Abstract
Hepatocellular carcinoma (HCC) typically develop in cirrhosis, a condition characterized by Hedgehog (Hh) pathway activation and accumulation of Hh-responsive myofibroblasts (MF). Although Hh signaling generally regulates stromal-epithelial interactions that support epithelial viability, the role of Hh-dependent MF in hepatocarcinogenesis is unknown. Here we used human HCC samples, a mouse HCC model, and hepatoma cell/MF co-cultures to examine the hypothesis that Hh signaling modulates MF metabolism to generate fuels for neighboring malignant hepatocytes. The results identify a novel paracrine mechanism whereby malignant hepatocytes produce HH-ligands to stimulate glycolysis in neighboring MF, resulting in release of MF-derived lactate that the malignant hepatocytes use as an energy source. This discovery reveals new diagnostic and therapeutic targets that might be exploited to improve the outcomes of cirrhotic patients with HCC.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common deadly forms of cancer worldwide (1). HCCs typically develop in cirrhotic livers (1). The latter compromises recovery from extensive liver resection, and restricts chemotherapy options and efficacy. Therefore, survival depends mainly upon detection of tumors that are small enough to be safely ablated. Better screening and preventative strategies are needed, however, because the number of HCCs that are already advanced at diagnosis is increasing, and the population at risk for HCC is growing due to the rising incidence of cirrhosis (1). Improved understanding of the early events in hepatocarcinogenesis would help to optimize prevention, early diagnosis, and treatment of HCC.
Evidence that HCC occur in 1-5% of cirrhotic patients annually suggests that the cirrhotic microenvironment promotes the outgrowth of malignant hepatocytes (2). However, the mechanisms involved remain obscure. One possibility is that that stromal-epithelial interactions fuel HCC growth because deregulated, and excessively fibrogenic, repair of liver injury causes cirrhosis itself (3). The major producers of fibrous matrix during liver injury are myofibroblasts (MF), and cirrhotic livers harbor large numbers of these cells. The role of MF in HCC pathogenesis/progression is unclear, however, despite evidence that MF-derived factors mediate key aspects of the wound healing response including matrix turnover, recruitment of inflammatory cells, vascular remodeling, and outgrowth of liver epithelial progenitors (4). The pivotal importance of MF in cirrhosis pathogenesis justifies evaluating their role in hepatocarcinogenesis.
A key regulator of MF is Hedgehog (Hh), a developmental morphogenic signaling pathway (5). Hh pathway activity is barely detectable in healthy livers but becomes robust during all types of liver injury. Injured liver epithelial cells are important drivers of this process because injury stimulates the wounded epithelia to produce and release Sonic hedgehog (Shh) and Indian Hedgehog (Ihh) ligands, as well as other soluble factors, that promote Hh signaling in neighboring Hh-responsive stromal cells (6, 7). Like other Hh-responsive stromal cells, MF express the Hh ligand transmembrane receptor, Patched (Ptc) (8). Interaction of epithelia-derived HH-ligands with Ptc results in the activation of Smoothened, the Hh signaling-competent co-receptor. This leads to accumulation and nuclear localization of Glioma-family proteins (Gli1, Gli2, Gli3) which regulate the transcription of Hh-responsive genes that control proliferation, viability, and differentiation of the stromal cells. Exchange of paracrine signals between Hh-producing epithelia and Hh-responsive stroma orchestrates organogenesis during development. Similar mechanisms are presumed to modulate some types of carcinogenesis based on findings in mouse models of pancreatic and prostate cancer (9, 10). Although increased Hh signaling has been documented in human HCC (11), and liver MF are known to be a Hh-responsive cell type, the possibility that HCC growth might be regulated by paracrine Hh signaling between MF and malignant hepatocytes has not, to our knowledge, been examined.
Recently, we demonstrated that treating a mouse model of fibrosis-associated HCC with a Hh signaling inhibitor caused advanced HCCs to regress (12). Tumor involution was accompanied by MF loss and fibrosis improvement. This suggests that the anti-cancer actions of the Smoothened antagonist may have resulted from deletion of Hh-responsive MF, and justifies further work to identify how MF might support the growth of malignant hepatocytes. Here we evaluate the hypothesis that Hh signaling modulates MF metabolism to generate fuels for neighboring malignant hepatocytes. Given that HCCs, like many other epithelial cancers, exhibit enhanced glycolysis (i.e., the Warburg effect) (13), we asked if HCC glycolytic activity was influenced by Hh signaling in tumor-associated MF.
Materials and Methods
Human subjects
The Duke Department of Pathology computer database was searched for cases of HCC arising in nonalcoholic fatty liver disease (NAFLD) patients from 2007 through 2011. Five cases were identified from resections, hepatectomies, and explants. Random tissue blocks containing both tumor and adjacent non-tumor were used.
Mice and cell culture
Ten Mdr2−/− mice (age 51-59 weeks) with advanced liver fibrosis and HCC were treated with either Vehicle (DMSO, n=5) or 40mg/kg GDC-0449 (n=5) for 9 days as described (12).
Liver MF (8B cells, M. Rojkind, George Washington University (14)) were cultured alone (monoculture) or in a Transwell co-culture system with HepG2 cells (ATCC), Huh 7.5 cells (C. Rice, Rockefeller University), or Panc 10.05 cells (Duke Cell Culture Facility). MF monocultures were also treated with PBS-control or 1000 ng/ml recombinant Shh ligand +/− DMSO-vehicle or 3μM GDC-0449, or conditioned medium from the other cells +/− IgG-control or 10ug/ml 5E1-antibody for 24 hours. HepG2 and Huh7.5 cells were grown alone or treated with lactate +/− FX11. See Supplemental Methods for details.
Statistical analyses
Mean data were compared using the Student’s t-test. Differences were considered significant when p<0.05.
Results and Discussion
Malignant epithelia produce HH-ligands and stroma is enriched with Hh-responsive, glycolytic MF in human HCC
To investigate Hh ligand expression and localization in HCC, we performed immunohistochemistry for Sonic Hedgehog (SHH) ligand in archived paraffin-embedded tissues from 5 patients with HCC and cirrhosis caused by nonalcoholic fatty liver disease (NAFLD). All of the HCCs demonstrated increased expression of SHH relative to their capsules and adjacent non-tumorous liver tissue (Figure 1A). Within tumor nodules, malignant hepatocytes were a major source of SHH ligand. In contrast, nuclear staining for the Hh-regulated transcription factor, GLI2, was confined to the tumor-associated stroma. Compared to adjacent nontumor liver, HCCs were also significantly enriched with GLI2(+) stromal cells (Figure 1B). These findings suggest that malignant hepatocytes produce HH-ligands that promote Hh signaling in adjacent stromal cells.
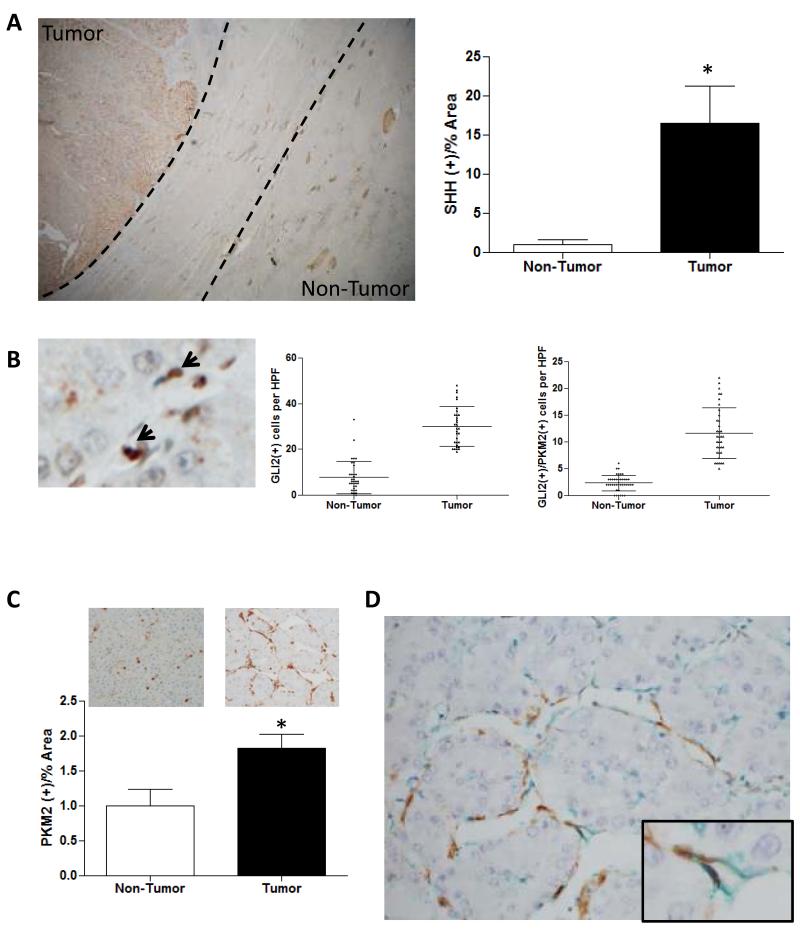
Figure 1. Evidence for paracrine Hedgehog signaling between malignant epithelia and tumor stroma in human HCC.
Tumor and adjacent nontumorous liver from 5 patients with HCC were evaluated by immunohistochemistry (IHC) and quantitative morphometry. Representative sections are shown and morphometric (mean +/− SEM) or cell count (mean +/− SD) data are graphed; *p<0.05 for tumor vs nontumorous tissue. (A) SHH ligand (brown, 50x). Dotted lines enclose fibrotic capsule. (B) GLI2 (arrows, 400x) (C) PKM2 (brown) in non-tumor and tumor nodules (400x). (D) Co-localization of PKM2 (green) with αSMA (brown) in tumor nodule (400x, 1000x insert).
To assess glycolytic activity in these HCC we stained sections for pyruvate kinase M2 (PKM2), a rate limiting glycolytic enzyme and well-validated marker of glycolysis (15). Unexpectedly, we found that PKM2 staining localized to HCC stroma, rather than to the malignant hepatocytes themselves (Figure 1C). Dual staining for GLI2 and PKM2 confirmed that the Hh-responsive stromal cells were glycolytic and showed that HCC stroma harbored greater numbers of glycolytic cells than adjacent nontumorous liver (Figure 1B, C). To further characterize these glycolytic stromal cells, we co-stained other sections for PKM2 and alpha smooth muscle actin (ASMA), a MF marker (4). Expression of PKM2 co-localized with ASMA, demonstrating that the glycolytic tumor-associated stromal cells were Hh-responsive MF (Figure 1D).
Hh inhibitor depletes glycolytic MF from tumor stroma in murine HCC model
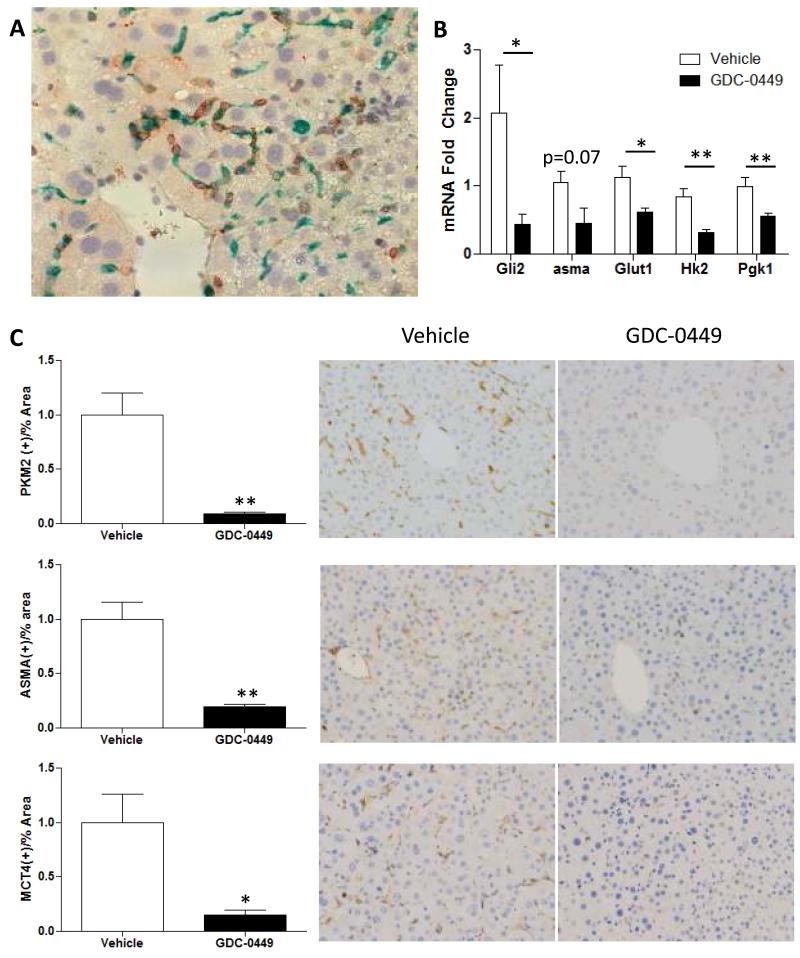
Progressive liver injury and fibrosis occur in Mdr2−/− mice due to deficient transport of phosphatidyl choline into bile. Primary hepatocellular carcinomas emerge spontaneously between 50-60 weeks of age, modeling the natural evolution of HCC during fibrogenic repair of various types of chronic liver injury (16). Therefore, we used immunohistochemistry to characterize the tumor-associated stroma in HCC that were micro-dissected from these mice. As noted in our human HCC cohort (Fig 1B-D), tumor-associated stroma in the mouse model was enriched with cells that co-expressed ASMA and PKM2 (Fig 2A). Therefore, glycolytic MF localize within the HCC-associated stroma in both species, and this seems to occur irrespective of the etiology of the underlying liver disease. In human HCC, the glycolytic MF co-stained for GLI2 (Fig 1B) and thus, were presumed to be Hh-responsive. To examine the role of Hh signaling in regulating glycolytic activity in the murine tumor stromal cells, we compared expression of Glut1, a glucose transporter, and several key glycolytic enzymes in mRNA isolated from HCCs of Mdr2-deficient mice that had been treated with either vehicle or the Smoothened antagonist, GDC0449, for 9 days prior to sacrifice. Treatment with the Hh inhibitor reduced expression of mRNAs encoding Gli2, Glut1, glycolytic enzymes, and αSma (Fig 2B). Immunohistochemistry confirmed that reduced expression of MF- and glycolysis-associated mRNAs was paralleled by depletion of tumor stromal cells that expressed ASMA, PKM2, and monocarboxylate transporter 4 (MCT4), a facilitator of lactate export (17) (Fig 2C).
Figure 2. Murine HCC stroma is enriched with Hh-dependent glycolytic MF.
Mdr2-deficient mice with HCC were treated with the Hh-inhibitor, GDC-0449, or vehicle (N=5 mice/group); effects on stroma of microdissected tumor nodules were evaluated by morphometry of immunostained sections and QRT-PCR. Representative sections and mean +/− SEM data are displayed (*p<0.05, **p<0.01 GDC-0449-vs. vehicle-treated groups). (A) Co-localization of PKM2 (green) with αSMA (brown) in tumor from vehicle-treated mouse (400x). (B) QRT-PCR for Gli2, αSma, Glut1, Hk2, Pgk1 in liver tumor mRNA. (C) Tumor sections from Vehicle- and GDC-0449 treated mice demonstrate (Top) αSMA, (Middle) PKM2, and (Bottom) MCT4 (brown, 200x)
Paracrine Hh signaling between hepatoma cells and MF stimulates glycolysis in MF
To determine if hepatoma cells generate soluble HH-ligands that might stimulate glycolytic activity in Hedgehog-responsive MF, we compared Gli-luciferase reporter activity in Shh-LightII cells that were exposed to conditioned medium from HepG2 cells, co-cultured with HepG2 cells in a Transwell system, or treated with control medium without or with recombinant SHH (Fig 3A). Like recombinant SHH, exposure to HepG2 cell-derived soluble factors significantly increased Hh signaling in the Shh-LightII cells. The stimulatory effect of HepG2 conditioned medium was abrogated by adding 5E1, a Hh neutralizing antibody that blocks HH ligand-Ptc interaction (18) (Fig 3A). Moreover, when HepG2 cells were replaced with cells that do not generate HH-ligands (Panc 10.05 cells) (19) and experiments were repeated, no change in Shh-LightII cell luciferase activity was observed (Supplemental Fig 1A). The aggregate data, therefore, indicate that HepG2 cells generate soluble, biologically-active HH-ligands.
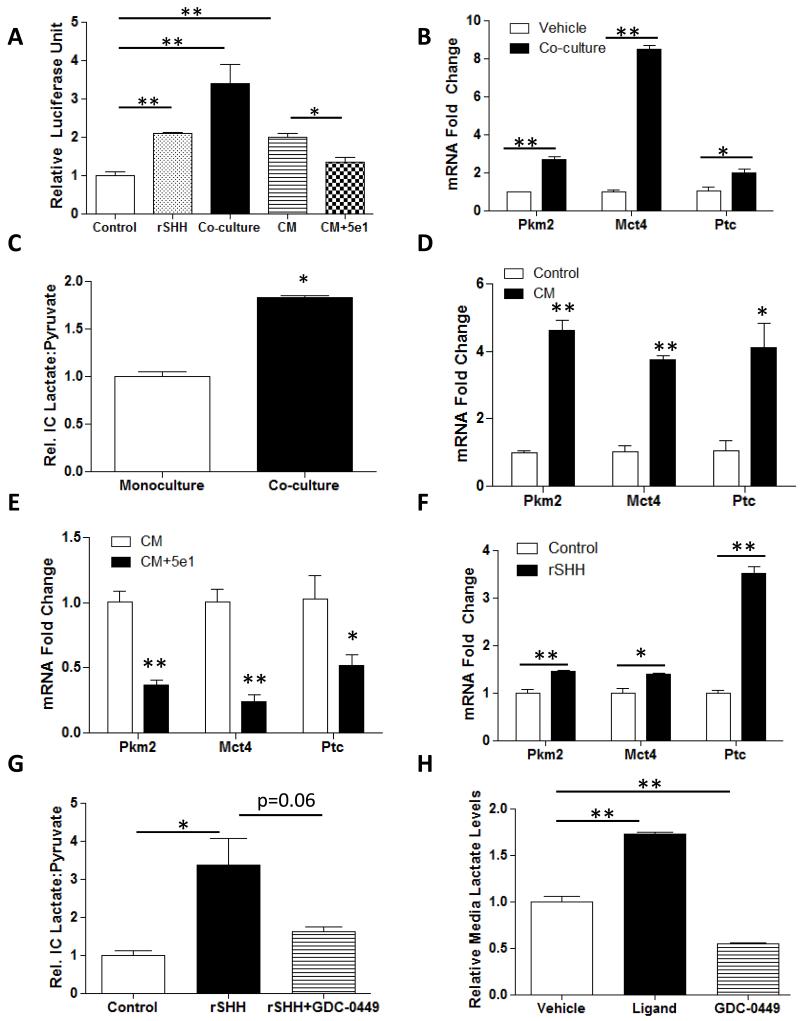
Figure 3. Paracrine Hh signaling stimulates MF glycolysis.
(A) Gli-luciferase reporter activity in Shh-LightII cells incubated with control medium, 1000ng/mL recombinant SHH ligand, co-cultured with HepG2 in a Transwell system, incubated with HepG2-conditioned media without or with 5E1 neutralizing antibody; (*p<0.05, **p<0.01). (B) Pkm2 and Mct4 mRNA levels in MFs grown in monoculture or co-cultured with HepG2s in Transwells. (C) Intracellular (I.C.) lactate/pyruvate ratio in MFs mono-cultured or co-cultured with HepG2 cells. (D) Pkm2, Mct4, and Patched mRNA levels in MFs grown in control-media or with HepG2-conditioned media (CM). (E) Pkm2, Mct4, and Patched mRNA levels in MFs grown in HepG2-conditioned media (CM) without or with 5E1 neutralizing antibody (F) Pkm2 and Mct4 mRNA levels in MFs treated with Vehicle or recombinant SHH ligand (rSHH-L). (G) Intracellular (I.C.) lactate/pyruvate ratio in MFs after treatment with rSHH-L. (H) Lactate exported into media by MFs treated with control media, rSHH-L or rSHH-L+GDC-0449. Mean +/− SEM data from triplicate experiments are graphed; *p<0.05, **p<0.01 vs. respective controls.
To determine if these HepG2-derived HH ligands functioned as inducers of MF glycolysis, we cultured a well-characterized rat liver MF line (8B cells) (14) alone (monoculture) or in the Transwell system with HepG2 cells, and assessed MF glycolytic activity. Co-culturing MF with HepG2 cells induced MF expression of mRNAs that encode key glycolytic enzymes (Fig 3B), and increased their lactate/pyruvate ratio, a measure of glycolytic activity (Fig 3C). Treating MF with HepG2 cell-conditioned media had similar effects (Fig 3D). The stimulatory effects of HepG2-conditioned medium on MF glycolysis were attenuated by adding 5E1 to block HH ligand-Ptc interactions (Fig 3E), suggesting that HH-ligands are the factors that malignant hepatocytes release to induce glycolytic activity in neighboring MF. To verify that activating Hh signaling in MF promotes glycolysis, we treated monocultures of liver MF with recombinant SHH (rSHH) ligand. Compared to vehicle-treated MF, MF treated with rSHH ligand had increased expression of genes encoding glycolytic enzymes (Fig 3F) and higher lactate/pyruvate ratios (Fig 3G). GDC-0449, a direct antagonist of the Hh signaling intermediate, Smoothened, reversed the effects of rSHH-ligand, confirming that MF glycolysis is regulated by canonical Hh signaling (Fig 3G). Treating MF with rSHH also significantly increased their secretion of lactate into the media, while adding GDC-0449 reduced media lactate below basal levels, demonstrating that Hh signaling also regulates MF secretion of lactate (Fig 3H). Finally, to determine if cancer cells that do not generate Hh ligands can induce MF glycolysis, we compared expression of pkm2 and mct4 mRNAs in MF that were exposed to soluble factors derived from Panc 10.05 cells (Supplemental Fig 1C, D). As predicted by the data shown in Supplemental Fig 1A, Panc 10.05 cells were unable to induce MF expression of Ptc, a known Hh-target gene (Supplemental Fig 1B). However, they did increase MF expression of both glycolysis markers, albeit at significantly lower levels than were induced by HepG2 cells. Thus, while malignant hepatocytes release Hh ligands to activate glycolysis in neighboring stromal cells, pancreatic cancer cells are able to achieve this via other mechanisms that do not require Hh-Ptc paracrine interactions.
Lactate generated by glycolytic MF fuels lipogenesis in HepG2 cells
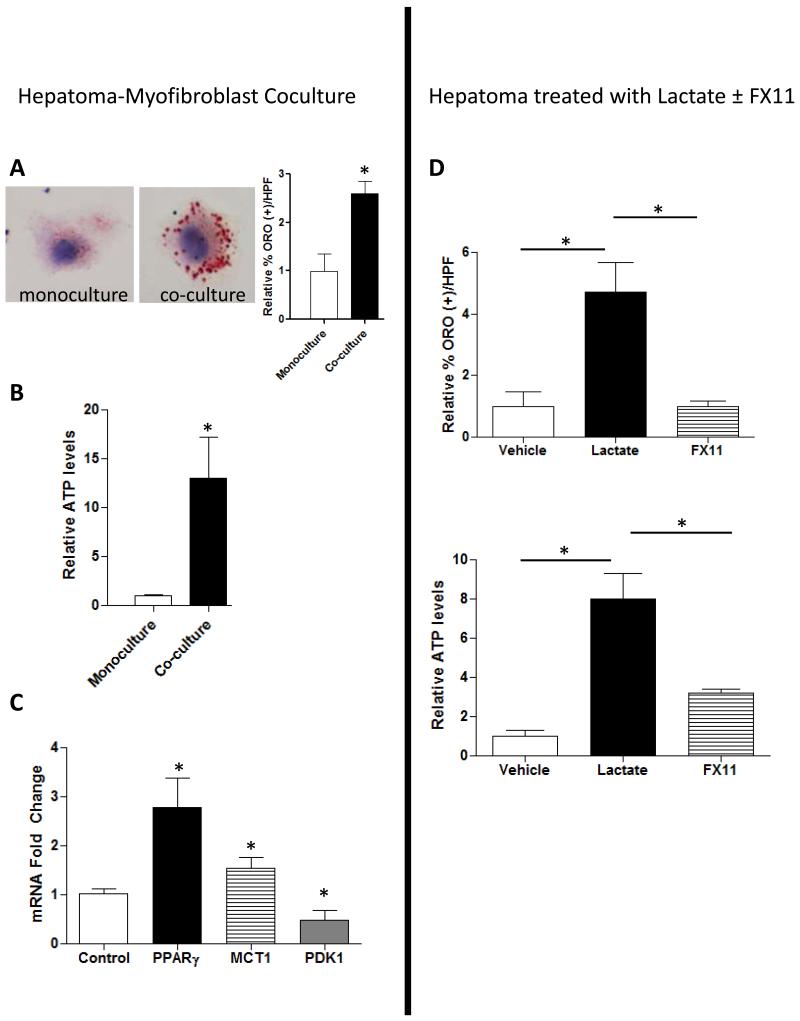
Never-the-less, our aforementioned findings identified a novel Hh-dependent mechanism whereby malignant hepatocytes modulate the metabolic activity of tumor-associated MF. Because tumor stroma is generally believed to support the growth of malignant epithelial cells, we used the Transwell co-culture system to evaluate the related hypothesis that MF-derived glycolytic end-products (such as lactate) enhance net energy homeostasis of malignant hepatocytes. Compared to mono-cultured HepG2 cells, HepG2 cells that were co-cultured with liver MF demonstrated significant accumulation of Oil Red O-stained lipid droplets (Fig 4A). Because lipid accumulation occurs during energy excess, we compared the ATP content of mono- and co-cultured HepG2 cells and found significantly higher ATP content in the co-cultured HepG2 cells (Fig 4B). Consistent with increased lipogenesis during co-culture, co-cultured HepG2 cells expressed higher mRNA levels of the lipogenic transcription factor, Pparγ, than mono-cultured HepG2 cells (Fig 4C). Co-culture also enhanced HepG2 expression of monocarboxylate transporter 1 (Mct1), which encodes a lactate transporter (17), but suppressed expression of pyruvate dehydrogenase kinase (Pdk1), which encodes an enzyme that gates entry of pyruvate into the tricarboxcylic acid (TCA) cycle (Supplemental Fig 3). These findings suggest that malignant hepatocytes import MF-derived lactate and convert it into pyruvate to fuel ATP and lipid biosynthesis. To assess this issue more directly, we treated monocultured HepG2 cells with lactate in the absence or presence of FX11. FX11 inhibits the activity of lactate dehydrogenase, thereby blocking the interconversion of lactate and pyruvate (20). Treating HepG2 cells with lactate significantly increased lipid accumulation and ATP content. Both responses were prevented when cells were pre-treated with FX11 to inhibit intracellular conversion of lactate into pyruvate (Fig 4D). Similar results were obtained when another hepatoma cell line, Huh7.5, was co-cultured with liver MF or treated directly with lactate (Supplemental Fig 4), providing reassurance that the findings were not restricted to a single liver cancer cell line.
Figure 4. Lactate generated by glycolytic MF fuels lipogenesis in HepG2 cells.
(A) Oil Red O staining of HepG2 cells grown alone or co-cultured in Transwells with MFs and quantified by morphometry. (B) Intracellular ATP and (C) MCT1, PPARγ, PDK1 mRNA levels in HepG2 cells cultured alone or in Transwells with MFs. (D) Change in Oil Red O staining and ATP in HepG2s after treatment with 40mM lactate or FX11. Mean +/− SEM data from triplicate experiments are graphed; *p<0.05, **p<0.01 vs. respective control
The aggregate data, therefore, support a model whereby malignant hepatocytes generate HH-ligands to orchestrate the construction of a Hh-responsive stroma that nurtures further growth of the malignant epithelia. The cancer-associated process resembles epithelial-stromal interactions that are triggered by injury to nonmalignant liver epithelial cells. When damaged, such cells begin to produce HH-ligands that also act in a paracrine fashion to promote accumulation of Hh-dependent MF (5). In nontumorous cirrhotic livers, MF are a major source of fibrous matrix, but also produce various factors that promote the survival of residual liver epithelial cells (4). Here we identify end-products of Hh-dependent changes in MF metabolism as novel trophic factors for malignant hepatocytes by showing that Hh signaling in MF stimulates glycolysis, and demonstrating that malignant hepatocytes use MF-derived lactate to generate ATP and fuel lipogenesis (Supplemental Fig 5). Evidence that the lactate-induced responses are blocked by treating malignant hepatocytes with an inhibitor of lactate dehydrogenase suggests that the improved epithelial energy balance occurs because malignant hepatocytes convert the assimilated lactate into pyruvate, which is then shunted into the TCA cycle to increase mitochondrial ATP production. In HCCs, therefore, increased aerobic glycolytic activity (i.e., the Warburg effect) is an end result of collaborations between malignant hepatocytes and glycolytic MF in the tumor associated stroma. Via this process, the malignant hepatocytes reap the benefits of the excess lactate generated by glycolysis without becoming glycolytic themselves, thereby fully retaining the capacity for oxidative phosphorylation and efficient ATP synthesis. Although this concept is contrary to conventional dogma which localizes the Warburg effect to the malignant cells themselves (15), it is consistent with other recent reports of lactate production by stroma in breast cancer (21), and raises the intriguing possibility that Hh-mediated switches in stromal cell metabolism also occur in cancers other than HCC. In any case, evidence for increased glycolytic activity in tumor-associated MF has important diagnostic and therapeutic implications. It suggests that positron emission tomography (PET) scans might be deployed to identify HCC that are particularly enriched with glycolytic stroma. The latter information might facilitate HCC detection, and could also have prognostic significance because highly glycolytic tumors tend to have more aggressive biology (22). Knowing which HCC are most enriched with glycolytic stroma would also justify, and help to refine, novel treatment approaches for HCC, supporting consideration of Hh inhibitors, LDH antagonists, and glycolysis inhibitors, as potential therapies for some patients with this life-threatening disease.
Supplementary Material
Acknowledgments
Grant Support: This work is supported in part by R01-DK-053792 and R01-DK-077794 (AMD).
Footnotes
Financial Disclosures: No conflict of interests to report.
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012 [Google Scholar]
- 2.Ye SL, Takayama T, Geschwind J, Marrero JA, Bronowicki JP. Current approaches to the treatment of early hepatocellular carcinoma. Oncologist. 2010;15(Suppl 4):34–41. doi: 10.1634/theoncologist.2010-S4-34. [DOI] [PubMed] [Google Scholar]
- 3.Jou J, Diehl AM. Epithelial-mesenchymal transitions and hepatocarcinogenesis. J Clin Invest. 2010;120:1031–4. doi: 10.1172/JCI42615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–72. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omenetti A, Choi S, Michelotti G, Diehl AM. Hedgehog signaling in the liver. J Hepatol. 2011;54:366–73. doi: 10.1016/j.jhep.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung Y, Witek RP, Syn WK, Choi SS, Omenetti A, Premont R, et al. Signals from dying hepatocytes trigger growth of liver progenitors. Gut. 2010;59:655–65. doi: 10.1136/gut.2009.204354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rangwala F, Guy CD, Lu J, Suzuki A, Burchette JL, Abdelmalek MF, et al. Increased production of sonic hedgehog by ballooned hepatocytes. J Pathol. 2011;224:401–10. doi: 10.1002/path.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi SS, Omenetti A, Witek RP, Moylan CA, Syn WK, Jung Y, et al. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1093–106. doi: 10.1152/ajpgi.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gipp J, Gu G, Crylen C, Kasper S, Bushman W. Hedgehog pathway activity in the LADY prostate tumor model. Mol Cancer. 2007;6:19. doi: 10.1186/1476-4598-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sicklick JK, Li YX, Jayaraman A, Kannangai R, Qi Y, Vivekanandan P, et al. Dysregulation of the Hedgehog pathway in human hepatocarcinogenesis. Carcinogenesis. 2006;27:748–57. doi: 10.1093/carcin/bgi292. [DOI] [PubMed] [Google Scholar]
- 12.Philips GM, Chan IS, Swiderska M, Schroder VT, Guy C, Karaca GF, et al. Hedgehog signaling antagonist promotes regression of both liver fibrosis and hepatocellular carcinoma in a murine model of primary liver cancer. PLoS One. 2011;6:e23943. doi: 10.1371/journal.pone.0023943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitamura K, Hatano E, Higashi T, Narita M, Seo S, Nakamoto Y, et al. Proliferative activity in hepatocellular carcinoma is closely correlated with glucose metabolism but not angiogenesis. J Hepatol. 2011;55:846–57. doi: 10.1016/j.jhep.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 14.Greenwel P, Schwartz M, Rosas M, Peyrol S, Grimaud JA, Rojkind M. Characterization of fat-storing cell lines derived from normal and CCl4-cirrhotic livers. Differences in the production of interleukin-6. Lab Invest. 1991;65:644–53. [PubMed] [Google Scholar]
- 15.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–3. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 16.Katzenellenbogen M, Pappo O, Barash H, Klopstock N, Mizrahi L, Olam D, et al. Multiple adaptive mechanisms to chronic liver disease revealed at early stages of liver carcinogenesis in the Mdr2-knockout mice. Cancer Res. 2006;66:4001–10. doi: 10.1158/0008-5472.CAN-05-2937. [DOI] [PubMed] [Google Scholar]
- 17.Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. The Biochemical journal. 1999;343(Pt 2):281–99. [PMC free article] [PubMed] [Google Scholar]
- 18.Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–51. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 19.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–10. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 20.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–42. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiavarina B, et al. Ketones and lactate “fuel” tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle. 2010;9:3506–14. doi: 10.4161/cc.9.17.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeluri S, Madhok B, Prasad KR, Quirke P, Jayne DG. Cancer’s craving for sugar: an opportunity for clinical exploitation. Journal of cancer research and clinical oncology. 2009;135:867–77. doi: 10.1007/s00432-009-0590-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.