Abstract
Purpose
Arsenic trioxide (ATO) as a single agent is used for treatment of acute promyelocytic leukemia (APL) with minimal toxicity but therapeutic effect of ATO in other types of malignancies has not been achieved. We tested whether a combination with ethacrynic acid (EA), a glutathione S-transferase P1-1 (GSTP1-1) inhibitor and a reactive oxygen species (ROS) inducer will extend the therapeutic effect of ATO beyond APL.
Experimental Design
The combined apoptotic effects of ATO plus EA were tested in non-APL leukemia and lymphoma cell lines. The role of ROS, GSTP1-1, glutathione, and Mcl-1 in apoptosis was determined. The selective response to this combination of cells with and without GSTP1-1 expression was compared.
Results
ATO/EA combination synergistically induced apoptosis in myeloid leukemia and lymphoma cells. This treatment produced high ROS levels, activated c-jun-NH2-terminal kinase and reduced Mcl-1 protein. This led to the decrease of mitochondrial transmembrane potential, release of cytochrome c and, subsequently, to activation of caspase 3 and 9. Induction of apoptosis in leukemia and lymphoma cells expressing GSTP1-1 required that high EA concentrations be combined with ATO. Silencing of GSTP1 in leukemia cells sensitized them to ATO/EA-induced apoptosis. In a sub-group of B-cell lymphoma which do not express GSTP1-1, lower concentrations of EA and its more potent derivative, ethacrynic acid butyl-ester, decreased intracellular glutathione levels and synergistically induced apoptosis when combined with ATO.
Conclusion
B-cell lymphoma cells lacking GSTP1-1 are more sensitive than myeloid leukemia cells to ATO/EA-induced apoptosis.
Keywords: Arsenic Trioxide, ethacrynic acid, leukemia, lymphoma, and apoptosis
Introduction
Arsenic trioxide (ATO) treatment induces complete remission in acute promyelocytic leukemia (APL) patients. It has been found that ATO could induce both apoptosis and partial differentiation in APL cells at therapeutic concentrations (1–2 μM) (1, 2). Although ATO-mediated degradation of a leukemia specific fusion protein PML-RARα in APL cells leads to differentiation (3), we and other groups found that ATO induced APL-cell apoptosis is independent of PML-RARα degradation (4–7). Therefore it is possible to use ATO to achieve therapeutic results in malignant cells based on apoptosis induction.
ATO-induced apoptosis in APL cells is partially mediated through H2O2 accumulation (6, 8), which is followed by a change in mitochondrial transmembrane permeability (MTP), cytochrome c release, and caspase activation (6). We showed that the remarkable sensitivity to ATO-induced apoptosis and H2O2 accumulation of APL-NB4 cells, compared to other types of myeloid leukemia such as HL-60 and K562 cells, was partly due to lower activity of glutathione S-transferase P1-1 (GSTP1-1) (6). GSTP1-1 is a detoxification enzyme which can determine cell sensitivity to ATO (9, 10). It catalyzes the conjugation of ATO with GSH causing its efflux from cells (11), it also scavenges intracellular reactive oxygen species (ROS) and detoxifies other products of oxidative damage. Furthermore, GSTP1-1 was found to regulate several signaling pathways, including that of c-jun NH2-teminal kinase 1 (JNK1) inhibition via direct protein-protein interactions (12, 13). We previously found that over-expression of GSTP1-1 reduces the intracellular level of free ATO and diminishes ROS-induced apoptosis (9), suggesting that agents that inhibit GSTP1-1 activity might extend the utility of ATO for treatment of malignancies beyond APL. Previously we and other groups have found that B-cell lymphoma cells are relatively responsive to ATO in vitro (14, 15). We suspect that the ineffectiveness of ATO in patients may be due to insufficient ROS levels which are incapable of triggering effective apoptosis. Thus we searched for a candidate that will improve ATO effect. Ethacrynic acid (EA), a diuretic drug, is an inhibitor of GSTP1-1 and an inducer of ROS production (16, 17). As a single agent, only at high concentrations, EA can inhibit cell growth and induce ROS-mediated apoptosis in cancer cells (17).
We tested and found that EA synergizes with ATO in apoptosis induction in non-APL leukemia and lymphoma cells and that the level of GSTP1-1 determines the effectiveness of this treatment.
Materials and Methods
Reagents
ATO solution was obtained from the pharmacy of our school hospital. EA, acridine orange (AO), ethidium bromide (EB), N-acetylcysteine (NAC), Trolox and JNK inhibitor SP600125 were purchased from Sigma Chemical Co. (St. Louis, MO). EA-GS and ethacrynic acid butyl-ester (EABE) were synthesized by Guisen Zhao (18). The general caspase inhibitor Z-VAD-FMK, caspase-8 inhibitor Z-IETD-FMK, and caspase-9 inhibitor Z-LEHD-FMK were obtained from Calbiochem (LA Jolla, CA). 3,3′-dihexy-loxacarbocyanine iodide [DiOC6(3)] and 5,6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) were obtained from Molecular Probes (Eugene, OR). Antibodies to poly-(ADP-ribose)-polymerase (PARP) were obtained from Boehringer Mannheim (Indianapolis, IN); pro-caspase-3 and caspase-8 from BD Biosciences (San Diego, CA), DR5 and c-FLIP from Alexis Biochemicals (San Diego, CA); Mcl-1, survivin, Bcl-2, GSTP1-1, Nrf2 and β-actin from Santa Cruz Biotechology, Inc. (Santa Cruz, CA); XIAP, ABL and Fas ligand from BD PharMingen (San Diego, CA); Fas from Upstate Biotechnology (Lake Placid, NY); Bcl-XL, cleaved caspase-9, -3, TRAIL, JNK, p-JNK, p-c-Jun (Ser63) II, and cytochrome c from Cell Signaling Technology, Inc. (Beverly, MA); catalase from Sigma Chemical Co. and VDAC/Porin from Abcam Inc. (Cambridge, MA).
Cell lines
Human acute myeloid leukemia HL-60 cells, chronic myeloid-derived K562 cells, lymphoma Raji, Namalwa, Daudi, and Jurkat cells were obtained from American Type Culture Collection. HP100-1, a H2O2-resitant derivative of HL-60 cell line (19), was obtained from the Japanese Cell Bank. Su-DHL-4 lymphoma cells were obtained from Dr. Ari Melnick (20). All cell lines were not authenticated by us once received in our lab. HL-60, HP100-1 and K562 cells were cultured in RPMI 1640 medium supplemented with 100 units/mL penicillin, 100 μg/mL streptomycin, 1 mmol/L L-glutamine, and 10% (v/v) heat-inactivated fetal bovine serum (FBS). Raji, Namalwa, Daudi, Jurkat and Su-DHL-4 cells were cultured in RPMI 1640 modified to contain 2 mmol/L L-glutamine, 10 mmol/L HEPES, 1.0 mmol/L sodium pyruvate, 4.5 g/L glucose, 1.5 g/L sodium bicarbonate, 100 unit/mL penicillin, 100 μg/mL streptomycin, and 10% (v/v) heat-inactivated FBS. RV4 and RG19 cells are clones of human Raji cells transfected with pcDNA3.1 empty vector and pcDNA3.1/GSTP1 plasmid which we have described previously (9).
Quantitation of apoptotic cells
For morphologic apoptosis quantification cells were stained with AO and EB as described previously (5), and the percentage of apoptotic cells was calculated from 300 cells. Annexin V–FITC Apoptosis Detection Kit (BD Biosciences, San Diego, CA) was used to quantify apoptosis by fluorescence-activated cell sorting (FACS) analysis. Data were analyzed using CELLQuest (BD Biosciences, San Diego, CA) software, on 10,000 events (9). Drug interaction was analyzed by Compusyn software(ComboSyn, Cambridge, MA) using a combination at a fixed ratio of two agents (21). A combination index (CI) less than 1 indicates synergism.
Measurement of mitochondrial transmembrane potential (MTP)
MTP was assessed by the retention of DiOC6(3), a membrane-permeable fluorescent cationic dye. The uptake of DiOC6(3) by mitochondria is proportional to the MTP (22). Briefly, cells under different treatments were incubated with 40 nmol/L DiOC6(3) for 15 min at 37oC. After washing with ice-cold PBS, the cells were analyzed by FACScan with excitation and emission wavelength of 484 and 500 nm, respectively.
Subcellular fractionation
Cytosolic and mitochondrial fractions were isolated with the Mitochondria Isolation Kit (Pierce/Thermo Fisher Scientific) following the manufacturer’s instructions using 2×107 cells. The mitochondria rich and cytosolic fractions were obtained by centrifugation. The mitochondria-rich fraction was washed once with 500 μl Mitochondrial Isolation Reagent C, followed by a final resuspension in lysis buffer [50mmol/L Tris-HCl (pH 7.4), 150mmol/L NaCl, 1% NP40, 0.25% sodium deoxycholate, 1mmol/L EGTA] with protease inhibitor mixture and stored at −80°C.
Determination of H2O2 production, Western blot analysis, GSTP1-1 activity, GSH content and siRNA interference were performed as we reported before (6, 23)
Statistical analysis
Data were analyzed for statistical significance using the Student’s t test (Microsoft Excel, Microsoft Corporation). A p-value of less than 0.05 was considered statistically significant.
Results
A combination of ATO with EA synergistically induces apoptosis in HL-60 and K562 myeloid leukemia cells
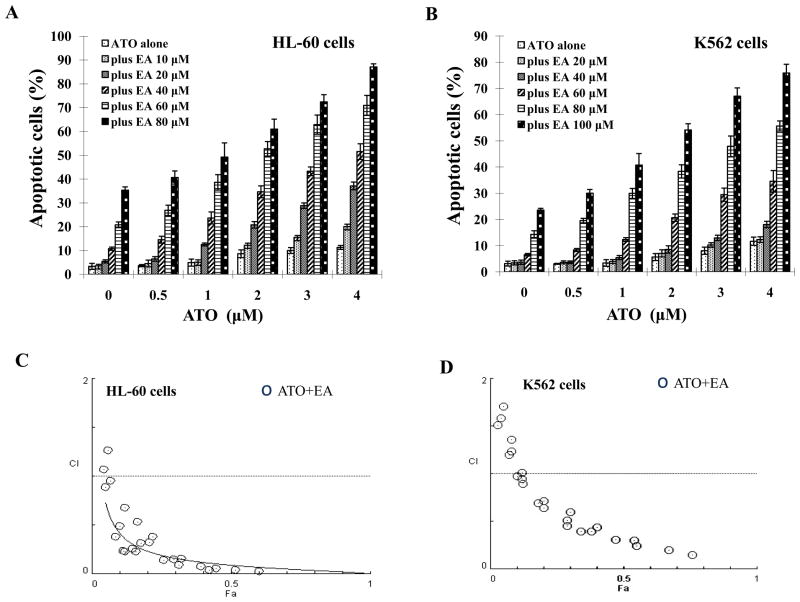
HL-60 and K562 cells were treated with ATO or EA or the combination and the apoptotic cells were identified by morphology after staining with AO and EB. ATO (0.5 to 4 μM) did not induce obvious apoptosis in neither of the two cell lines (Fig. 1A and B). EA alone was ineffective below 40μM and at 80 μM, it induced apoptosis in 30% of HL-60 cells and in 14% of K562 cells (Fig. 1A and B). EA at concentrations of 40 μM to 80 μM in HL-60 cells (Fig. 1A) and at concentrations of 60 μM to 100 μM in K562 cells augmented ATO-induced apoptosis (Fig. 1B). Using Compusyn software the combined apoptotic effects of ATO with EA were analyzed for synergy. Combination Index (CI) values were calculated for different dose-effect levels based on parameters derived from median-effect plots of ATO alone, EA alone, or their combinations. As shown in Fig. 1C and D, simultaneous exposure of cells to ATO (1 μM to 5 μM) and EA (40 μM to 80 μM in HL-60 cells and 60 μM to 100 μM in K562 cells) showed CIs of less than 1, indicating synergistic effects. Although the antagonism seems to be obtained in cells treated with combinations of lower concentrations, it should be considered as ineffectiveness. The apoptosis induction by low concentrations of EA (10–20 μM), ATO (0.5 μM) and their combinations is minimal (<10%) (Fig. 1C and D). Synergistic results were confirmed in HL-60 and K562 cells when annexin V and FACS were used to measure apoptosis. The combination produced ~36% apoptosis in each of the cell lines (Suppl. Fig. 1).
Figure 1. ATO/EA has synergistic effect on apoptosis in HL-60 and K562 cells.
(A & B) Quantification of apoptosis. HL-60 (A) and K562 (B) cells were treated for 24 h with a fixed ratio of ATO (0.5–4 μM) and EA (10–100 μM). Apoptotic cells were quantified by microscopic detection and counting of AO and EB-stained cells. (C & D) Combination index (CI) of ATO with EA on apoptosis induction in HL-60 (C) and K562 (D) cells. The nature of interaction between ATO and EA was characterized by median dose effect analysis using CompuSyn software. CI values less than 1.0 (horizontal line) corresponds to a synergistic interaction. Fa on the x-axis denotes the fraction affected (e.g. Fa of 0.5 is equivalent to a 50% apoptosis induction.)
ATO/EA induces apoptosis in HL-60 and K562 cells through the mitochondrial pathway
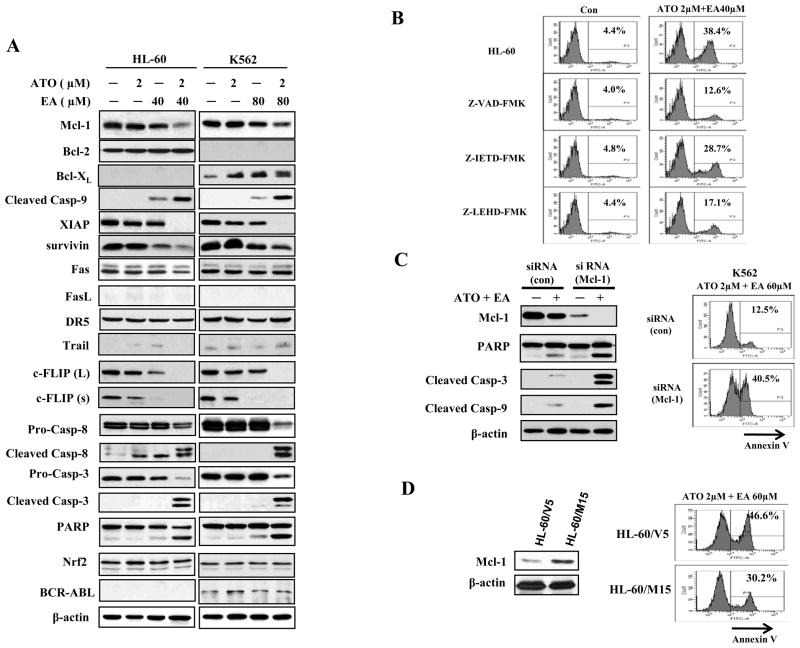
We analyzed HL60 and K562 cells for protein levels of the Bcl-2 family members (mitochondrial pathway) and for death receptors (extrinsic pathway), in control cells and cells treated with ATO (2 μM) or EA (40 μM in HL-60 cells and 80 μM in K562 cells) alone, or the combination. The anti-apoptotic, Bcl-2 family member, Mcl-1 protein was somewhat reduced by EA alone in both cell lines which was further reduced by the combination treatment (Fig. 2A). AML HL-60 cells express Bcl-2, but not Bcl-xL. The levels of Bcl-2 were not changed by treatment with either agent alone or their combination. CML K562 cells contain fusion protein BCR-ABL which is known to upregulate Bcl-xL (24). K562 cells express Bcl-xL, but not Bcl-2. The levels of BCR-ABL were not changed after treatment with each agent and the combination, however, the levels of Bcl-xL were slightly increased by treatments with each agent alone or their combination (Fig. 2A). The expression of inhibitors of caspase-9 and 3, was either completely blocked (XIAP), or strongly reduced (survivin) by the ATO/EA combination. Compatible with these changes, cleaved caspase-9 was strongly increased. Of the extrinsic pathway only a reduction in c-FLIP (an inhibitor of caspase-8) and elevated cleaved caspase-8 were detected (Fig. 2A). The levels of death receptors and their ligands, such as Fas, FasL, DR5 and TRAIL were unchanged, yet there was an increase in cleaved caspase-3 (the target of both caspase 8 and 9) in both cell lines treated with the combination (Fig. 2A). Nrf2, a transcriptional factor, mediates cellular defense against oxidative stress. High levels of Nrf2 are correlated with relative resistance to ATO treatment and Nrf2 is increased in ATO-treated myeloma cells (25, 26). Thus we determined whether the effectiveness of ATO/EA treatment regulates Nrf2 levels. We found that in either HL60 or K562 the Nrf2 level was not regulated by EA or ATO treatment alone or by their combination (Fig. 2A), suggesting that Nrf2-mediated defense system is not activated in this combination treatment
Figure 2. ATO and EA combination induces activation of caspase-3, caspase-8, and caspase-9 and dowregulates antiapoptotic proteins.
(A) Modulation of apoptotic proteins by ATO/EA. HL-60 and K562 cells were treated with EA and ATO at the indicated concentrations for 24 h. The relative levels of indicated proteins were determined by Western blotting using specific antibodies. β-actin served as loading control. (B) Caspase 9 (but not caspase 8) is the major effector of ATO/E-induced apoptosis. HL-60 cells were pretreated for 4 h with 50 μM Z-VAD-FMK (a general-caspase inhibitor), 50 μM Z-IETD-FMK (a caspase-8 inhibitor), 50 μM Z-LEHD-FMK (a caspase-9 inhibitor), and then incubated with 2 μM ATO and 40 μM EA for another 24 h. Apoptotic cells were quantified using annexin V-FITC staining and FACS analysis. Con.-control. (C) Silencing of Mcl-1 enhances ATO/EA-induced apoptosis in K562 cells. K562 cells were transfected with Mcl-1siRNAor negative control siRNA and after 18 h, treated with 2 μM ATO and 60 μM EA for additional 24 h. The protein levels were determined by Western blotting and the apoptotic cells were determined by FACS after staining with annexin V-FITC. (D) Increased Mcl-1 level attenuated ATO/EA-induced apoptosis in HL-60 cells. HL-60 cells transfected with an empty vector (HL-60/V5) and Mcl-1 expression plasmid (HL-60/M15) were treated with 2 μM ATO and 60 μM EA for additional 24 h. The Mcl-1 level was determined by Western blotting and the apoptotic cells were determined by FACS after staining with annexin V-FITC.
To identify which apoptotic pathway is activated, control or ATO/EA treated HL-60 were incubated with a general caspase inhibitor, Z-VAD-FMK, a caspase-9 inhibitor, Z-LEHD-FMK, and a caspase-8 inhibitor, Z-IETD-FMK. Fig. 2B shows that the general caspase inhibitor and caspase-9 inhibitor reduced apoptosis from ~38% to ~13% and 17%, respectively, while inhibition of caspase-8 (extrinsic pathway) had a much lesser effect on apoptosis induction by ATO/EA. Thus, the mitochondrial pathway appears to be the principle mechanism of apoptosis induction by ATO/EA combination.
As shown in Fig. 2A, 3 proteins of the mitochondrial pathway, a Bcl-2 family member, Mcl-1, and 2 inhibitors of caspases, XIAP and survivin, are down-regulated by ATO/EA. These proteins were individually knocked down in K562 cells using specific siRNA. The levels of each of the 3 proteins were significantly reduced (Fig. 2C and Suppl. Fig. 2), but only knockdown of Mcl-1 sensitized K562 cells to ATO/EA apoptosis. The Mcl-1 knockdown-K562 cells, treated with ATO/EA, had more cleaved PARP, caspase-3 and caspase-9 (Fig. 2C). The percent of annexin V positive cells rose ~3.5 fold over the control (intact Mcl-1) treated with 2μM ATO/60μM EA, which had only 12% of apoptotic cells (Fig. 2C). To further test the role of Mcl-1 in this combination treatment, subclones of HL-60 cells transfected with the pcDNA3.1 empty vector (HL-60/V5) and the pcDNA3.1/Mcl-1 plasmid (HL-60/M15) were generated. HL-60/M15 cells with much higher levels of Mcl-1 than HL-60/V5 cells were less sensitive to ATO/EA induced apoptosis (Fig. 2D). To test if Bcl-xL expression has a negative role in ATO/EA-induced apoptosis in K562 cells, Bcl-xL was silenced using siRNA. This intervention did not sensitize K562 cells to ATO/EA-induced apoptosis (Suppl. Fig. 2E and F). Taken together, this suggests that the down-regulation of Mcl-1, but not of XIAP or survivin, is a crucial step in apoptosis induction by ATO/EA.
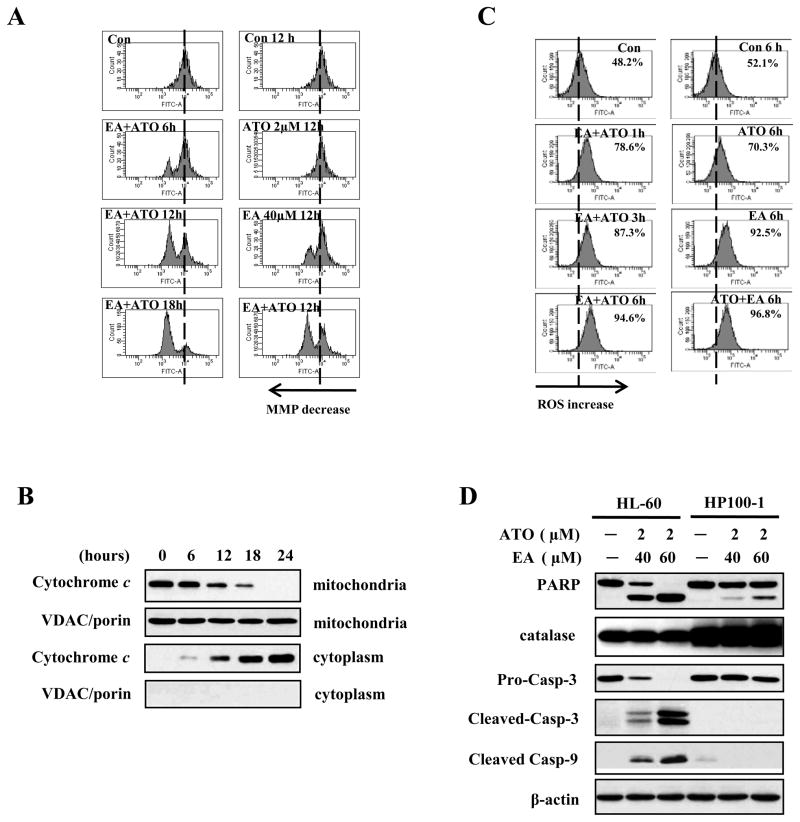
Figure 3. ROS production is involved in the mitochondria-mediated apoptotic pathway activated by ATO/EA treatment.
(A) Combination treatment reduces mitochondrial transmembrane potential (MTP). MTP was measured using changes in fluorescence density upon DiOC6(3) loading (see Material and Methods) of untreated HL-60 cells, or cells treated with 2 μM ATO alone, 40 μM EA alone, or the combination, for the indicated times. The peak shift to the left indicates lower MTP. (B) Time-course of cytochrome c release from mitochondria to cytoplasm. HL-60 cells were treated with the combination of 2 μM ATO/40 μM EA for the indicated times, cytosolic and mitochondrial fractions were obtained and analyzed by Western blotting with anti-cytochrome c antibody. VDAC/porin was used as control for purity of the mitochondrial fraction. (C) EA alone and ATO/EA combination increase the H2O2 (ROS) content. HL-60 cells untreated, or treated for the indicated times with 2 μM ATO, or 40 μM EA, or the combination were used to determine the intracellular H2O2 content by DCFH-DA. The peak shift to the right indicates increased levels of H2O2 content. (D) High catalase level blocks ATO/EA-0induced mitochondrial apoptotic pathway. HL-60 and its derivative, catalase over-expressing HP100-1 cells, untreated or treated with 2 μM ATO/40 μM or 60 μM EA for 24 h. The relative levels of PARP, catalase, caspase-3, -9, and β-actin were determined with Western blotting using specific antibodies.
ATO/EA combination decreases mitochondrial MTP, causes release of cytochrome c, and increases ROS
Following ATO/EA treatment, MTP in HL-60 cells was analyzed using FACS after staining with the cationic dye DOiC6 (3). The combination treatment produced a time-dependent reduction in MTP, which culminated after 18 h (Fig. 3A, left panel). When compared to ATO or EA alone, 12 h treatment with ATO/EA elicited a much more pronounced MTP reduction (Fig. 3A, right panel). The decrease in MTP was followed by a time-dependent release of cytochrome c from mitochondria into cytosol (Fig. 3B). We previously showed that ATO induces apoptosis in APL via a H2O2-dependent pathway (6) and others showed ROS involvement in EA-induced apoptosis (17). We measured the levels of H2O2 in HL-60 cells using a peroxide-sensitive fluorescent probe, DCFH-DA and showed (Fig. 3C, right panel) that ATO alone generated only a minimal increase in H2O2, while EA produced an increase in H2O2 content similar to that induced by the combination at 6 h of treatment. However the ATO/EA combination attained the maximal level of H2O2 already at 1 h (Fig. 3C, left panel). Thus, EA is a major contributor to the ROS generation. Moreover, the rapid kinetics (1 h) of ROS generation by the ATO/EA suggests that ROS is an early response to treatment followed by changes in anti-apoptotic protein and activation of caspases.
To further test the role of ROS in ATO/EA-induced apoptosis we used an H2O2-resistant subclone of HL-60 cells, HP100-1 (19). ATO (2μM) combined with EA (40 μM) induced apoptosis in ~40% of HL-60 cells but only in less than 10% of HP100-1 cells (Results not shown). Likewise, as compared to parental HL-60, PARP cleavage, and caspase-9 and -3 activation in HP100-1 cells were greatly reduced (Fig. 3D) and ROS production was not significantly elevated (Suppl. Fig. 3A). The HP100-1 cells have high catalase activity (27), a finding we confirmed in this cell line (Fig. 3D). Knockdown of catalase in HP100-1 cells produced an increase in cleaved PARP, caspase 9 and caspase 3 and reduced levels of Mcl-1, XIAP and survivin (Suppl. Fig. 3B). In addition we found that the antioxidant Trolox attenuated ATO/EA-induced apoptosis in HL-60 cells (Suppl. Fig. 3C). These data suggest that ROS play important role in ATO/EA-induced apoptosis.
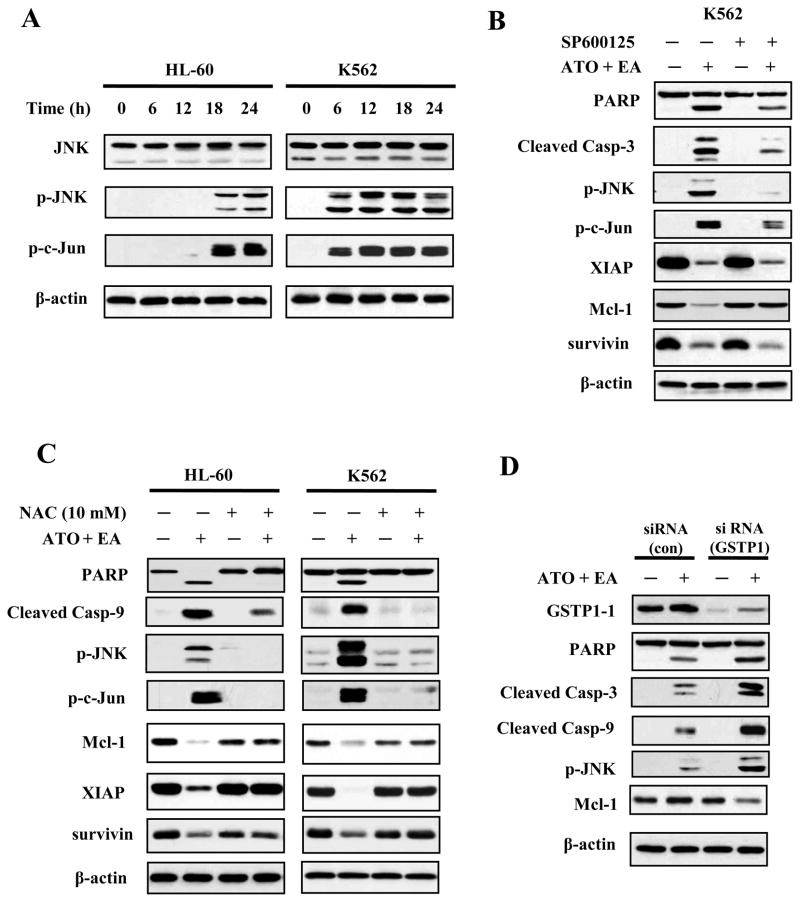
JNK activation is in the pathway of ATO/EA induced ROS generation
JNK is activated by ROS, both through activation of upstream kinases and through inactivation of a JNK-specific phosphatase (28). We found that ATO/EA induced a time-dependent increase in the level of p-JNK in both HL-60 and K562 cell lines, followed by an increase in phosphorylated c-Jun, its target (Fig. 4A). To examine whether JNK activation was an integral part of the ATO/EA induced apoptotic program, cells were pretreated with a JNK inhibitor SP600125 (29) prior to being treated with ATO/EA. As expected, SP600125 prevented the phosphorylation of JNK, reduced the phosphorylation of c-Jun (Fig. 4B) and suppressed by 50% ATO/EA-induced apoptosis (Suppl. Fig. 4A). Inhibition of JNK activity also blocked the cleavage of PARP and caspase-3 and restored the Mcl-1 level, but not the level of XIAP or survivin (Fig. 4B). To determine the pathways of Mcl-1 reduction by ATO/EA treatment, K562 cells were pretreated with the general caspase inhibitor Z-VAD-FMK and the proteasome inhibitor MG132 followed by ATO/EA treatment. Z-VAD-FMK blocked EA/ATO-induced PARP cleavage without preventing Mcl-1 reduction, suggesting the down-regulation of Mcl-1 is not mediated by caspase activation (Suppl. Fig. 4B). MG132 prevented ATO/EA-induced loss of Mcl-1 (Suppl. Fig. 4C).
Figure 4. JNK signaling is activated by ATO/EA treatment.
(A) Activation of JNK. HL-60 and K562 cells treated with ATO/EA combinations for the indicated times, were lysed and tested for levels of total JNK protein, p-JNK and p-c-Jun, a target of JNK, using specific antibodies in a Western blot analysis. β-actin was used as a loading control. The combinations of ATO/EA are: 2μM ATO and 40 μM EA for HL-60 cells, and 2 μM ATO and 80 μl EA for K562 cells. (B) JNK activation is crucial for ATO/EA induced apoptotic pathway. K562 cells pretreated with 40 μM JNK inhibitor (SP600125) followed by treatment with ATO/EA for 24 h were used to determine the levels of PARP, caspase-3, p-JNK, p-c-Jun, XIAP, Mcl-1, survivin, and β-actin using Western blotting. (C) Treatment with an anti-oxidant NAC inhibits the apoptotic pathway. HL-60 and K562 cells were pretreated with 10 mM NAC for 4 h, followed by ATO/EA treatment for 24 h. The levels of PARP, caspase-3, -9, p-JNK, p-c-Jun, XIAP, Mcl-1, surviving and β-actin were determined using Western blotting. (D) Knock-down of GSTP1-1 enhances the activation of caspases and JNK. K562 cells were transfected with GSTP1siRNA or control siRNA and after 18 h treated with 2 μM ATO/60 μM EA for additional 24 h. Protein levels of GSTP1-1, PARP, caspase-3, -9, p-JNK, Mcl-1 and β-actin were determined with Western blotting using specific antibodies.
GSTP1-1 has been shown to inhibit JNK activation through a protein-protein interaction (12, 13). We prepared lysates of K562 cells untreated, or treated with the ATO/EA combination, immunoprecipitated the lysates with an anti-JNK antibody and determined the amount of JNK-bound GSTP1-1. The treatment did not reduce the GSTP1-1/JNK association (data not shown) suggesting that this was not the mechanism of JNK activation. In contrast, pre-treatment of cells with NAC, an anti-oxidant and glutathione inducer, prevented both ATO/EA induced JNK phosphorylation (Fig. 4C) in both HL-60 and K562 cells and restored PARP and Mcl-1, proteins which are considered the hallmark of ATO/EA induced apoptosis, to nearly normal levels (Fig. 4C). Thus the state of JNK activation has a profound impact on the apoptotic pathway probably through affecting proteasomal degradation of Mcl-1.
GSTP1-1 and catalase are roadblocks to induction of apoptosis by ATO/EA in K562 cells
To test whether GSTP1-1 was responsible for treatment resistance, it was knocked down with siRNA and the cells were subjected to ATO/EA treatment. Western blot analysis confirmed that the GSTP1-1 knockdown was effective (Fig. 4D). Cells with reduced GSTP1-1 treated with ATO/EA produced much more ROS (data not shown). The expression or activation of other components of the apoptotic pathway, including p-JNK, was enhanced above that seen in K562 with intact GSTP1-1 treated with ATO/EA (Fig. 4D). Lowering GSTP1-1 levels increased apoptosis from 14% to 34% in K562 cells treated for 24 h with 2 μM ATO and 60 μM EA. Together these data suggest that high GSTP1-1 level, by preventing ROS accumulation, made the cells resistant to ATO/EA. Using an additional approach to confirm this conclusion we targeted catalase with siRNA and found that cells with reduced catalase behave in a way similar to that of K562 cells with knocked-down GSTP1-1, i.e., displaying enhanced apoptosis, increased levels of p-JNK, and decreased levels of Mcl-1 (Suppl. Fig. 5A and B).
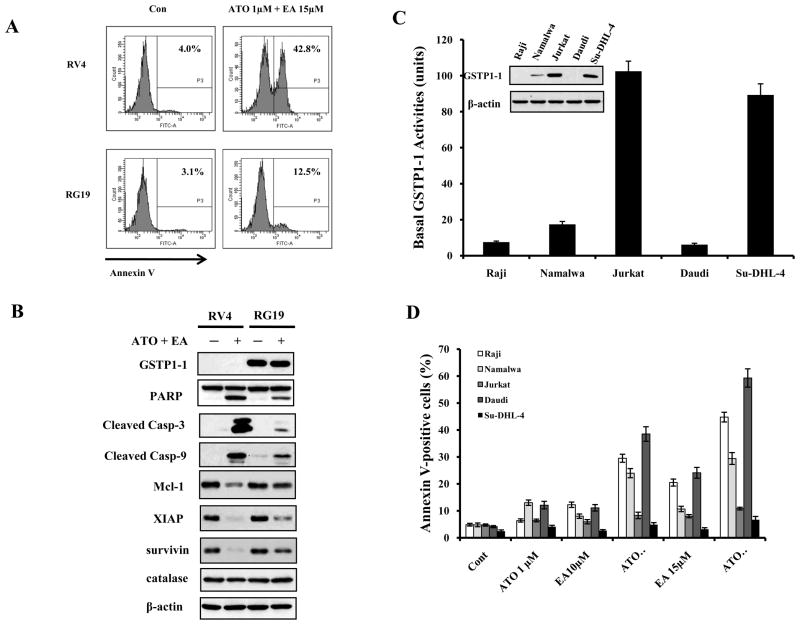
Absence of GSTP1-1expression in lymphoma cells defines a sub-group which is highly sensitive to ATO/EA induced apoptosis
We showed (Fig. 4D) that knockdown of GSTP1-1 in K562 cells profoundly increased sensitivity to ATO/EA induced apoptosis. We previously found that while Burkitt’s lymphoma (Raji) cell line, which does not express GSTP1-1, was relatively sensitive to ATO alone (14), forced expression of GSTP1-1 caused resistance to ATO (9). We tested whether GSTP1-1 is also a determinant of ATO/EA sensitivity in lymphoma cells by comparing a clone of Raji transfected to express GSTP1-1 (RG19) and a clone transfected with the vector alone (RV4). RV4 and RG19 cells were treated with ATO/EA and the apoptosis was quantified. Remarkably, a high degree of apoptosis (40%, Fig. 5A) was induced in the GSTP1-1 negative RV4 cells with 1 μM ATO and only 15 μM EA, a concentration 3–5 fold lower than needed to induce apoptosis in leukemia cells (Suppl. Fig. 1); only 12% of GSTP1-1 expressing, RG19 cells underwent apoptosis (Fig. 5A). Apoptosis was paralleled by a much stronger ROS production (data not shown) and Mcl-1 down-regulation in RV4 as compared to RG19 (Fig. 5B). These results opened a possibility that this combination might selectively induce apoptosis in B-cell lymphoma without GSTP1-1 expression. To test this possibility, we examined the protein level and activity of GSTP1-1 in four B cell lymphoma lines (Raji, Namalwa, Daudi and Su-DHL-4) and in one T cell lymphoma (Jurkat). Both Jurkat and Su-DHL-4 cells expressed higher levels of GSTP1-1 protein associated with higher GSTP1-1 activities (Fig. 5C); Namalwa cells had much lower levels than Jurkat cells, and Raji and Daudi cells had undetectable levels (Fig. 5C). Individually, ATO, or low concentration of EA (10μM), was ineffective in apoptosis induction, but a combination of 1 μM ATO with 10 μM EA induced strong apoptosis in the three GSTP1-1 low lymphoma cell lines, Raji, Daudi and Namalwa (Fig. 5D), but not in Jurkat and Su-DHL-4 cells (Fig. 5D). These results show that relatively low levels of ATO/EA can selectively induce apoptosis in malignant cells.
Figure 5. GSTP1-1 levels determine the sensitivity of lymphoma cells to ATO/EA treatment-induced apoptosis.
(A) RV4 lymphoma cells which lack GSTP1-1 expression are sensitive to ATO/EA induced apoptosis at low EA concentration. RV4 cells and RG19 (high GSTP1-1 expression) cells were treated with 1 μM ATO/15 μM EA for 24 h and the apoptotic cells were detected by FACS after staining with annexin V-FITC. (B) ATO/EA induced mitochondrial apoptosis pathway is muted in RG19 cells. RV4 and RG19 cells were treated with 1 μM ATO/15 μM EA for 24 h and the protein levels of the mitochondrial apoptotic pathways were analyzed by Western blot analysis. (C) Lymphoma cell lines differ widely in GSTP1-1protein and activity. The protein levels of GSTP1-1 were determined by Western blotting and the activity was determined biochemically. (D) Low GSTP1-1 activity lymphoma cells, Raji, Namalwaand Daudi, are highly sensitive to apoptosis induction by low concentrations of ATO/EA; Jurkat and Su-DHL-4 cells with high GSTP1-1activity are resistant. Raji, Namalwa, Daudi, Su-DHL-4 and Jurkat cells were treated with 1 μM ATO together with EA at the indicated concentrations for 24 h. Apoptotic cells were detected by FACS after staining with annexin V-FITC.
EA decreases intracellular levels of GSH which augments ATO-induced apoptosis in non-GSTP1-1 expression cells
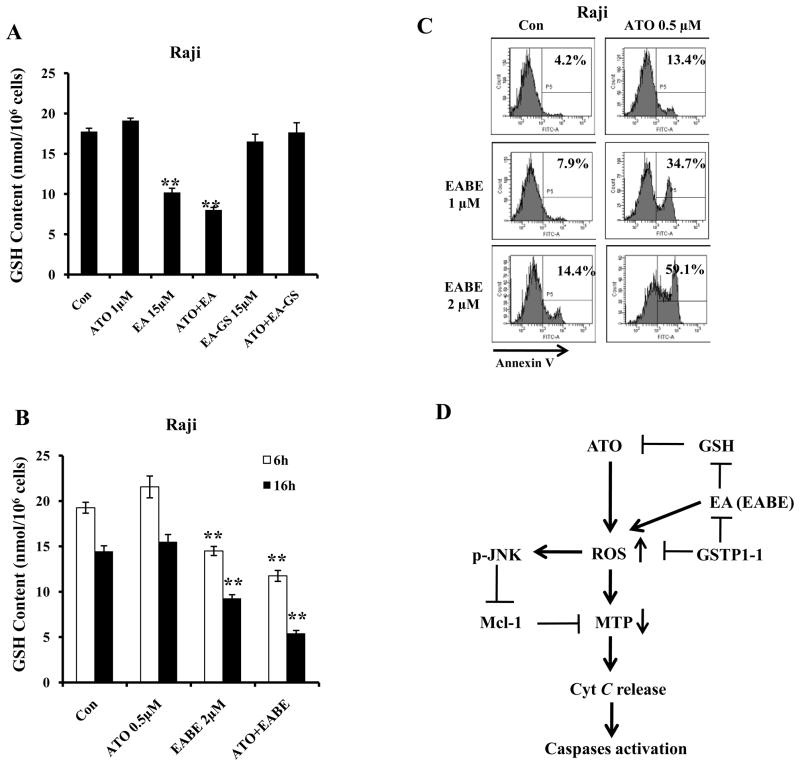
The observation that EA enhanced ATO-induced apoptosis only in non-GSTP1-1 expressing cells (Fig. 5D) suggested that EA acts through a pathway which is independent of GSTP1-1 inhibition. Previously we also found that the basal levels of GSH control cell sensitivity to ATO-induced apoptosis (5). Using the Raji and Jurkat cells with or without expression of GSTP1-1, we tested the intracellular levels of GSH after treatment with EA, ATO alone and their combination. As shown in Fig. 6A and Suppl. Fig. 6A, EA at 15 μM decreased the levels of GSH equally in both cell lines; ATO did not decrease the levels of GSH and the effect of EA/ATO on GSH was similar to that of EA alone. We synthesized GSH-conjugated EA, EA-GS. EA-GS neither decreased the levels of GSH nor enhanced ATO-induced apoptosis in either of the cell lines (Fig. 6A and Suppl. Fig. 6). EA-GS alone or in combination with ATO failed to increase the levels of ROS (data not shown). Previously we have modified EA structure and found that EABE was more effective than EA in apoptosis induction in leukemia cells (18). We tested the combined effects of ATO with EABE in Raji cells. EABE at low concentration of 1 μM and 2 μM decreased the levels of GSH (Fig. 6B) and augmented apoptosis induction by ATO even at as low as 0.5 μM (Fig. 6C). ATO plus EABE at the same concentrations did not induce apoptosis in Jurkat cells which express GSTP1-1 (data not shown). These data suggest that the EA or EABE-mediated reduction in GSH level plays an important role in increasing the ATO-induced apoptosis.
Figure 6. GSH level is crucial for synergistic apoptosis induction by a combination of ATO with EA and its derivatives in lymphoma cells without GSTP1-1 expression. (A) EA, but not EA-GS, decreases the levels of intracellular GSH.
Raji cell were treated with 1 μM ATO, 15 μM EA, 15 μM EA-GS for 6 h. (B) EABE decreases GSH levels in Raji cells. Raji cells were treated with 0.5 μM ATO, 2 μM EABE or their combination for 6 h and 16 h. The intracellular GSH levels were determined as described in Materials and Methods. (C) EABE enhances ATO apoptosis in Raji cells. Raji cells were treated with 0.5 μM ATO, 1 μM or 2 μM EABE or their combination for 3 days with; medium with fresh drug supplements was changed daily. The apoptotic cells were detected by FACS after staining with annexin V-FITC. (D) A working model of ATO/EA-induced apoptosis in lymphoma cells with or without GSTP1-1 expression. ATO or EA/EABE, can individually increase ROS through separate pathways. When combined, the two drugs strongly increase ROS causing reduction in MTP as well as activation of JNK. Active JNK participates in down-regulation of Mcl-1, leading to maximal cytochrome c release, activation of caspase-9 and induction of apoptosis. EA enhances ATO ability to produce ROS by decreasing intracellular GSH levels. GSTP1-1 detoxifies EA/EABE, ROS and ATO and blocks apoptosis induction. ATO combined with low concentrations of EA/EABE selectively induces apoptosis in B-cell lymphoma lacking GSTP1-1 expression.
Discussion
We found that the effective concentrations of EA required to enhance the pro-apoptotic activity of ATO are dependent on the GSTP1-1 level (Fig. 5C and D). To induce apoptosis in AML cells (Fig. 1 and Suppl. Fig. 7A), which have high GSTP1-1 protein (Suppl. Fig. 7B), required several fold greater EA concentration than that required for lymphoma cells, which have either lower, or entirely absent, GSTP1-1 activity. The observation that EA, with a known GSTP1-1 inhibitor, can synergistically enhance ATO-induced apoptosis in non-GSTP1-1 expressing lymphoma cells indicates that EA must have also a GSTP1-1 independent function. If this dependence of ATO/EA effect on GSTP1-1 levels found in cell lines will translate to patient samples, it will provide a novel approach to stratification of lymphoma patients and selection of those with low or absent GSTP1-1 as best candidates for ATO/EA combination therapy.
Several questions arise from these studies: a) what is the mechanism of ATO/EA induced apoptosis in GSTP1-1 expressing cells and what role GSTP1-1 plays in these processes? b) How does EA contribute to the synergistic enhancement of the pro-apoptotic effect of ATO? c) What is the GSTP1-1 independent role of EA in enhancing ATO-induced apoptosis in lymphoma cells without GSTP1-1 expression?
GSTP1-1 has been found to oppose ATO-induced apoptosis by detoxification of ATO and ROS (6, 9, 30) and by inhibition of JNK activity through a protein-protein interaction (12, 13). We found that GSTP1-1 knockdown in K562 cells enhanced ATO/EA induced ROS production, significantly increased apoptosis (data not shown), and substantially activated proteins of the pro-apoptotic pathway (Fig. 4D). Individually, ATO or EA has been shown to induce both the extrinsic and the intrinsic pathways of apoptosis (17, 18, 31) and ROS were shown to be involved (5, 6, 8, 17, 18), but the combination of ATO/EA on both pathways was not tested. Activation of the intrinsic pathway involves MTP, ROS accumulation and release of proteins, including cyctochrome c, which form the caspase activating complex, apoptosome(6). As shown in Fig. 3 we found that ATO/EA induced ROS accumulation represents the crucial and early step in apoptosis induction. Their increase precedes the changes in the pro-apoptotic protein levels. ROS did not accumulate and apoptosis was not induced by ATO/EA in HP100-1 cells expressing high level of catalase, a resistance that was reversed by knockdown of catalase (Fig. 3D and Suppl. Fig. 3B). Moreover, we found that the antioxidant Trolox blocked ATO/EA-induced apoptosis in HL-60 cells (Suppl. Fig. 3C). These data indicate that ROS play an important role in this combination-induced apoptosis. We found that ROS accumulation was followed by a decrease in MTP, cytochrome c release and activation of JNK. GSTP1-1 can bind JNK and inhibit its activity (12, 13), but ATO/EA treatment did not affect this interaction (data not shown), suggesting that ROS activation of JNK proceeds through a mechanism that is not due to the JNK dissociation from GSTP1-1. It has been found that JNK phosphorylates Mcl-1 and induces its degradation through proteasome (32). This event contributes to Mcl-1 down-regulation (33, 34). We found that Mcl-1 reduction by ATO/EA was blocked by proteasomal inhibitor MG132, but not by the general-caspase inhibitor Z-VAD-FMK (Suppl. Fig. 4 B and C). We show that ATO/EA activation of JNK is responsible for the decrease in Mcl-1 level, because inhibition of JNK with a specific inhibitor, SP600125, restores the Mcl-1 level (Fig. 4B) and blocks apoptosis (Suppl. Fig. 4A). Silencing of Mcl-1 sensitized K562 cells to EA/ATO-induced apoptosis (Fig. 2C) and increased levels of Mcl-1 in HL-60 cells attenuated ATO/EA-induced apoptosis (Fig. 2D). These data suggest that JNK activation-mediated Mcl-1 proteasomal degradation contributes to ATO/EA-induced apoptosis. In addition to decreasing Mcl-1, ATO/EA combination abolished XIAP expression and reduced the levels of survivin (Fig. 2A), 2 inhibitors of caspase 9 and 3 (35), but their knockdown did not sensitize cells to ATO/EA-induced apoptosis (Suppl. Fig. 2A, C). In cells with high catalase activity (HP100-1) ATO/EA decreased the levels of XIAP and survivin, but not of Mcl-1, and did not induce apoptosis (Suppl. Fig. 3B). JNK inhibition restored the Mcl-1 level, but neither XIAP nor survivin (Fig. 4B). Thus XIAP and survivin might aid in ATO/EA induced apoptosis, but the mechanism of their regulation by ATO/EA, unlike that of Mcl-1, does not require activation of JNK.
Our results showed that sensitivity of lymphoma cells to ATO/EA depends strictly on GSTP1-1 levels and that low concentration of EA in combination with ATO has the ability to produce more ROS and synergistically induce apoptosis in cells that do not have GSTP1-1, such as Raji, Namalwa and Daudi cells (Fig. 5). EA can interact directly with GSH and by forming EA-GS conjugate, reduce the level of GSH. This interaction could also be catalyzed by GSTP1-1 (36). We found that EA decreased GSH levels in both Raji and Jurkat cells suggesting that GSTP1-1 is not absolutely required for EA to interact with GSH (Fig. 6A and Suppl. Fig. 6A). The fact that EA-GS did not decrease GSH levels and did not enhance ATO-induced apoptosis suggests that the decrease in the levels of GSH by EA plays an important role in enhancing ATO-induced apoptosis. Therefore, GSTP1-1 is considered as an enzyme to detoxify EA. Our results revealed an intriguing insight into the mechanism of ATO/EA synergy in lymphoma cells that do not express GSTP1-1 (Fig. 6D). Previously several groups, including our own, showed that buthionine sulfoximine (BSO) decreased the levels of GSH and enhanced ATO induced apoptosis (37, 38), but in an unselective manner. We find that addition of BSO further enhances ATO/EA-induced apoptosis in AML cells (data not shown). Overall, these findings indicate that the GSTP1-1 blockade of EA/ATO effect resides in the ability of GSTP1-1 to detoxify ROS, EA and/or ATO. EA has been included in a clinical trial with thiotepa in advanced cancer patients (39). When given orally at a tolerable dose the peak plasma concentration of EA is only ~ 3 μM, while an i.v. injection of the same dose produces a peak plasma concentration about 30 μM. It is believed that high plasma concentrations can be maintained by i.v. infusion (40) making the EA enhanced ATO-therapy of B-cell lymphoma lacking GSTP1-1 a clinical reality. We have performed structure modifications of EA and found that EA esters had an increased ability to inhibit leukemia cell growth (41) and that one of these EA derivatives, EABE, was more rapidly taken up by leukemia cells and was a more active apoptosis inducer in leukemia cells (18). EABE at 1–2 μM in combination with ATO, even at 0.5 μM, induced apoptosis in Raji cells (Fig. 6C). Thus, EABE could be considered as an alternative enhancer of ATO-induced apoptosis in B-cell lymphoms that do not express GSTP1-1. Recently it has been found that, with the exception of mantle cell lymphoma, the majority of other B-cell lymphomas, belonging to different phenotypes, do have low levels, or entirely absent GSTP1-1 (42, 43). In Raji cells the GSTP1-1 is silenced due to GSTP1 promoter methylation (44). The GSTP1promoter methylation in lymphomas has been reported (45) and it appears that the gene is silenced in 55.5% of follicular lymphoma, 52.0% Burkitt’s lymphoma, and 50.0% of MALT lymphoma (46). Combined with the results of our studies, these findings indicate that patients whose cells lack GSTP1-1 expression or have methylated GSTP1promoter, can be selected to benefit from this novel ATO/EA combination therapy without suffering severe side effects.
Supplementary Material
Translational Relevance.
Arsenic trioxide (ATO) is effective for treatment of acute promyelocytic leukemia with minimal toxicity. Although B-cell lymphoma cell lines are sensitive to ATO-induced apoptosis in culture, the effect in patients is minimal. We found that a novel combination of ATO with low doses of ethacrynic acid (EA) and a more potent derivative, ethacrynic acid butyl-ester (EABE), can synergistically and selectively induce apoptosis in B-cell lymphoma cell lines lacking GSTP1-1 expression. Both EA and EABE decrease glutathione levels leading to enhanced ATO-induced ROS production and Mcl-1 reduction. This effect is blocked by forced expression of GSTP1-1. Since, with the exception of mantle cell lymphoma, the majority of B-cell lymphomas have low or undetectable levels of GSTP1-1, the combination of ATO with EA/EABE has the potential to be translated into a novel therapy for B-cell lymphoma patients stratified without GSTP1-1 expression.
Acknowledgments
We thank Dr. Liliana Ossowski for critical reading of this manuscript.
Grant Support: This work was supported by NIH grant R01-CA93533 to Y.J.
Footnotes
Conflict of interest disclosure:JG is one of named inventors for the formulation of arsenic trioxide and receives royalty payment for the use of arsenic trioxide in patients with acute promyelocytic leukemia.
References
- 1.Niu C, Yan H, Yu T, Sun HP, Liu JX, Li XS, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94:3315–24. [PubMed] [Google Scholar]
- 2.Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852–60. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- 3.Jing Y, Wang L, Xia L, Chen GQ, Chen Z, Miller WH, et al. Combined effect of all-trans retinoic acid and arsenic trioxide in acute promyelocytic leukemiacells in vitro and in vivo. Blood. 2001;97:264–9. doi: 10.1182/blood.v97.1.264. [DOI] [PubMed] [Google Scholar]
- 4.Chen GQ, Zhou L, Styblo M, Walton F, Jing Y, Weinberg R, et al. Methylated metabolites of arsenic trioxide are more potent than arsenic trioxide as apoptotic but not differentiation inducers in leukemia and lymphoma cells. Cancer Res. 2003;63:1853–9. [PubMed] [Google Scholar]
- 5.Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood. 1999;93:268–77. [PubMed] [Google Scholar]
- 6.Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102–11. [PubMed] [Google Scholar]
- 7.Shao W, Fanelli M, Ferrara FF, Riccioni R, Rosenauer A, Davison K, et al. Arsenic trioxide as an inducer of apoptosis and loss of PML/RAR alpha protein in acute promyelocytic leukemia cells. J Natl Cancer Inst. 1998;90:124–33. doi: 10.1093/jnci/90.2.124. [DOI] [PubMed] [Google Scholar]
- 8.Chou WC, Jie C, Kenedy AA, Jones RJ, Trush MA, Dang CV. Role of NADPH oxidase in arsenic-induced reactive oxygen species formation and cytotoxicity in myeloid leukemia cells. Proc Natl Acad Sci U S A. 2004;101:4578–83. doi: 10.1073/pnas.0306687101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou L, Jing Y, Styblo M, Chen Z, Waxman S. Glutathione-S-transferase pi inhibits As2O3-induced apoptosis in lymphoma cells: involvement of hydrogen peroxide catabolism. Blood. 2005;105:1198–203. doi: 10.1182/blood-2003-12-4299. [DOI] [PubMed] [Google Scholar]
- 10.Miller WH, Jr, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–903. [PubMed] [Google Scholar]
- 11.Wang Z, Dey S, Rosen BP, Rossman TG. Efflux-mediated resistance to arsenicals in arsenic-resistant and -hypersensitive Chinese hamster cells. Toxicol Appl Pharmacol. 1996;137:112–9. doi: 10.1006/taap.1996.0062. [DOI] [PubMed] [Google Scholar]
- 12.Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, et al. Regulation of JNK signaling byGSTp. EMBO J. 1999;18:1321–34. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang T, Arifoglu P, Ronai Z, Tew KD. Glutathione S-transferase P1-1 (GSTP1-1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J Biol Chem. 2001;276:20999–1003. doi: 10.1074/jbc.M101355200. [DOI] [PubMed] [Google Scholar]
- 14.Zhu XH, Shen YL, Jing YK, Cai X, Jia PM, Huang Y, et al. Apoptosis and growth inhibition in malignant lymphocytes after treatment with arsenic trioxide at clinically achievable concentrations. J Natl Cancer Inst. 1999;91:772–8. doi: 10.1093/jnci/91.9.772. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Ohnishi K, Shigeno K, Fujisawa S, Naito K, Nakamura S, et al. The induction of apoptosis and cell cycle arrest by arsenic trioxide in lymphoid neoplasms. Leukemia. 1998;12:1383–91. doi: 10.1038/sj.leu.2401112. [DOI] [PubMed] [Google Scholar]
- 16.Ploemen JH, van Ommen B, van Bladeren PJ. Inhibition of rat and human glutathione S-transferase isoenzymes by ethacrynic acid and its glutathione conjugate. Biochem Pharmacol. 1990;40:1631–5. doi: 10.1016/0006-2952(90)90465-w. [DOI] [PubMed] [Google Scholar]
- 17.Aizawa S, Ookawa K, Kudo T, Asano J, Hayakari M, Tsuchida S. Characterization of cell death induced by ethacrynic acid in a human colon cancer cell line DLD-1 and suppression by N-acetyl-L-cysteine. Cancer Sci. 2003;94:886–93. doi: 10.1111/j.1349-7006.2003.tb01371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang R, Li C, Song D, Zhao G, Zhao L, Jing Y. Ethacrynic acid butyl-ester induces apoptosis in leukemia cells through a hydrogen peroxide mediated pathway independent of glutathione S-transferase P1-1 inhibition. Cancer Res. 2007;67:7856–64. doi: 10.1158/0008-5472.CAN-07-0151. [DOI] [PubMed] [Google Scholar]
- 19.Yamada M, Hashinaka K, Inazawa J, Abe T. Expression of catalase and myeloperoxidase genes in hydrogen peroxide-resistant HL-60 cells. DNA Cell Biol. 1991;10:735–42. doi: 10.1089/dna.1991.10.735. [DOI] [PubMed] [Google Scholar]
- 20.Polo JM, Juszczynski P, Monti S, Cerchietti L, Ye K, Greally JM, et al. Transcriptional signature with differential expression of BCL6 target genes accurately identifies BCL6-dependent diffuse large B cell lymphomas. Proc Natl Acad Sci U S A. 2007;104:3207–12. doi: 10.1073/pnas.0611399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–81. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 22.Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, et al. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med. 1995;182:367–77. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang R, Xia L, Gabrilove J, Waxman S, Jing Y. Downregulation of Mcl-1 through GSK-3beta activation contributes to arsenic trioxide-induced apoptosis in acute myeloid leukemia cells. Leukemia. 2012 doi: 10.1038/leu.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horita M, Andreu EJ, Benito A, Arbona C, Sanz C, Benet I, et al. Blockade of the Bcr-Abl kinase activity induces apoptosis of chronic myelogenous leukemia cells by suppressing signal transducer and activator of transcription 5-dependent expression of Bcl-xL. J Exp Med. 2000;191:977–84. doi: 10.1084/jem.191.6.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q, Zhang H, Smeester L, Zou F, Kesic M, Jaspers I, et al. The NRF2-mediated oxidative stress response pathway is associated with tumor cell resistance to arsenic trioxide across the NCI-60 panel. BMC Med Genomics. 2010;3:37. doi: 10.1186/1755-8794-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morales AA, Gutman D, Cejas PJ, Lee KP, Boise LH. Reactive oxygen species are not required for an arsenic trioxide-induced antioxidant response or apoptosis. J Biol Chem. 2009;284:12886–95. doi: 10.1074/jbc.M806546200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasugai I, Yamada M. High production of catalase in hydrogen peroxide-resistant human leukemia HL-60 cell lines. Leuk Res. 1992;16:173–9. doi: 10.1016/0145-2126(92)90129-u. [DOI] [PubMed] [Google Scholar]
- 28.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–61. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 29.Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–6. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernardini S, Nuccetelli M, Noguera NI, Bellincampi L, Lunghi P, Bonati A, et al. Role of GSTP1-1 in mediating the effect of As2O3 in the Acute Promyelocytic Leukemia cell line NB4. Ann Hematol. 2006;85:681–7. doi: 10.1007/s00277-006-0139-8. [DOI] [PubMed] [Google Scholar]
- 31.Mann KK, Colombo M, Miller WH., Jr Arsenic trioxide decreases AKT protein in a caspase-dependent manner. Mol Cancer Ther. 2008;7:1680–7. doi: 10.1158/1535-7163.MCT-07-2164. [DOI] [PubMed] [Google Scholar]
- 32.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrialouter membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–60. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez-Perez T, Ortiz-Ferron G, Lopez-Rivas A. Mitotic arrest and JNK-induced proteasomal degradation of FLIP and Mcl-1 are key events in the sensitization of breast tumor cells to TRAIL by antimicrotubule agents. Cell Death Differ. 2010;17:883–94. doi: 10.1038/cdd.2009.176. [DOI] [PubMed] [Google Scholar]
- 34.Inoshita S, Takeda K, Hatai T, Terada Y, Sano M, Hata J, et al. Phosphorylation and inactivation of myeloid cell leukemia 1 by JNK in response to oxidative stress. J Biol Chem. 2002;277:43730–4. doi: 10.1074/jbc.M207951200. [DOI] [PubMed] [Google Scholar]
- 35.Schimmer AD, Dalili S. Targeting the IAP family of caspase inhibitors as an emerging therapeutic strategy. Hematology Am Soc Hematol Educ Program. 2005:215–9. doi: 10.1182/asheducation-2005.1.215. [DOI] [PubMed] [Google Scholar]
- 36.Awasthi S, Srivastava SK, Ahmad F, Ahmad H, Ansari GA. Interactions of glutathione S-transferase-pi with ethacrynic acid and its glutathione conjugate. Biochim Biophys Acta. 1993;1164:173–8. doi: 10.1016/0167-4838(93)90245-m. [DOI] [PubMed] [Google Scholar]
- 37.Davison K, Cote S, Mader S, Miller WH. Glutathione depletion overcomes resistance to arsenic trioxide in arsenic-resistant cell lines. Leukemia. 2003;17:931–40. doi: 10.1038/sj.leu.2402876. [DOI] [PubMed] [Google Scholar]
- 38.Chen D, Chan R, Waxman S, Jing Y. Buthionine sulfoximine enhancement of arsenic trioxide-induced apoptosis in leukemia and lymphoma cells is mediated via activation of c-Jun NH2-terminal kinase and up-regulation of death receptors. Cancer Res. 2006;66:11416–23. doi: 10.1158/0008-5472.CAN-06-0409. [DOI] [PubMed] [Google Scholar]
- 39.O’Dwyer PJ, LaCreta F, Nash S, Tinsley PW, Schilder R, Clapper ML, et al. Phase I study of thiotepa in combination with the glutathione transferase inhibitor ethacrynic acid. Cancer Res. 1991;51:6059–65. [PubMed] [Google Scholar]
- 40.Lacreta FP, Brennan JM, Nash SL, Comis RL, Tew KD, O’Dwyer PJ. Pharmakokinetics and bioavailability study of ethacrynic acid as a modulator of drug resistance in patients with cancer. J Pharmacol Exp Ther. 1994;270:1186–91. [PubMed] [Google Scholar]
- 41.Zhao G, Yu T, Wang R, Wang X, Jing Y. Synthesis and structure-activity relationship of ethacrynic acid analogues on glutathione-s-transferase P1-1 activity inhibition. Bioorg Med Chem. 2005;13:4056–62. doi: 10.1016/j.bmc.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 42.Thieblemont C, Rolland D, Baseggio L, Felman P, Gazzo S, Callet-Bauchu E, et al. Comprehensive analysis of GST-pi expression in B-cell lymphomas: Correlation with histological subtypes and survival. Leuk Lymphoma. 2008;49:1403–6. doi: 10.1080/10428190802094245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bennaceur-Griscelli A, Bosq J, Koscielny S, Lefrere F, Turhan A, Brousse N, et al. High level of glutathione-S-transferase pi expression in mantle cell lymphomas. Clin Cancer Res. 2004;10:3029–34. doi: 10.1158/1078-0432.ccr-03-0554. [DOI] [PubMed] [Google Scholar]
- 44.Borde-Chiche P, Diederich M, Morceau F, Puga A, Wellman M, Dicato M. Regulation of transcription of the glutathione S-transferase P1 gene by methylation of the minimal promoter in human leukemia cells. Biochem Pharmacol. 2001;61:605–12. doi: 10.1016/s0006-2952(00)00581-5. [DOI] [PubMed] [Google Scholar]
- 45.Esteller M, Corn PG, Urena JM, Gabrielson E, Baylin SB, Herman JG. Inactivation of glutathione S-transferase P1 gene by promoter hypermethylation in human neoplasia. Cancer Res. 1998;58:4515–8. [PubMed] [Google Scholar]
- 46.Rossi D, Capello D, Gloghini A, Franceschetti S, Paulli M, Bhatia K, et al. Aberrant promoter methylation of multiple genes throughout the clinico-pathologic spectrum of B-cell neoplasia. Haematologica. 2004;89:154–64. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.