers in the TIL, tumor microenvironment, or differences in systemic factors may ultimately play a defining role. However, measurement of anti-tumor reactivity of TIL in the actual infusion products (pos-REP) will be needed to make a more definitive conclusion regarding this issue.
Recently, there has been an increasing interest in identifying additional predictive biomarkers in the TIL associated with the induction objective clinical responses and durable survival. A number of markers such as telomere length of the infused T cells, the type of T cells (CD4+ versus CD8+) and their differentiation status, as well as the differential expression of positive and negative T-cell costimulatory molecules, can affect both the persistence of adoptively transferred T cells and their effector function in vivo. In this regard, one striking observation besides the significantly higher number of TIL infused into responding patients was that the CD8+ T-cell lineage seemed to be the key active component in the large majority of patients. Both a higher percentage and higher total number of infused CD8+ T cells was significantly associated with response, while the opposite trend was found with CD4+ T cells. This suggests that CD8+ T cells are the key driving force behind the anti-tumor activity of TIL. In certain regards, this is expected due to CTL activity attributed to CD8+ T cells, but formal proof of this association in TIL therapy has been lacking until now.
Overall, our results suggest that TIL therapy protocols should aim to maximize the number of CD8+ T cells generated. This can be achieved through by the selective isolation of CD8+ T cells at the pre-REP stage, or providing additional factors in the REP that can facilitate CD8+ T-cell activation and division. In this regard, we have recently found that provision of costimulatory signals through CD137 (4–1BB) could significantly increase the survival and yield of CD8+ TIL (34). Despite the positive association of CD8+ T cells in TIL and clinical response, there was however a minority of responders (2/15 patients) that had CD4+ T cells making up >50% of the infused TIL (#2054/2256 and #2267). Adoptively transferred CD4+ T cells can in some circumstances mediate tumor regression by providing help to CD8+ T cells or having a direct anti-tumor effector function by secreting T-helper 1 (Th1) cytokines (e.g., IFN-γ and TNF-α) or by direct killing of HLA class II-expressing tumor targets by expressing granzymes and perforin (35). It will be important to determine what is the mechanism of action of these CD4+ T cells in these select patients and whether they act on their own or cooperate with other CD8+ T cells in TIL or recruit endogenous CD8+ T-cell responses to kill tumors as a results of antigen or epitope spreading.
The positive role of CD8+ T cells leads to another critical question regarding the state of CD8+ CTL differentiation in the infused TIL and how it is related to response. We found that most of the CD8+ T-cells in the infused TIL had the phenotype of either TEM (CD45RA−CD62L−CD27+CD28+) or more-differentiated TEFF (CD45RA−CD62L−CD27−CD28−) cells, with negligible levels of naïve and few central memory cells. Although previous data have suggested that the number and percentage of infused CD8+CD27+ TEM cells are critical components (36), we found that CD8+ TEFF cells that are CD27− seem to be more clinically relevant than TEM cells in mediating anti-tumor responses. One possible caveat that has been raised previously is that the conditions during the rapid expansion protocol (REP) of the TIL induces transient CD27 down-modulation (37), but in two published reports on multiple patient TIL subjected to the REP we have found no significant down-modulation of CD27 expression on the CD8+ T cells (8, 34).
The reason why CD8+ TEFF phenotype cells seem to be critical in our clinical trial over TEM is unclear and seems counterintuitive. It is possible that the TEFF cells have improved migratory properties into tumors by expressing more chemokine receptors, such as CXCR3 (38, 39). However, we have not formally tested this question yet. Human CD8+CD27− TEFF have been characterized as having a shorter lifespan than TEM (18, 40), but the situation in a prior lymphodepleted host may be different where these cells may be exposed to higher amounts of homeostatic cytokines, such as IL-15 and costimulatory signals, that may facilitate their persistence and telomerase expression. Many CD8+ T cell clones specific for chronic viruses such as CMV and EBV infections in adulthood are highly differentiated cells that nevertheless can persist for long periods of time and are spontaneously cytolytic, or rapidly re-express cytolytic granule molecules upon antigen exposure, and kill target cells; these cells are critical in protecting the host against viral reactivation for years (20). Another emerging issue is that adoptively transferred CD8+ T cells in lymphodeleted hosts can exhibit plasticity, with either CD8+ T-cells with a TEM profile rapidly losing CD27, or with some TEFF cells gaining CD27 expression (41). Finally, our data here does not preclude a critical role for less-differentiated CD8+ TEM. These cells may cooperate with TEFF cells and have a critical role in the long-term control of tumor growth not readily apparent in our study here.
One of the most unexpected findings of this study came after examining the association of the “negative” costimulatory molecules, PD-1, BTLA and TIM-3 (30, 42, 43) on infused TIL with clinical response. Significant heterogeneity in PD-1, BTLA, and TIM-3 expression was found on the CD8+ T cells. Surprisingly, we found that an increased frequency of CD8+BTLA+ TIL as well as higher numbers of infused CD8+BTLA+ TIL were both highly associated with a favorable clinical response. Interestingly, the extent of PD-1 expression on CD8+ TIL was not observed to be related to clinical response, and, in fact, TIL from responding patients tended to have higher frequencies of PD-1+ T cells. Similarly, TIM-3 was also highly expressed in CD8+ TIL infused into responding patients, but on its own was not observed to be predictive of response without co-expression of BTLA. These results suggest that BTLA expression could be an important new biomarker in T-cell adoptive cell therapy that needs to be studied further in future TIL clinical trials to verify its predictive value and its possible biological role.
BTLA is a more distant member of the Ig family expressed binding to an unusual ligand called herpes virus entry mediator (HVEM) leading to SHP1 and SHP2 phosphatase activity dampening T-cell activation signals (44). BTLA is constitutively on naïve T cells and tends to be down-modulated in CD8+ T cells as they mature into effector cells (42). Interestingly, BTLA has been found to be continually expressed (not down-modulated) during differentiation of certain melanoma antigen-specific (e.g., MART-1) CD8+ T cells towards effector cells (42). The reasons for this however are unclear. Whether BTLA plays a functional role in CD8+ TIL or whether it is simply is a marker for TIL associated with improved anti-tumor activity in vivo after cell transfer will need to be determined. In addition, although BTLA is considered a negative costimulatory molecule (32), its role at different stages of CD8+ T-cell differentiation has not been clearly defined and recent data suggests that it may in fact may also play a pro-survival function by binding to HVEM on activated CD8+ T cells and triggering NFκB activation (45, 46).
Another correlative parameter studied in T-cell therapy is the telomere repeat lengths of infused TIL. Telomere length has been linked to shorter persistence of transferred TIL in vivo (47). Using the Southern blotting approach, we found no significant difference between the mean telomere length in TIL between responders and non-responders. Responders had a tendency to have slightly longer telomere lengths, but this was found not to be significant. It is also important to point out that the median telomere lengths found in our study for both responders and non-responders here (median of 5.9 Kb and 5.4, respectively), as well as in previous TIL therapy studies (36), are in fact within the same range as those found in original studies on human lymphocyte senescence showing telomeric repeat lengths in the range of 4.7–6.3 Kb in senescing T cells by Southern blotting (23). However, our Southern blotting analysis also revealed that a considerable heterogeneity existed in the telomere lengths of infused TIL, with a range of 3 and 11 Kb found in the blots of both responders and non-responders. Thus, it is possible that smaller sub-populations of TIL still retain relatively long telomeres above this senescent range (>6.3 Kb) with a higher capacity to persist and expand in vivo, while other TIL populations with shorter telomere lengths have a reduced cell division potential and lifespan. Overall, our data underscores the difficulty in interpreting the impact of telomere length as a biomarker because there is not one single telomere length that can be assigned to the entire TIL population and considerable heterogeneity exists.
In summary, we have found that adoptive transfer of TIL expanded ex vivo can induce a high rate of objective tumor regression in unresectable metastatic melanoma patients and that these responses can be long-lasting in a significant fraction of treated patients. Moreover, our laboratory correlative studies have revealed potential biomarkers in adoptively transferred TIL associated with clinical response, such as the expression of BTLA. Future efforts will focus on further defining the role of BTLA in the antitumor response and enhancing predictive assays of response, developing strategies to generate more effective T-cells, identifying ideal patient candidates, and exploring future TIL clinical trials in combination with new FDA approved agents for melanoma.
Supplementary Material
Translational Relevance.
In this study, we report on a Phase II clinical trial showing that adoptive cell therapy with autologous tumor-infiltrating lymphocytes (TIL) is a powerful regimen to treat metastatic melanoma patients after failing first- and second- line therapies. We also performed comprehensive phenotypic analysis of T cells in the infused TIL and found a strong association between clinical response and the total number and percentage of CD8+ T cells. CD8+ T cells with a more differentiated effector phenotype seemed to be the most highly active component. In addition, for the first time we screened for the expression of negative T-cell costimulatory molecules (PD-1, BTLA, and TIM-3) in TIL and how these are associated with clinical response. Unexpectedly, we found that CD8+ T cells expressing BTLA (B- and T- lymphocyte attenuator) were highly associated with clinical response. This cell subset may be a potentially powerful new biomarker in TIL therapy.
Acknowledgements
The authors would like to thank all the additional clinical and research staff in the Melanoma Medical Oncology Department and GMP Facility at MD Anderson Cancer Center for their help throughout the study. The authors are especially thankful to Prometheus Therapeutics & Diagnostics (San Diego, CA) and Novartis (East Hanover, NJ) for providing human recombinant IL-2 (Proleukin™) used to expand TIL for therapy. We also thank Drs. Mark Dudley, Steve Rosenberg, Ena Wang and Franco Marincola (National Cancer Institute, Bethesda, MD) for helpful discussions. Additional consultation with Dr. Laurence Cooper (Pediatric Department, MD Anderson Cancer Center) is also greatly appreciated. This study was supported by NIH research grants CA111999 (P. Hwu), CA093459-DRP21 (L. Radvanyi), and grants from the Melanoma Research Alliance (L. Radvanyi and P. Hwu), the Dr. Miriam and Sheldon Adelson Medical Research Foundation/AMRF (P. Hwu and L. Radvanyi), and the Gillson Longenbough Foundation (P. Hwu).
Footnotes
Disclosures: The authors declare no competing financial or other conflicts of interest.
References
- 1.Trinh VA. Current management of metastatic melanoma. Am J Health Syst Pharm. 2008;65:S3–8. doi: 10.2146/ajhp080460. [DOI] [PubMed] [Google Scholar]
- 2.Quirt I, Verma S, Petrella T, Bak K, Charette M. Temozolomide for the treatment of metastatic melanoma: a systematic review. Oncologist. 2007;12:1114–23. doi: 10.1634/theoncologist.12-9-1114. [DOI] [PubMed] [Google Scholar]
- 3.Fecher LA, Amaravadi RK, Flaherty KT. The MAPK pathway in melanoma. Curr Opin Oncol. 2008;20:183–9. doi: 10.1097/CCO.0b013e3282f5271c. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty KT, McArthur G. BRAF, a target in melanoma: implications for solid tumor drug development. Cancer. 2010;116:4902–13. doi: 10.1002/cncr.25261. [DOI] [PubMed] [Google Scholar]
- 5.Flaherty KT, Yasothan U, Kirkpatrick P. Vemurafenib. Nat Rev Drug Discov. 10:811–2. doi: 10.1038/nrd3579. [DOI] [PubMed] [Google Scholar]
- 6.Flaherty KT. Targeting Metastatic Melanoma. Annu Rev Med. 2011 doi: 10.1146/annurev-med-050410-105655. [DOI] [PubMed] [Google Scholar]
- 7.Weber J. Immunotherapy for melanoma. Curr Opin Oncol. 2011;23:163–9. doi: 10.1097/CCO.0b013e3283436e79. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Liu S, Hernandez J, Vence L, Hwu P, Radvanyi L. MART-1-specific melanoma tumor-infiltrating lymphocytes maintaining CD28 expression have improved survival and expansion capability following antigenic restimulation in vitro. J Immunol. 2010;184:452–65. doi: 10.4049/jimmunol.0901101. [DOI] [PubMed] [Google Scholar]
- 9.Hoos A, Ibrahim R, Korman A, Abdallah K, Berman D, Shahabi V, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2011;37:533–46. doi: 10.1053/j.seminoncol.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–55. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 12.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332–42. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph RW, Peddareddigari VR, Liu P, Miller PW, Overwijk WW, Bekele NB, et al. Impact of clinical and pathologic features on tumor-infiltrating lymphocyte expansion from surgically excised melanoma metastases for adoptive T-cell therapy. Clin Cancer Res. 2011;17:4882–91. doi: 10.1158/1078-0432.CCR-10-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(Suppl 1):S11–4. [PubMed] [Google Scholar]
- 16.Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–85. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 19.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 20.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–45. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Khong HT, Dudley ME, El-Gamil M, Li YF, Rosenberg SA, et al. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J Immunother. 2005;28:258–67. doi: 10.1097/01.cji.0000158855.92792.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura M, Stone RC, Hunt SC, Skurnick J, Lu X, Cao X, et al. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat Protoc. 2010;5:1596–607. doi: 10.1038/nprot.2010.124. [DOI] [PubMed] [Google Scholar]
- 23.Vaziri H, Schachter F, Uchida I, Wei L, Zhu X, Effros R, et al. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet. 1993;52:661–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher RA. On the interpretation of X2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society. 1922;85:87–94. [Google Scholar]
- 25.Randles R, Wolfe D. Introduction to the Theory of Nonparametric Statistics. John Wiley; 1979. [Google Scholar]
- 26.Arkenau HT, Kefford R, Long GV. Targeting BRAF for patients with melanoma. Br J Cancer. 2010;104:392–8. doi: 10.1038/sj.bjc.6606030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu T, Saijo N. [Common toxicity criteria: version 2.0, an improved reference for grading the adverse reaction of cancer treatment] Nihon Rinsho. 2003;61:937–42. [PubMed] [Google Scholar]
- 28.Dafni U. Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes. 2011;4:363–71. doi: 10.1161/CIRCOUTCOMES.110.957951. [DOI] [PubMed] [Google Scholar]
- 29.Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM, et al. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol. 2007;178:4112–9. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- 30.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–86. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulos CM, June CH. Putting the brakes on BTLA in T cell-mediated cancer immunotherapy. J Clin Invest. 2010;120:76–80. doi: 10.1172/JCI41811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Effros RB, Allsopp R, Chiu CP, Hausner MA, Hirji K, Wang L, et al. Shortened telomeres in the expanded CD28-CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. Aids. 1996;10:F17–22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez-Chacon JA, Li Y, Wu RC, Bernatchez C, Wang Y, Weber JS, et al. Costimulation through the CD137/4–1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances antitumor effector function. J Immunother. 2011;34:236–50. doi: 10.1097/CJI.0b013e318209e7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qui HZ, Hagymasi AT, Bandyopadhyay S, St Rose MC, Ramanarasimhaiah R, Menoret A, et al. CD134 plus CD137 dual costimulation induces Eomesodermin in CD4 T cells to program cytotoxic Th1 differentiation. J Immunol. 2011;187:3555–64. doi: 10.4049/jimmunol.1101244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang J, Kerstann KW, Ahmadzadeh M, Li YF, El-Gamil M, Rosenberg SA, et al. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006;176:7726–35. doi: 10.4049/jimmunol.176.12.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dengel LT, Norrod AG, Gregory BL, Clancy-Thompson E, Burdick MD, Strieter RM, et al. Interferons induce CXCR3-cognate chemokine production by human metastatic melanoma. J Immunother. 2010;33:965–74. doi: 10.1097/CJI.0b013e3181fb045d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wenzel J, Bekisch B, Uerlich M, Haller O, Bieber T, Tuting T. Type I interferon-associated recruitment of cytotoxic lymphocytes: a common mechanism in regressive melanocytic lesions. Am J Clin Pathol. 2005;124:37–48. doi: 10.1309/4EJ9KL7CGDENVVLE. [DOI] [PubMed] [Google Scholar]
- 40.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–62. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 41.Powell DJ, Jr, Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–50. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Derre L, Rivals JP, Jandus C, Pastor S, Rimoldi D, Romero P, et al. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J Clin Invest. 2010;120:157–67. doi: 10.1172/JCI40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–10. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy KM, Nelson CA, Sedy JR. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat Rev Immunol. 2006;6:671–81. doi: 10.1038/nri1917. [DOI] [PubMed] [Google Scholar]
- 45.Cheung TC, Steinberg MW, Oborne LM, Macauley MG, Fukuyama S, Sanjo H, et al. Unconventional ligand activation of herpesvirus entry mediator signals cell survival. Proc Natl Acad Sci U S A. 2009;106:6244–9. doi: 10.1073/pnas.0902115106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakoda Y, Park JJ, Zhao Y, Kuramasu A, Geng D, Liu Y, et al. Dichotomous regulation of GVHD through bidirectional functions of the BTLA-HVEM pathway. Blood. 2011;117:2506–14. doi: 10.1182/blood-2010-08-301325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–52. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.