Abstract
Childhood autologous hematopoietic cell transplant (AHCT) survivors can be at risk for secondary malignant neoplasms (SMNs). We assembled a cohort of 1,487 pediatric AHCT recipients to investigate the incidence and risk factors for SMNs. Primary diagnoses included neuroblastoma (39%), lymphoma (26%), sarcoma (18%), CNS tumors (14%), and Wilms tumor (2%). Median follow-up was 8 years (range, <1–21 years). SMNs were reported in 35 patients (AML/MDS=13, solid cancers=20, subtype missing=2). The overall cumulative incidence of SMNs at 10 years from AHCT was 2.60% (AML/MDS=1.06%, solid tumors=1.30%). We found no association between SMNs risk and age, gender, diagnosis, disease status, time since diagnosis, or use of total body irradiation or etoposide as part of conditioning. Overall survival at 5-years from diagnosis of SMNs was 33% (95% CI, 16–52%). When compared to age- and gender-matched general population, AHCT recipients had 24 times higher risks of developing SMNs (95% CI, 16.0–33.0). Notable SMN sites included bone (N=5 SMNs, observed (O)/expected (E)=81), thyroid (N=5, O/E=53), breast (n=2, O/E=93), soft tissue (N=2, O/E=34), AML (N=6, O/E=266), and MDS (N=7, O/E=6603). Risks of SMNs increased with longer follow-up from AHCT. Pediatric AHCT recipients are at considerably increased risk for SMNs and need life-long surveillance for SMNs.
Keywords: Hematopoietic cell transplantation, Autologous, Pediatric, Second Cancers, Risk factors
INTRODUCTION
Autologous hematopoietic-cell transplantation (AHCT) is a well-established treatment option for some pediatric patients with aggressive malignancies, including neuroblastoma(1–3), brain tumors(4), Hodgkin’s disease(5), and certain sarcomas.(6) The long-term toxicities, especially the incidence and risk factors for second malignant neoplasms (SMNs), have not been well characterized in this population.
The risk of developing SMNs is higher in childhood cancer survivors than in the general population.(7, 8) A recent study of the Childhood Cancer Survivor Study (CCSS) cohort (n=14,358 childhood cancer survivors) showed increased risk of SMNs among all primary childhood cancer diagnoses.(8) When compared to the general population, the overall standardized incidence ratio of developing SMNs was 6.4 with an estimated 30-year cumulative incidence of 9.3%. The use of high dose chemotherapy to eradicate disease in these aggressive pediatric malignancies, specifically alkylating agents, anthracyclines, and epipodophyllotoxins increased the risk of SMNs. Radiation has also been shown to increase the risk of SMNs. Revelations of the long-term effects of radiation therapy began to emerge in the 1970–1980’s with development of SMNs in known radiation fields.(9) It has been shown that there is a close relationship between the risk of SMNs and radiation dose in childhood cancer survivors.(8) Central nervous system (CNS) SMNs, specifically subsequent gliomas and meningiomas, have been associated with prior radiation therapy.(10, 11)
Allogeneic hematopoietic stem cell transplantation also increases the risk for SMNs in children.(12–15) In one of the largest studies to date, the Center for International Blood and Marrow Transplant Research (CIBMTR) assembled a large multi-institutional cohort of allogeneic transplant recipients with a median age of 27 years (58% of the cohort was <30 years of age). New solid malignancies occurred at twice the rate of expected general population rates, with risk increasing over time.(15) Furthermore, exposure to radiation was specifically associated with risks of SMNs in pediatric transplant recipients.
In comparison to childhood cancer survivors in general and pediatric recipients of allogeneic HCT, the incidence, characteristics, and risks of SMNs after AHCT in children have not been well described. We present a study of the incidence and risk factors for SMNs among pediatric AHCT survivors using a large cohort from the CIBMTR with extended followup.
PATIENTS AND METHODS
Data Sources
The CIBMTR, is a research affiliation of the International Bone Marrow Transplant Registry (IBMTR), Autologous Blood and Marrow Transplant Registry (ABMTR), and the National Marrow Donor Program (NMDP). The CIBMTR comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic cell transplantation to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively. Patients are followed longitudinally. Computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected Health Information used in such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the HIPAA Privacy Rule.
Patients
Our study analyzed a cohort of patients who underwent AHCT from 1987 to 2003 in transplant centers located in the United States or Canada and for whom CRF-level data were reported to the CIBMTR. Patients were included in this study if they were younger than 21 years of age at time of AHCT. The cohort was restricted to survivors of first AHCT for lymphoma or solid tumors (neuroblastoma, CNS tumors, rhabdoid tumors, Ewing’s sarcoma, primitive neuro-ectodermal tumors, Wilms tumor, or other sarcomas).
Overall, 1,626 patients met the study eligibility criteria. Since follow-up information regarding long-term survival and secondary malignancies was required, patients from centers with a follow-up completeness index (ratio of total observed to potential person-time of follow-up) of <80% at 5 years after transplantation were excluded (139 patients from 21 centers).(16) The final study population consisted of 1,487 patients from 111 centers.
Pathology and physician reports of second cancers were reviewed centrally, and if necessary, tumors were reclassified.
Statistical Analysis
The objectives of this study were to determine the incidence of SMNs in pediatric AHCT survivors, to evaluate risk-factors for SMNs in this population and to compare the risks of SMNs with an age- and gender-matched general population. The cumulative probability of a new cancer was estimated by the cumulative incidence function that accounted for the competing risk of death among patients who did not develop a second malignancy.(17) Univariate probabilities of overall survival were calculated by the Kaplan-Meier estimator.(18) Among patients who had received more than one AHCT, follow-up and time to event were considered from the first transplant. Thirty-nine patients received an allogeneic transplant after their first AHCT; follow-up time for these patients was censored at the time of their allogeneic transplant.
Potential risk factors for second cancers were analyzed with the use of Cox regression models.(19) Variables considered included age at AHCT, gender, diagnosis (lymphoma, sarcoma, CNS tumor, Wilms tumor and neuroblastoma), remission status at AHCT (complete remission and not in complete remission), time from diagnosis to AHCT (<6 months, 6–12 months and >12 months), use of TBI, use of etoposide as part of conditioning, use of any irradiation pre- or post-transplantation, number of transplants (1 and >1), and year of transplantation. Factors were tested for their association with development of SMNs by means of backward selection of variables. Multivariate analyses were also conducted using Poisson regression to compare risks of solid cancers for various subgroups of HCT recipients.(20, 21) Both Cox and Poisson regression analyses yielded similar results. Analyses for cumulative incidence and risk factors were conducted for the whole cohort as well as separately for secondary acute myeloid leukemia/myelodysplastic syndromes (AML/MDS) and secondary solid tumors (including new lymphomas).
We also evaluated the excess risk of SMNs compared to the general population using methods described previously.(13–15) Briefly, for each transplant recipient, the number of person-years at risk was calculated from the date of transplantation until the date of last contact, death, or diagnosis of a new cancer, whichever occurred first. Age-, sex-, and country-specific incidence rates for all SMNs combined and for cancers at specific anatomical sites were applied to the appropriate person-years at risk to compute the expected numbers of cancers. Incidence rates for all cancers were obtained from national registries.(22, 23) Observed-to-expected (O/E) ratios, also called standardized incidence ratios (SIRs), were calculated, and Poisson distribution was used to calculate 95% confidence intervals (CI).(20)
All P-values are two-sided. All analyses were carried out using SAS statistical software (SAS Institute Inc., Cary, NC, USA).
RESULTS
Patient Characteristics
Among the 1,487 patients included in our study, the median age at AHCT was 8 years (range, <1–21 years). Primary diagnoses were neuroblastoma (39%), lymphoma (26%), sarcoma (18%), CNS tumors (14%), and Wilms tumor (2%). TBI was part of the conditioning regimen in 19% of patients, 3% received other irradiation (e.g., total lymphoid irradiation or thoraco-abdominal irradiation) as part of conditioning, and 68% received etoposide. Fifteen percent of patients (N=227) had received more than one transplant; among these, 39 had received a subsequent allogeneic transplant. Median follow-up of survivors was 8 years (range, <1–21 years) from transplantation. Overall, 80% of patients had received chemotherapy prior to AHCT. Overall survival was 43% (95% CI, 40–45) at 5 years and 36% (33–39) at 10 years after AHCT.
As expected, there were differences in patient demographics based on the underlying disease (Table 1). Patients with lymphoma and sarcoma were more likely to be older at AHCT (median age 17 and 15 years, respectively) compared to patients with CNS tumors, Wilms tumor, and neuroblastoma (median age 7, 8, and 4 years, respectively). Similarly, the pre-AHCT exposures (e.g., pre-transplant chemotherapy, number of chemotherapy regimens, and pre-transplant radiation therapy) varied for each disease. Patients with lymphoma, sarcoma, and neuroblastoma were more likely to have received TBI as part of conditioning regimen.
Table 1.
Patient, disease, and transplant characteristics by diagnosis
| Variable | Lymphoma | Sarcoma | CNS tumor | Wilms tumor | Neuroblastoma |
|---|---|---|---|---|---|
| Number of patients | 394 | 271 | 207 | 31 | 584 |
| Number of centers | 84 | 57 | 43 | 18 | 59 |
| Median age at transplant, years (range) | 17 (3–21) | 15 (1–21) | 7 (1–20) | 8 (2–21) | 4 (<1–21) |
| Age at transplant, years | |||||
| <5 yrs | 12 ( 3) | 25 ( 9) | 73 (35) | 10 (32) | 419 (72) |
| 6–10 yrs | 26 ( 7) | 40 (15) | 53 (26) | 14 (45) | 138 (24) |
| 11–15 yrs | 72 (18) | 74 (27) | 54 (26) | 3 (10) | 18 ( 3) |
| 16–21 yrs | 284 (72) | 132 (49) | 27 (13) | 4 (13) | 9 ( 2) |
| Recipient gender | |||||
| Male | 232 (59) | 173 (64) | 135 (65) | 9 (29) | 346 (59) |
| Female | 162 (41) | 98 (36) | 72 (35) | 22 (71) | 238 (41) |
| Disease status prior to transplant | |||||
| Lymphoma, CR | 154 (42) | -- | -- | -- | -- |
| Lymphoma, relapsed/refractory | 215 (58) | -- | -- | -- | -- |
| Solid tumor, CR/VGPR | -- | 153 (58) | 123 (67) | 18 (60) | 373 (65) |
| Solid tumor, PR/SD/PD | -- | 111 (42) | 60 (33) | 12 (40) | 200 (35) |
| Missing | 25 | 7 | 24 | 1 | 11 |
| Pre-transplant chemotherapy | |||||
| No | 0 | 1 (<1) | 18 (29) | 0 | 6 (1) |
| Yes | 353 | 232 (100) | 44 (71) | 0 | 511 (99) |
| Missing | 41 | 38 | 145 | 31 | 67 |
| Median number of chemotherapy cycles (range) | 7 (1–21) | 6 (1–44) | 4 (1–8) | - | 5 (1–34) |
| Pre-transplant radiation therapy | |||||
| No | 79 (34) | 94 (41) | 42 (66) | 0 | 287 (56) |
| Yes | 153 (66) | 135 (59) | 22 (34) | 0 | 229 (44) |
| Missing | 162 | 42 | 143 | 31 | 68 |
| Median time from diagnosis to transplant, months (range) | 15 (2–157) | 9 (3–226) | 9 (<1–165) | 15 (2–108) | 7 (2–88) |
| Time from diagnosis to transplant, months | |||||
| <6 | 44 (11) | 71 (26) | 78 (38) | 2 ( 6) | 141 (24) |
| 6–12 | 110 (28) | 94 (35) | 43 (21) | 7 (23) | 355 (61) |
| >12 | 240 (61) | 106 (39) | 86 (42) | 22 (71) | 88 (15) |
| Irradiation (TBI or other radiation) as part of conditioning regimen | |||||
| No | 296 (75) | 203 (75) | 204 (99) | 30 (97) | 421 (72) |
| Yes | 98 (25) | 68 (25) | 3 ( 1) | 1 ( 3) | 163 (28) |
| Median TBI dose, cGy (range) | 1200 (500– 1440) | 1200 (800– 1500) | -- | -- | 1200 (982–2520) |
| TBI dose, cGy | |||||
| No TBI | 303 (77) | 206 (77) | 206 (100) | 31 (100) | 454 (78) |
| <1200 | 14 ( 4) | 2 ( 1) | 0 | 0 | 61 (10) |
| ≥ 1200 | 76 (19) | 61 (23) | 0 | 0 | 68 (12) |
| Missing | 1 | 2 | 1 | 0 | 1 |
| Etoposide as part of conditioning regimen | |||||
| No | 79 (20) | 96 (35) | 131 (63) | 9 (29) | 156 (27) |
| Yes | 315 (80) | 175 (65) | 76 (37) | 22 (71) | 428 (73) |
| Graft type | |||||
| Bone marrow | 186 (47) | 48 (18) | 69 (33) | 5 (16) | 287 (49) |
| Peripheral blood | 208 (53) | 223 (82) | 138 (67) | 26 (84) | 297 (51) |
| Planned post-transplant irradiation | |||||
| No | 316 (83) | 205 (85) | 82 (95) | 3 | 351 (66) |
| Yes | 67 (17) | 35 (15) | 4 ( 5) | 0 | 181 (34) |
| Missing | 11 | 31 | 121 | 28 | 52 |
| Year of transplant | |||||
| 1987–1990 | 59 (15) | 8 ( 3) | 7 ( 3) | 0 | 44 ( 8) |
| 1991–1995 | 203 (52) | 61 (23) | 68 (33) | 11 (35) | 215 (37) |
| 1996–2000 | 114 (29) | 179 (66) | 88 (43) | 18 (58) | 253 (43) |
| 2001–2003 | 18 ( 5) | 23 ( 8) | 44 (21) | 2 ( 6) | 72 (12) |
| Number of transplants | |||||
| 1 | 359 (91) | 230 (85) | 150 (72) | 31 | 490 (84) |
| 2 | 32 ( 8) | 27 (10) | 17 ( 8) | 0 | 74 (13) |
| ≥ 3 | 3 ( 1) | 14 ( 5) | 40 (19) | 0 | 20 ( 3) |
Abbreviations: CR – complete remission; VGPR – very good partial remission; PR – partial remission; SD – stable disease; PD – progressive disease; TBI – total body irradiation; CNS – central nervous system
Incidence and Risk-factors for SMNs
SMNs were reported in 35 patients. Confirmatory pathology reports were available for 33 patients; for 2 patients, the transplant center confirmed that a SMN had occurred, but the pathology report was not provided for review. The two patients were included in the descriptive analyses but excluded from the multivariate analyses.
Thirteen patients had secondary AML/MDS (AML=6, MDS=7), and 20 had a secondary solid tumor. Secondary solid cancers included cancers of the bone (N=5), thyroid (N=5), breast (N=2), soft tissue (N=2), brain (N=1), head and neck (N=1), testis (N=1), and uterine cervix (N=1); 1 patient had Kaposi’s sarcoma, and 1 patient had 2 separate SMNs (soft tissue sarcoma and hepatocellular carcinoma). The median time from AHCT to occurrence of SMNs was 6.3 years (range, 4.5 months–20.4 years). The median time from AHCT to occurrence of AML/MDS was 2 years (range, 5 months–10 years) and for solid tumors was 7 years (range, 4.5 months–20.4 years).
The 5-year and 10-year cumulative incidence of all SMNs was 1.04% (95% CI, 0.58–1.63%) and 2.60% (95% CI, 1.69–3.70%). The corresponding cumulative incidence of AML/MDS was 0.56% (95% CI, 0.24–1.01%) and 1.06% (95% CI, 0.52–1.77%) and for solid tumors was 0.41% (95% CI, 0.15–0.81%) and 1.30% (95% CI, 0.69–2.10%), respectively (Figure 1).
Figure 1.
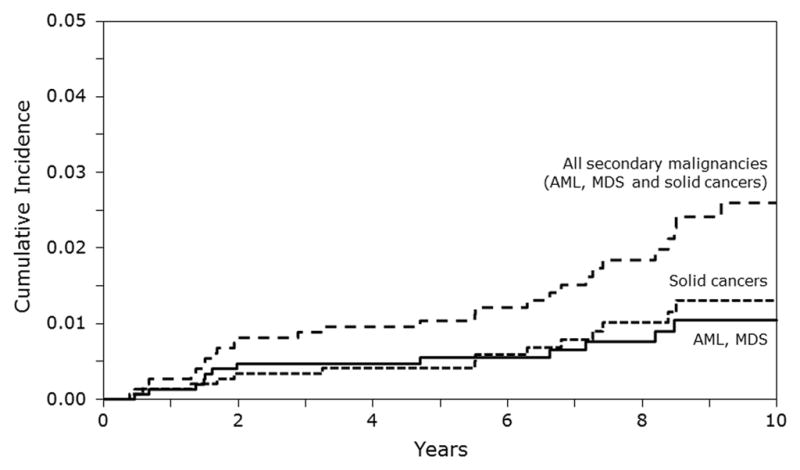
In multivariate risk-factor analysis, we did not find any association between risks of SMNs and age, gender, diagnosis, remission status at AHCT, time from diagnosis to AHCT, use of TBI, use of etoposide as part of conditioning, use of any irradiation, number of transplants, and year of transplantation. Although the number of specific SMNs was small, we also investigated whether these risk factors were associated with risks of cancer of the bone, thyroid, or AML/MDS. None of these factors significantly increased risks of secondary cancers at these specific sites.
Exposures among Patients with SMNs
Tables 2 and Supplemental Table 1 describe characteristics of patients with and without SMNs.
Table 2.
Selected characteristics of patients without secondary malignant neoplasm, with – secondary AML/MDS and with secondary solid cancers
| Variable | No SMN | Secondary AML/MDS | Secondary solid cancers |
|---|---|---|---|
| N | 1452 | 13 | 20 |
| Age, years | |||
| <10 | 791 (54) | 6 (46) | 13 (65) |
| 11–21 | 661 (46) | 7 (54) | 7 (35) |
| Disease | |||
| Non-Hodgkin lymphoma | 177 (12) | 1 ( 8) | 2 (10) |
| Hodgkin lymphoma | 204 (14) | 5 (38) | 3 (15) |
| Sarcoma | 263 (18) | 4 (31) | 4 (20) |
| CNS tumor | 204 (14) | 1 ( 8) | 2 (10) |
| Wilms tumor | 30 ( 2) | 0 | 1 ( 5) |
| Neuroblastoma | 574 (40) | 2 (15) | 8 (40) |
| Irradiation as part of conditioning regimen | |||
| No | 1128 (78) | 10 (77) | 13 (65) |
| Yes | 324 (22) | 3 (23) | 7 (35) |
| Pre-transplant radiation therapy | |||
| No | 486 (48) | 5 (45) | 9 (53) |
| Yes | 525 (52) | 6 (55) | 8 (47) |
| Missing | 441 | 2 | 3 |
| Etoposide as part of conditioning regimen | |||
| No | 459 (32) | 2 (15) | 10 (50) |
| Yes | 993 (68) | 11 (85) | 10 (50) |
| Planned post-transplant irradiation | |||
| No | 929 (77) | 10 (83) | 17 (94) |
| Yes | 284 (23) | 2 (16) | 1 ( 6) |
| Missing | 239 | 1 | 2 |
Abbreviations: SMN – secondary malignant neoplasm, AML/MDS – acute myeloid leukemia/myelodysplastic syndrome; CNS – central nervous system
Although the numbers were small, we also looked at treatment exposures among patients with specific SMNs. Among the 5 patients with secondary cancers of the bone, indications for AHCT were neuroblastoma (N=2), Hodgkin lymphoma (N=1), sarcoma (N=1), and CNS tumor (N=1). SMN of the bone occurred at a median of 5.5 years (range, 1.3–8.4 years) after AHCT. Out of these 5 patients, 2 had pre-transplant radiation exposure: 1 patient with secondary osteosarcoma of the fibula received radiation to the tumor bed after resection of neuroblastoma followed by TBI as part of AHCT conditioning, and 1 patient with secondary osteosarcoma of the femur received mantle field irradiation for the treatment of Hodgkin lymphoma. Secondary thyroid cancer was reported in 5 patients at a median of 6.8 years (range, 1.9–12.5 years) after AHCT; among these, indications for AHCT were neuroblastoma (N=2), Hodgkin lymphoma (N=1), sarcoma (N=1) and Wilms tumor (N=1). Radiation exposure to the thyroid region was documented in 3 patients (1 prior to AHCT, 1 received TBI and 1 received TBI plus post HCT irradiation at primary tumor bed). Of the 2 patients with breast cancer, 1 patient had non-Hodgkin lymphoma and had received pre-transplant irradiation and TBI as part of conditioning, and 1 patient had Hodgkin lymphoma and received mantle field irradiation pre-transplantation. Of the 2 patients with secondary cancer of the soft tissue, 1 patient received TBI as part of AHCT conditioning, and 1 patient had no radiation exposures.
Among the 13 patients with secondary AML/MDS, indications for AHCT were Hodgkin lymphoma (N=5), sarcoma (N=4), neuroblastoma (N=2), non-Hodgkin lymphoma (N=1), and CNS tumor (N=1). Only 3/13 (23%) patients had received TBI, while 11/13 (65%) had received etoposide as part of AHCT conditioning regimen. As noted above, the median time to occurrence of AML/MDS after AHCT was 2 years (range, 5 months-10 years).
Five patients with SMNs received more than one AHCT. Among these, 3 patients developed secondary AML/MDS, and 2 patients had solid cancers (bone and testis each).
Outcomes of Patients with SMNs
Overall survival at 2 years and 5 years from the time of diagnosis of all SMNs (N=35) was 65% (95% CI, 48–91%) and 33% (95% CI, 46–52%), respectively. Among patients with secondary solid tumors, 2-year and 5-year survival from time of diagnosis of SMNs was 69% (44–89) and 41% (16–70), respectively. Survival at 2 years and 5 years after diagnosis of AML/MDS was 54% (28–79) and 18% (2–45), respectively. Among patients with secondary AML/MDS, 4 received a subsequent transplant (myeloablative allogeneic=3, reduced-intensity allogeneic=1). Of these 4 patients, 2 died at 1 and 32 months after second transplant, and 2 are still alive with follow-up of 2 and 9 years after second transplant, respectively (both patients received myeloablative allogeneic transplants).
SMN Risk Compared to General Population
When compared to an age- and gender-matched population, AHCT recipients had 24 times higher risk of developing SMNs (SIR 24.0 [95% CI, 16.0–33.0]) (Table III). Specifically, AHCT recipients had significantly higher risks of cancers of the bone (N=5, SIR 81), thyroid (N=5, SIR 53), breast (N=2, SIR 93), soft tissue (N=2, SIR 34), AML (N=6, SIR 266), and MDS (N=7, SIR 6603). Higher risks were also observed for cancers of the pharynx and for Kaposi’s sarcoma; however, only 1 patient each had SMNs at these sites. Risks of SMNs increased over time since AHCT; SIR were 17, 18, 29, and 39 for patients followed for <1 year, 1–4 years, 5–9 years, and ≥10 years after transplantation (Table 3). The 2 patients with unknown SMN type were excluded from these analyses.
Table 3.
Ratio of observed (O) to expected (E) cases of secondary cancers - overall and according to time since HCT
| Time since transplantation†
|
Overall | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| < 1 yr | 1–4 yr | 5–9 yr | ≥ 10 yr | ||||||||
|
| |||||||||||
| Number of patients | 1485 | 1036 | 510 | 166 | 1485 | ||||||
|
| |||||||||||
| Person-years at risk | 1240 | 2858 | 1614 | 449 | 6160 | ||||||
|
| |||||||||||
| Secondary cancer site | O | O/E | O | O/E | O | O/E | O | O/E | O | O/E | (95% CI) |
| Pharynx | 0 | -- | 1 | -- | 0 | -- | 0 | -- | 1 | 11803* | 299–65762 |
| Liver | 0 | -- | 0 | -- | 0 | -- | 1 | -- | 1 | 89 | 2–498 |
| Bone | 0 | -- | 2 | 73* | 3 | 167* | 0 | -- | 5 | 81* | 26–189 |
| Kaposi’s sarcoma | 1 | -- | 0 | -- | 0 | -- | 0 | -- | 1 | 275* | 7–1534 |
| Connective, soft tissue | 0 | -- | 0 | -- | 1 | 67 | 1 | 187 | 2 | 34* | 4.06–121 |
| Breast | 0 | -- | 0 | -- | 0 | -- | 2 | 226* | 2 | 93* | 11–336 |
| Cervix uteri | 1 | -- | 0 | -- | 0 | -- | 0 | -- | 1 | 48 | 1.2–270 |
| Testis | 0 | -- | 0 | -- | 1 | -- | 0 | -- | 1 | 7 | 0.2–38 |
| Brain, nervous system | 0 | -- | 0 | -- | 1 | -- | 0 | -- | 1 | 5 | 0.1–30 |
| Thyroid | 0 | -- | 1 | 31 | 2 | 55* | 2 | 116* | 5 | 53* | 17–123 |
| Myeloid leukemia | 0 | -- | 3 | 309* | 2 | 325* | 1 | 481* | 6 | 266* | 98–580 |
| MDS | 2 | 9972* | 3 | 5740* | 2 | 8043* | 0 | -- | 7 | 6603* | 2655–13604 |
| All sites | 4 | 17* | 10 | 18* | 12 | 29* | 7 | 39* | 33 | 24* | 16–33 |
P-value<0.01
Observed/Expected (O/E) ratios for cancer sites with only ≤1 SMN are not show
DISCUSSION
AHCT is a curative treatment modality for selected hematologic and solid tumors in children. The conditioning regimens in AHCT use intensive, high dose chemotherapy with or without radiation to achieve tumor kill and consolidate therapy. The aggressive nature of these conditioning regimens raises concern for the development of SMNs. Our large, multi-institution study with extended followup adds important information to the emerging knowledge of secondary cancer risks among childhood AHCT survivors. Although the overall incidence of SMNs among pediatric recipients of AHCT is low, they are at increased risk for SMNs compared to the general population. The risk of SMNs increases with longer survival since transplantation and does not plateau after 10 years of follow-up. Our findings reinforce the importance of long-term follow-up of pediatric AHCT survivors and continued vigilance and surveillance for SMNs in this population.
These increased risks for SMNs have also been noted in other pediatric cancer studies. In the CCSS study, SIR for SMNs remained elevated over time, despite aging in both the case and control group.(7) Although the two studies cannot be compared directly, the SIR’s for SMNs overall and for specific cancers were notably higher in our study than the CCSS study. Due to lack of sufficient data, our study could not address whether AHCT imparts any incremental risks for SMNs over pre-transplant chemotherapy and radiation exposures. We found no association between SMNs risk and age, gender, diagnosis, disease status, time since diagnosis, or use of total body irradiation or etoposide as part of conditioning. In comparison, the CCSS study observed a modest association between SMNs and female gender (RR=1.5), older age at cancer diagnosis (RR=1.3), radiation exposure (RR=2.7) and primary diagnosis of Hodgkin lymphoma (RR=1.5).(7) More studies are still needed to understand the role of inherited and acquired genetic factors in modulating risks for SMNs. Also, since AHCT continues to be an integral part of therapy in selected children with high-risk malignancies, more studies are still needed to better understand the risks associated with chemotherapy and radiation versus AHCT for SMNs in this population.
Although the development of SMNs in this relatively large cohort was rare, the incidence doubled from 5 years post-transplant (1.04%) to 10 years post-transplant (2.60%), and it did not plateau over time. Another important finding from our study was the progressive increase in SIR’s of SMNs with increasing follow-up since AHCT. These increased risks in very long-term survivors have also been noted in other pediatric cancer survivor studies.(7–9, 24)
The major limitations of our study relate to the fact that these are registry data that did not capture the full details of pre-AHCT treatment regimens and exposures. Of the evaluable SMNs (N=33), the majority of both secondary solid tumors (15/20, 75%) and secondary AML/MDS (11/13, 85%) occurred in patients with underlying neuroblastoma, sarcoma, and Hodgkin lymphoma. These pediatric malignancies have known chemotherapeutic backbones, including high-dose alkylating agents, high-dose topoisomerase inhibitors, and radiation; these exposures have all been implicated in increasing risk for SMNs, and we could not tease out the contribution of AHCT versus these previous exposures and the risks of SMNs. Since these data were generated by reporting from the transplant centers and long-term survivors may no longer be in contact with the transplant center, it is possible that our study may have under-reported the incidence of SMNs in this population and that the actual incidence and risks of SMNs may even be higher. The absolute number of SMNs in our study was relatively small, and we could not evaluate the risks and risk factors for cancers at specific sites.
Our study has important implications for future research and clinical practice related to pediatric AHCT survivors. It emphasizes the need for ongoing life-long surveillance for SMNs in this population. It also highlights the need for more investigation on whether pediatric AHCT survivors have higher risks of SMNs than childhood cancer survivors in general and whether the former require specific recommendations for secondary cancer screening and prevention (e.g., more frequent screening, targeted screening for specific cancer sites based on risk factors). Studies with detailed information describing pre- and post-AHCT therapeutic exposures and genetic risk factors are critical to understand the risk of SMNs in these patients.(25)
Supplementary Material
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Allos, Inc.; Amgen, Inc.; Angioblast; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Genzyme Corporation; GlaxoSmithKline; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Millennium Pharmaceuticals, Inc.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; RemedyMD; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
CONFLICT OF INTEREST: None.
Supplementary information related to this study is available at BMT’s website.
References
- 1.Matthay KK. Intensification of therapy using hematopoietic stem-cell support for high-risk neuroblastoma. Pediatr Transplant. 1999;3 (Suppl 1):72–7. doi: 10.1034/j.1399-3046.1999.00070.x. [DOI] [PubMed] [Google Scholar]
- 2.Kletzel M, Katzenstein HM, Haut PR, Yu AL, Morgan E, Reynolds M, et al. Treatment of high-risk neuroblastoma with triple-tandem high-dose therapy and stem-cell rescue: results of the Chicago Pilot II Study. J Clin Oncol. 2002;20(9):2284–92. doi: 10.1200/JCO.2002.06.060. [DOI] [PubMed] [Google Scholar]
- 3.Stram DO, Matthay KK, O’Leary M, Reynolds CP, Haase GM, Atkinson JB, et al. Consolidation chemoradiotherapy and autologous bone marrow transplantation versus continued chemotherapy for metastatic neuroblastoma: a report of two concurrent Children’s Cancer Group studies. J Clin Oncol. 1996;14(9):2417–26. doi: 10.1200/JCO.1996.14.9.2417. [DOI] [PubMed] [Google Scholar]
- 4.Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–20. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 5.Lieskovsky YE, Donaldson SS, Torres MA, Wong RM, Amylon MD, Link MP, et al. High-dose therapy and autologous hematopoietic stem-cell transplantation for recurrent or refractory pediatric Hodgkin’s disease: results and prognostic indices. J Clin Oncol. 2004;22(22):4532–40. doi: 10.1200/JCO.2004.02.121. [DOI] [PubMed] [Google Scholar]
- 6.Burke MJ, Walterhouse DO, Jacobsohn DA, Duerst RE, Kletzel M. Tandem high-dose chemotherapy with autologous peripheral hematopoietic progenitor cell rescue as consolidation therapy for patients with high-risk Ewing family tumors. Pediatr Blood Cancer. 2007;49(2):196–8. doi: 10.1002/pbc.21182. [DOI] [PubMed] [Google Scholar]
- 7.Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102(14):1083–95. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meadows AT, Friedman DL, Neglia JP, Mertens AC, Donaldson SS, Stovall M, et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27(14):2356–62. doi: 10.1200/JCO.2008.21.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meadows AT, Baum E, Fossati-Bellani F, Green D, Jenkin RD, Marsden B, et al. Second malignant neoplasms in children: an update from the Late Effects Study Group. J Clin Oncol. 1985;3(4):532–8. doi: 10.1200/JCO.1985.3.4.532. [DOI] [PubMed] [Google Scholar]
- 10.Hijiya N, Hudson MM, Lensing S, Zacher M, Onciu M, Behm FG, et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA. 2007;297(11):1207–15. doi: 10.1001/jama.297.11.1207. [DOI] [PubMed] [Google Scholar]
- 11.Walter AW, Hancock ML, Pui CH, Hudson MM, Ochs JS, Rivera GK, et al. Secondary brain tumors in children treated for acute lymphoblastic leukemia at St Jude Children’s Research Hospital. J Clin Oncol. 1998;16(12):3761–7. doi: 10.1200/JCO.1998.16.12.3761. [DOI] [PubMed] [Google Scholar]
- 12.Curtis RE, Metayer C, Rizzo JD, Socie G, Sobocinski KA, Flowers ME, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood. 2005;105(10):3802–11. doi: 10.1182/blood-2004-09-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socie G, Travis LB, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336(13):897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 14.Majhail NS, Brazauskas R, Rizzo JD, Sobecks RM, Wang Z, Horowitz MM, et al. Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood. 2011;117(1):316–22. doi: 10.1182/blood-2010-07-294629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizzo JD, Curtis RE, Socie G, Sobocinski KA, Gilbert E, Landgren O, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113(5):1175–83. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002;359(9314):1309–10. doi: 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- 17.Klein J, Moeschberger M. Survival Analysis: Techniques of censored and truncated data. 2. Springer-Verlag; New York, N.Y: 2003. [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from in complete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 19.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–202. [Google Scholar]
- 20.Breslow NE, Day NE. The design and analysis of cohort studies. In: Statistical Methods in Cancer Research. II. International Agency for Research on Cancer Scientific Publications No. 82; Lyon, France: 1987. [PubMed] [Google Scholar]
- 21.Preston DL, Lubin JH, Pierce DA. Epicure user’s guide. Seattle, WA: Hirosoft International; 1993. [Google Scholar]
- 22.Curado MP, Edwards B, Shin HR, et al. Cancer Incidence in Five Continents. IX. International Agency for Research on Cancer Scientific Publications No. 160; Lyon, France: 2007. [Google Scholar]
- 23.Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2006. National Cancer Institute; Bethesda, MD: 2009. http://seer.cancer.gov/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site. [Google Scholar]
- 24.Nottage K, Lanctot J, Li Z, Neglia JP, Bhatia S, Hammond S, et al. Long-term risk for subsequent leukemia after treatment for childhood cancer: a report from the Childhood Cancer Survivor Study. Blood. 117(23):6315–8. doi: 10.1182/blood-2011-02-335158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hake CR, Graubert TA, Fenske TS. Does autologous transplantation directly increase the risk of secondary leukemia in lymphoma patients? Bone Marrow Transplantation. 2007;39 :59–70. doi: 10.1038/sj.bmt.1705547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


