Summary
The bacterial cell wall is conserved in prokaryotes, stabilizing cells against osmotic stress. Beta-lactams inhibit cell wall synthesis and induce lysis through a bulge-mediated mechanism; however, little is known about the formation dynamics and stability of these bulges. To capture processes of different timescales, we developed an imaging platform combining automated image analysis with live cell microscopy at high time resolution. Beta-lactam killing of Escherichia coli cells proceeded through four stages: elongation, bulge formation, bulge stagnation and lysis. Both the cell wall and outer membrane (OM) affect the observed dynamics; damaging the cell wall with different beta-lactams and compromising OM integrity cause different modes and rates of lysis. Our results show that the bulge formation dynamics is determined by how the cell wall is perturbed. The OM plays an independent role in stabilizing the bulge once it is formed. The stabilized bulge delays lysis, and allows recovery upon drug removal.
Introduction
Beta-lactam antibiotics have been widely used in the clinic for more than half a century and account for the largest share in the worldwide antibiotic market (Hamad, 2010). The cellular target of beta-lactams is the peptidoglycan (PG). This thin layer of biopolymer mesh, which serves to maintain cell morphology and balance turgor pressure, is composed of long glycan chains and peptide cross-links (Holtje, 1998). Peptide cross-links are formed by the transpeptidase activity of penicillin-binding proteins (PBPs). Beta-lactam antibiotics covalently bind to PBPs and inhibit cross-link formation, the last stage of PG synthesis (Tipper and Strominger, 1965; Wise and Park, 1965). Inhibition of PG synthesis by beta-lactams has various effects on cell shape due to their ability to bind to one or more PBPs involved in cell division, elongation, and shape maintenance (Spratt, 1975). It has been proposed that inhibition of cross-link formation by beta-lactams combined with mis-regulated cell wall degradation by PG hydrolases results in the accumulation of PG defects, which ultimately leads to cell lysis (Chung et al., 2009).
The physical process of PG defect formation and subsequent lysis is poorly understood. Previous literature suggested a bulge-mediated process (Chung et al., 2009; Huang et al., 2008) and the reported rates of lysis have been loosely characterized as slow and fast (de Pedro et al., 2002). However, in the absence of systematic characterization of bulge formation dynamics and its variability across individual cells, it is unclear whether lysis occurs uniformly within isogenic cell populations, or whether distinct physical processes act in different cells. Furthermore, it is unknown whether different beta-lactam antibiotics cause similar or distinct modes of lysis.
In addition to the PG layer, the cell envelope also includes inner and outer membranes (IM and OM), both of which are essential for cell viability. Unlike the IM, which is a simple phospholipid bilayer, the OM is asymmetric (Funahara and Nikaido, 1980; Kamio and Nikaido, 1976). Its outer leaflet lipopolysaccharide layer (LPS) serves as a protective barrier against detergents and hydrophobic antibiotics (e.g. vancomycin), with embedded porins that allow diffusion of small hydrophilic molecules including nutrients and beta-lactams (Pages et al., 2008). An LPS molecule consists of lipid A, LPS core and the O-antigen. Absence of O-antigen makes Gram-negative bacteria hypersensitive towards hydrophobic antibiotics, detergents and host proteins (Silhavy et al., 2010). Although PG is covalently attached to the OM, and recent studies have shown that OM lipoproteins regulate PG synthesis (Paradis-Bleau et al., 2010; Typas et al., 2010), the potential role of the OM in beta-lactam induced cell lysis has not been studied.
In order to study bulge formation and lysis in greater detail, we developed a live-cell imaging platform to monitor morphological dynamics of E. coli cells under beta-lactam treatment at high time resolution. This platform allows a high-throughput study of single cell shape dynamics over long periods of time, and at a time resolution that captures the fast lysis dynamics (~2 hr, at ~8 frame/second). We used this platform to characterize variability in bulge formation and lysis within isogenic cells under different beta-lactams. We chose to primarily focus on cephalexin (Keflex), because it targets PBP3 (FtsI), the only essential PBP involved in cell division, which is the best understood pathway for PG biogenesis (Chung et al., 2009). We also tested cefsulodin, which targets elongation-specific PBP1a/1b, and ampicillin, which broadly targets all PBPs. Finally we applied genetic and chemical perturbations to test the possible role of OM in beta-lactam killing.
Results
Beta-lactam induced bulges are enclosed by both IM and OM
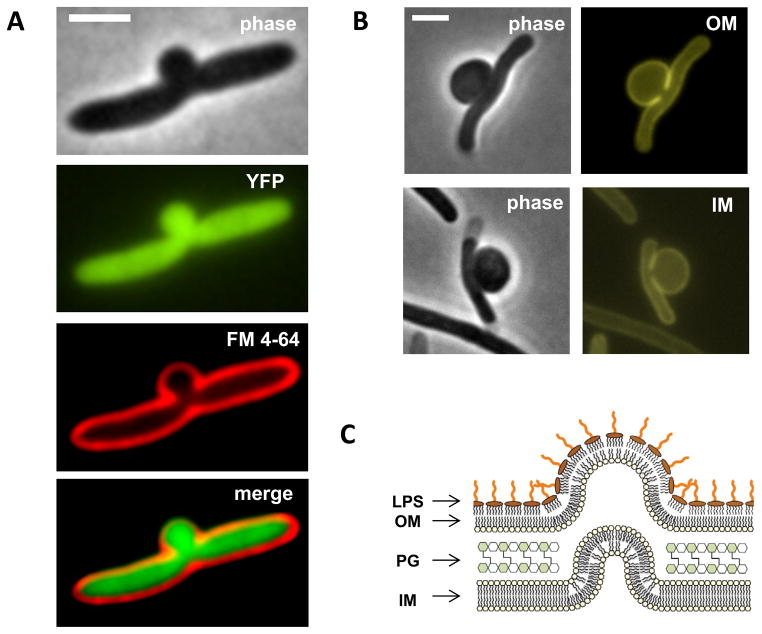
To understand the physical processes behind bulge formation, we determined the structural components of the bulge. First, we asked whether beta-lactam induced bulges contain the same materials as cytoplasm. We used an E. coli strain expressing cytoplasmic yellow fluorescent protein (YFP) and examined morphology of these cells grown in liquid culture under cephalexin treatment. YFP and membrane stain images suggest that cytosol materials leak through PG defects and deform the double membranes at the midcell site (Fig. 1A). Next, to examine whether both membranes are intact, we used two additional E. coli strains with mCherry fused to an IM protein and an OM protein (Paradis-Bleau et al., 2010). Fluorescence images showed that both IM and OM remain intact in bulging cells (Fig. 1B and Fig. S1). We conclude that bulges are protrusions of cytoplasmic materials surrounded by both IM and OM (Fig. 1C).
Figure 1. Both inner and outer membranes are intact in bulging cells.
(A, B) Phase contrast and fluorescence images of bulging cells under cephalexin treatment (A, cytoplasmic YFP and FM 4–64 membrane staining; B, ZipA-mCherry, IM marker; ssPal-mCherry, OM marker). Membrane protrusions indicate PG defects at the potential division site. Leakage of cytoplasmic YFP into the bulge suggests redistribution of cytosol materials from cell filament to the midcell bulge. (C) Schematic illustration of a bulge as a cell wall-less cytosolic protrusion surrounded by both IM and OM. LPS is exclusively located at the outer leaflet of the OM and its long glycan chains facilitate a micelle-like close packing structure. All scale bars represent 2 μm. See also Figure S1.
Four distinct physical phases leading to cell lysis
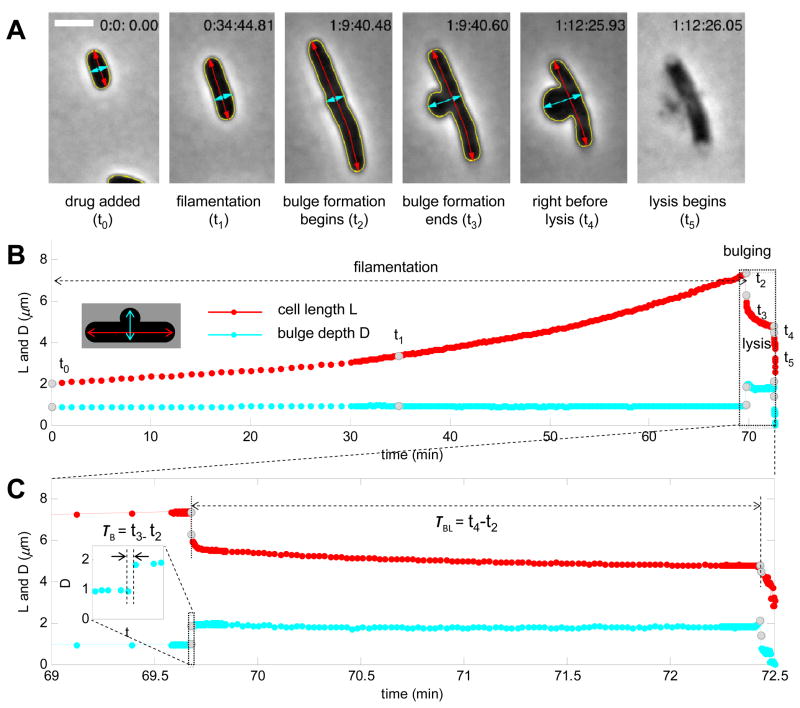
Beta-lactam induced cell lysis requires disintegration of all three layers of the cell envelope, which involves processes that take place at a range of timescales. In order to characterize each of these processes, it is necessary to acquire time-lapse movies with adaptive time resolutions in accordance with different stages leading to cell lysis (Experimental Procedures). The resulting time resolution of our imaging platform varies from ~125 ms/frame to ~12.5 s/frame for fast and slow processes, respectively. Quantitative image analysis allows continuous measurement of cell length and bulge depth throughout beta-lactam treatment (Fig. 2A and Movie S1).
Figure 2. Live cell microscopy with automated imaging analysis reveals bulge formation as an intermediate step towards lysis.
(A) Selected images of a representative E. coli cell at different stages of cephalexin treatment (yellow, cell contour; red, cell length; cyan, bulge depth). The time of these snapshots (t0 ~ t5) is indicated in gray dots in panel B. (B, C) Measurement of cell length and bulge depth of a representative E. coli cell throughout beta-lactam treatment (B) and during bulging and lysis (C, zoom-in view of the box in panel B). Both bulge formation time (τB) and bulge lifetime (τBL, zoom-in inset in panel C) are defined based on bulge depth measurements. Adaptive time resolutions were adopted to characterize processes with different kinetics: 1 min/frame for the first 30 minutes after adding drug, 12.5 s/frame for filamentation and bulge stagnation, and ~125 ms/frame for bulge formation and final lysis. Take notice of the simultaneous cell length shrinkage during the abrupt bulge formation and the second increase in bulge depth right before lysis. Scale bar represents 2 μm. See also Figure S2 and Movie S1.
A typical progression to cell lysis observed by time-lapse microscopy of cells under cephalexin involves four distinct physical phases: filamentation, bulge formation, bulge stagnation, and lysis (membrane rupture). Following exposure to the drug (t0=0), we observed a long period of cell elongation (Fig. 2B, ~70 minutes), during which cell length kept increasing at a normal rate without change in cell width (Fig. 2A, t1; Fig. 2B). Then a bulge formed abruptly at the potential division site. We define the time between the beginning (t2) and end (t3) of bulge formation as bulge formation time τB (Fig. 2C, τB=t3-t2 ~150 ms; higher time resolution in Fig. S2). This second phase is marked by a large increase in bulge depth and simultaneous shrinkage of cell length, indicating that bulges form out of existing cytosol and membranes instead of newly synthesized materials. Some of the cells also underwent “cracking” during bulge formation, which we quantified by the change in the angle (Δθ) measured at the bulging site. After its abrupt formation, the midcell bulge is typically stable for an intermediate time (~ minutes) before final lysis. We define this delay between the onset of bulge formation (t2) and final lysis (t4) as bulge lifetime τBL (Fig. 2C, τBL= t4-t2 ~ 3 min). Finally, after a period of stagnation, we observed a fast increase in bulge depth followed by membrane rupture and lysis (t5). Cells treated with ampicillin showed similar characteristics with additional swelling at the midcell site (Fig. S3).
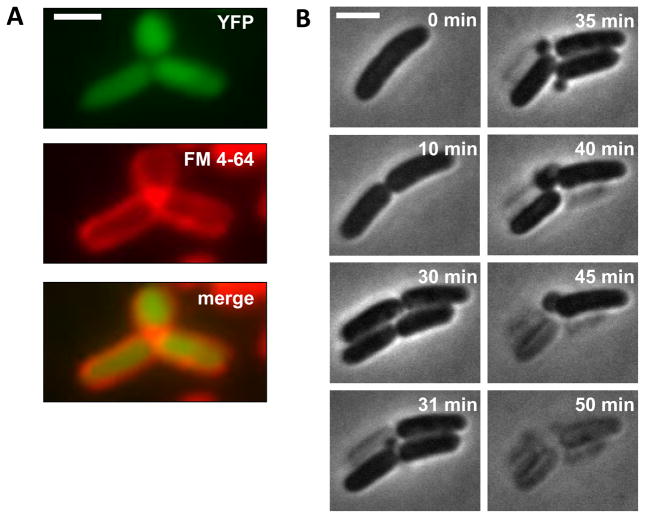
To evaluate the generality of the observed progression for other beta-lactams, we carried out similar experiments for cefsulodin, a beta-lactam with a different profile from cephalexin and ampicillin. Cefsulodin targets PBP1a and PBP1b in E. coli, and it is generally considered to be elongation specific (Jacoby and Young, 1991). We first examined cells grown in liquid culture under cefsulodin treatment. Bulges still formed at the midcell site; however, YFP and membrane stain images of these cells did not show membrane gaps near the bulging site (Fig. 3A). Selective images of a representative cell show that, unlike cephalexin and ampicillin, cefsulodin does not block septation; the representative cell underwent two rounds of division before lysis. Bulges formed at nascent poles on septated filaments, and lysis occurred separately in daughter cells (Fig. 3B; Movie S2). We conclude that there is a common pathway to cell lysis under all beta-lactams tested. However, morphological characteristics of each step vary for different classes of beta-lactams based on their specific cellar targets.
Figure 3. Cefsulodin-induced lysis shows shared as well as distinct features.
(A) Fluorescence images of bulging cells treated with cefsulodin (cytoplasmic YFP and FM 4–64 membrane staining). No obvious membrane gap was observed between the bulge and the filament; (B) Selected snapshots of a representative E. coli cell at different stages of cefsulodin treatment. Bulge formation and separate lysis events occur at nascent poles on septated filaments. Scale bar represents 2 μm. See also Figure S3 and Movie S2.
Three modes of bulge formation within isogenic cell populations
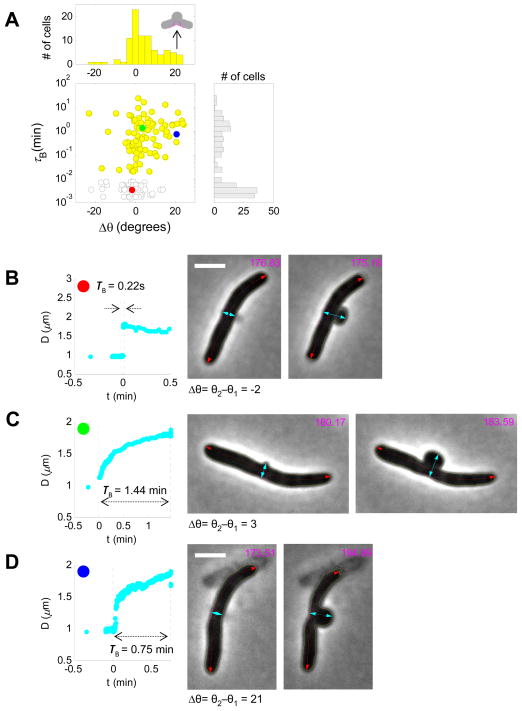
Do all E. coli cells follow the same morphological dynamics as shown in representative cells? To answer this question, we recorded and analyzed morphological dynamics of ~200 wildtype cells under cephalexin treatment, and focused on bulge formation. We found that cells separated into three distinct patterns in terms of how fast the bulge is formed and its final morphology. Histogram plots of both bulge formation time (τB) and cracking angle (Δθ) showed bimodal distribution (Fig. 4A). Approximately half of the cells developed bulges abruptly, within less than 0.5 s (Fig. 4A, red dots; Fig. 4B), while the rest of the population formed bulges gradually, over the course of several minutes (Fig. 4A, yellow and blue dots; Fig. 4C, D). Many of the fast bulging cells completed bulge formation within ~150 ms, the time resolution limit of our imaging platform. Further measurements using a faster camera (~5 ms/frame) resolved the bulge formation time of a representative fast bulging cell to less than 100 ms (Fig. S2). A small fraction of the slow bulging cells displayed a cracking pattern, where filaments bent at the potential division site as bulges were slowly formed (Fig. 4D). The typical cracking angle is ~20° (Fig. 4A, top panel). We hypothesized that the heterogeneity of bulge formation observed in isogenic cell populations might be due to different elongation states upon exposure to the drug. To test this hypothesis, we took initial cell length as a proxy for elongation states; however no correlation was found between initial cell length and bulge formation time (Fig. S4). Moreover these three distinct bulging patterns were also observed in cells treated with a different concentration of cephalexin and with ampicillin (Fig. S4).
Figure 4. Three distinct bulging patterns and dynamics within isogenic cell populations.
(A) Scatter and histogram plots of bulge formation time (τB) and cracking angle (Δθ) reveal three different bulging patterns in response to cell wall damage by cephalexin: fast bulging without cracking (b, red dot, τB<10−2 min), slow bulging without cracking (c, green dot, τB>10−2 min), and slow bulging with cracking (d, blue dot, τB>10−2 min). Yellow dots and bar graphs represent slow bulging cells (top and middle panels). Gray bars represent all isogenic cells examined (N=195, right panel). (B–D) Representative bulge depth measurement and snapshots of cells before (t2) and after (t3) bulge formation for each bulging pattern. Magenta lines represent measurement of cracking angles. All scale bars represent 2 μm. See also Figure S4 and online supplementary movies for cell 6, cell 75 and cell 123.
The mode of bulge formation affects bulge stability
Do distinct modes of bulge formation affect bulge stability? To answer this question, we quantified bulge lifetime of the same ~ 200 cephalexin-treated cells. Again we observed significant variability within isogenic cell populations. The distribution of bulge lifetime varies by almost three orders of magnitude (Fig. 5A). This variability correlates with the rate of bulge formation; slow bulging cells are much more stable (τBL>100 min) than fast bulging cells (Fig. 5A). To verify this correlation, we examined whether cells treated with cefsulodin, which showed a different mechanism of bulge formation, would also exhibit different bulge stability. The percentage of fast-lysing cells increased significantly under cefsulodin treatment (Fig. 5B). Alternatively, variability in bulge stability could also arise from different bulge sizes in fast and slow bulging cells; however, measurements of change in cell length and bulge depth before and after bulge formation excluded such possibility (Fig. S4). We conclude that the mode of cell wall damage, through its effect on the rate of bulge formation, can affect bulge stability and ultimately cell lysis.
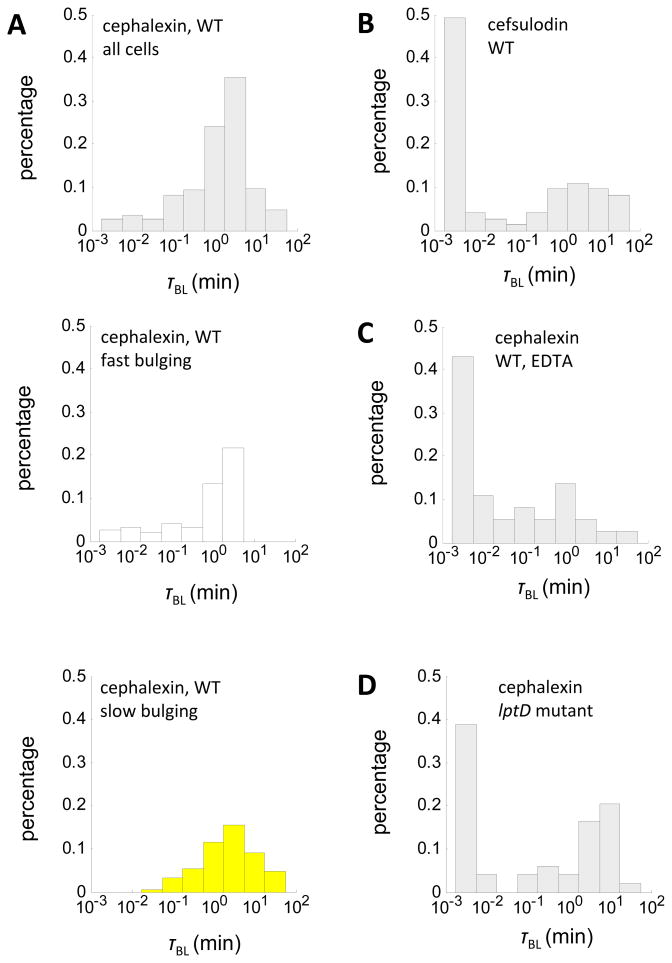
Figure 5. Both modes of bulge formation and integrity of the OM affect bulge stability.
(A) Bulge lifetime distribution of all wildtype cells (left, N=195), and subpopulation of fast bulging cells (middle, N=95) and slow bulging cells (right, N=100) under cephalexin treatment (50 μg/mL). (B) Bulge lifetime distribution of wildtype cells (N=73) under cefsulodin treatment (50 μg/mL). (C–D) Bulge lifetime distribution of EDTA-treated cells (N=37) and lptD mutants (N=49) under cephalexin treatment (50 μg/mL). See also Figure S5 and Movie S3.
Outer membrane integrity affects bulge stability
While bulge stability correlates with the rate of bulge formation, not all of its large variability is explained. Since the OM encloses the bulge, we hypothesized that its strength may affect bulge stability and identified two ways to influence OM integrity. First, we used a chelating agent (EDTA) to remove Mg2+, which stabilizes the electrostatic interactions among adjacent LPS molecules in the OM (Nikaido and Vaara, 1985). Second, we used an E. coli strain carrying a mutation in lptD, which compromises its efficiency in the assembly of LPS molecules at the OM (Freinkman et al., 2011). While EDTA may have non-specific effects on other cellular processes, the mutation in lptD is specific to OM biogenesis. Indeed, the measurement of Minimal Inhibitory Concentration (MIC) for lptD mutants showed a 30-fold increase in susceptibility towards vancomycin, but it also showed unchanged susceptibility towards hydrophilic antibiotics, including cephalexin (Fig. S5). Without additional stress, cells with a leaky OM increase in biomass at a normal rate with only mild defects in daughter cell separation (Fig. S5). These results confirmed that the lptD mutation compromises OM integrity without major effect on other aspects of cell growth.
We applied our imaging platform to monitor the progression to lysis of EDTA-treated wildtype cells and lptD mutants under cephalexin treatment. Both OM perturbations -2 reduced bulge stability dramatically (Fig. 5C, D). For many fast-lysing cells (τBL<10−2min), bulge formation did not reach completion in both scenarios and membranes ruptured prematurely (Fig. S5). Unchanged susceptibility to cephalexin ruled out higher effective drug concentration as the cause for reduced bulge lifetime in lptD mutants. Conversely, stabilizing the LPS layer by adding Mg2+ lengthened bulge lifetime and generated stable spheroplasts (Fig. 6A and Movie S4) (Birdsell and Cota-Robles, 1967; Joseleau-Petit et al., 2007; Lederberg and St Clair, 1958). Lysis still occurred when 0.3 M sucrose was used to generate similar osmolarity; therefore, the hypertonic environment alone cannot explain the protective effect of Mg2+. These results suggested that, in Gram-negative bacteria, mechanical support provided by the LPS layer prevents the bulge from rupturing despite internal turgor pressure.
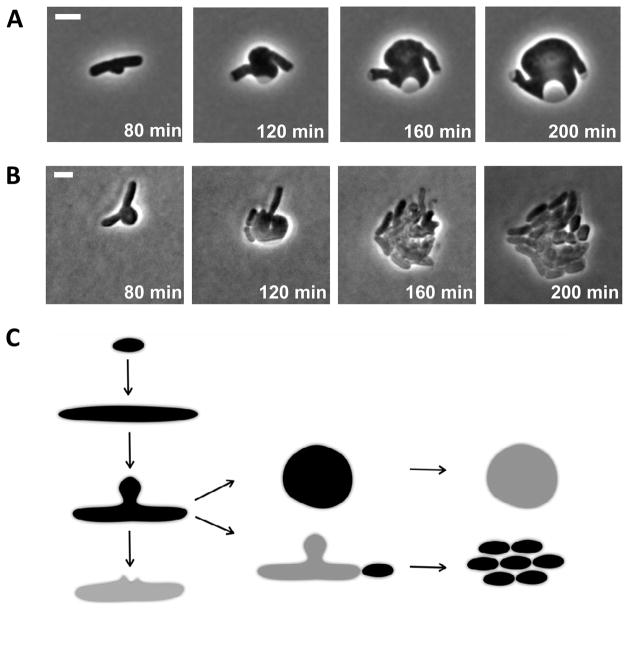
Figure 6. Alternative cell fates of bulging cells.
(A) Snapshots of spheroplast formation induced by cephalexin. Initial stages of spheroplast formation are identical with those of beta-lactam induced cell lysis. These remarkably stable spheroplasts lysed immediately upon Mg2+ removal, as judged by optical density drop of the liquid culture. (B) Snapshots of bulging cells reverting to rod shape cells upon drug removal. Cells with stabilized bulges escaped lysis by initiating septation near old poles and pinching off portions of the filaments. (C) Proposed model of beta-lactam induced bulges as a meta-stable state towards cell lysis, spheroplast formation, or reversion to rod-shaped cells. All scale bars represent 2 μm. See also Movie S4 and S5.
Bulging cells resume normal growth upon drug removal
Is bulge formation a reversible process? Are bulging cells able to revert to rod shape upon drug removal? To answer these questions, we acquired time-lapse microscopy of bulging cells upon drug removal. Mg2+ was added throughout to prevent lysis from washing, re-suspension and transfer onto our imaging platform (Experimental Procedures). After 2 hours, all bulging cells (20 out of 20) escaped lysis and formed rapidly growing micro-colonies. Notably, such rapid recovery was initiated by pinching off poles of the rod-shape filaments (Fig. 6B and Movie. S5). The bulge itself, which continued to grow, did not contribute to the reversion of new, healthy rod-shaped cells. Consistently, all spheroplasts examined (20 out of 20) never recovered upon drug removal and ultimately lysed. To summarize, bulging cells, unlike spheroplasts, are in a meta-stable state, which can lead either to cell lysis, or recovery upon drug removal (Fig. 6C).
Discussion
Using live cell microscopy at high time resolution combined with automated image analysis, this study characterized morphological dynamics of single cells under the treatment of beta-lactam antibiotics. We found that cell lysis induced by beta-lactam antibiotics shares a common progression through four physical phases: elongation, bulge formation, bulge stagnation and lysis. Two of these stages, bulge formation and bulge stagnation, showed large variability within isogenic cell populations. Understanding the factors contributing to the variability in bulge stability could be critical, because cells with stabilized bulges demonstrated capacity to escape lysis upon drug removal.
We identified two factors that influence the stability of the bulge. First, genetic and chemical perturbations to the OM strongly affect the stability of the bulge. It is conceivable that isogenic cells can vary in the abundance and activity of cellular components that affect OM integrity, such as the LPS transport pathway or the Tol-Pal complex (Gerding et al., 2007). Second, the stability of the bulge is affected by the mode of bulge formation, with fast-formed bulges being less stable. Bulge formation is ultimately a cell wall dependent process; membrane eruption requires the formation of a cell wall lesion exceeding a critical size (Daly et al., 2011). Indeed, we find that perturbing the cell wall with different classes of beta-lactams leads to substantially different modes of bulge formation. This is also consistent with the fact that glycopeptides antibiotics, which inhibit cell wall synthesis non-specifically, induce diverse modes of bulge formation (Huang et al., 2008).
The fact that we also observed variability in bulge formation across isogenic cells treated with the same drug, suggests that cells vary in cellular states or components that affect the cell wall damage caused by beta-lactams. We tested whether the rate of bulge formation depends on the cell elongation state upon exposure to the drug. However, we found that initial cell length, a proxy for cell states, is not correlated with either bulge formation time or bulge lifetime. This lack of correlation is likely because cephalexin does not act until the localization of FtsI, a late event in bacterial cell cycle (Goehring and Beckwith, 2005). This observation suggests that cell-cell variability in other components that affect PG biogenesis and metabolism (e.g. PG hydrolases) might underlie the observed heterogeneity in the bulge formation across isogenic cells. The ability to quantitatively characterize bulge stability enabled by our imaging platform can be used as a tool to explore the contribution of such cellular components to cell lysis.
The meta-stability of the bulge and its dependence on the integrity of the OM suggest that an intact OM can support some level of turgor pressure. It is interesting to speculate that this mechanical strength of the OM may also play a role in counterbalancing turgor pressure in intact cells, adding to the mechanical support provided by the cell wall. It is worth noting two observations supporting this idea. First, Gram-positive bacteria, which lack the added support from the OM, are known to have a much thicker cell wall compared to their Gram-negative counterparts. Second, studies have shown that host cell lysis by bacteriophage lambda requires the Rz-Rz1 complex, which disrupts the OM after cell wall destruction (Berry et al., 2008). Taken together, these observations suggest a mechanical role for the OM in stabilizing the cell envelop in bulging cells and possibly also in intact cells under normal physiological condition. Since lysis requires failure of both PG and the OM, OM-targeting therapeutics combined with beta-lactams antibiotics might promote their efficacy and reduce the incidence of resistance. The recognition that the OM stabilizes bulging cells may therefore have important clinical implications.
Experimental Procedures
Bacterial strains, plasmids and media
Wildtype strain used in this study is JOE309 (Chen and Beckwith, 2001), derived from E. coli K-12 strain MC4100. lptD mutant (MC4100 .lptD::kan pET23/42 lptD .529–538) and the fluorescent strain with an outer membrane marker (TB28(attHK MM47) Plac::ssPal-mCherry) are described in our recent work (Freinkman et al., 2011) and work from the Bernhardt lab (Paradis-Bleau et al., 2010). The fluorescent strain with an inner membrane marker (ZipA-mCherry) was kindly provided by the Bernhardt lab. All live cell time-lapse experiments were performed in Miller’s LB/agarose pads at 37 °C. Beta-lactam antibiotics were all freshly prepared and added into melted LB/agarose before each experiment. All cells were treated with 50 μg/mL cephalexin, unless noted otherwise.
Fluorescence microscopy
Cells were taken from an overnight culture, diluted by 100 fold and allowed for further growth in LB for 2 hours at 37 °C to reach early exponential phase (OD600nm ~ 0.05). After that, beta-lactam antibiotics were added into the liquid culture and cells were grown in the presence of antibiotic treatment for 30 ~ 90 min before imaging. Cells were sampled periodically throughout the treatment to examine their morphology. For membrane staining, FM 4–64 dye was added at the appropriate concentration. Cells were transferred from liquid culture onto drug-free LB agarose pads for imaging. Fluorescence images were taken immediately with appropriate filter sets to visualize YFP, FM 46-4 and mCherry.
Live cell time-lapse microscopy
Cells were first grown as described above into early exponential phase (OD600nm ~ 0.05). After that, cells were concentrated (10 fold) by centrifuge and re-suspension. 1 μL of the concentrated cell culture was then transferred onto a drug-containing LB agarose pad (200 μL) for live cell microscopy with a 100× oil immersion objective. For spheroplast formation, 100 mM Mg2+ was also added into these LB/agarose pads. All LB/agarose pads were discs with a diameter of 1 cm and a height of 0.5 cm. The pads were also surrounded by polydimethylsiloxane (PDMS) mold to create an air pocket and sealed by a cover glass to prevent evaporation. The final cell density on agarose pads was ~106 cells/mL. Phase contrast images were collected from the same field of view (containing ~25 cells) with auto focus on. All live cell microscopy was performed using NIS-Elements software to control a digital imaging system coupled to an inverted microscope (model TE2000-E; Nikon). Image acquisition was carried out at 1 min/frame for the first 30 min after adding the drug and ~125 ms/frame after that until final lysis (~ 2 hours after addition of the drug).
Single-cell movies with adaptive time resolutions
In order to analyze the morphological dynamics of individual cells at adaptive time resolutions in accordance with processes with different kinetics, the complete dataset of ~30000 frames of full field of view (~ 25 cells) were reduced to ~500 frames for each individual cell by applying a variable sampling algorithm. The following two steps were implemented to generate these single-cell movies: (1) crop the regions containing individual cells within the full field of view and generate separate movies containing each single cell; (2) apply varying time resolutions to four different phases of beta-lactam killing. 1 out of every 100 images was sampled for filamentation leading to bulge formation and 1 out of every 10 for bulge stagnation. For bulge formation and the lysis process, every image was selected for further analysis. This second step produced ~ 500 selected frames of single-cell images and established adaptive time resolutions. All image analysis, including computational analysis of single-cell shape dynamics, was implemented in Matlab (The Mathworks).
Image analysis of single-cell morphological dynamics
The overall strategy of image analysis takes advantage of the dramatic change in pixel intensity across the cell boundary of the phase contrast images (Guberman et al., 2008; Reshes et al., 2008). The following four steps were implemented to characterize morphological features of single cells throughout beta-lactam treatment: (1) cell area (number of pixels within the cell contour) was plotted against a wide range of threshold values. The plot showed a plateau region, where changing threshold values produces little cell area change. The yellow cell contour was generated based on the medium value of this plateau range; (2) cyan and red lines were drawn manually for each single cell movie to measure bulge depth and cell length, respectively. Two intersection points between those lines and the cell contour were generated, and the distance between two intersection points yielded the value of bulge depth and cell length for each frame; (3) bulge depth was plotted against time, and t2, t3 and t4 were marked based on bulge depth measurement to calculate bulge formation time and bulge lifetime; (4) for cracking angle, two angle lines were drawn on both sides of the cyan line for bulge depth. Intersection points of two angle lines and line for bulge depth were used to calculate the cracking angle around the bulging region.
Recovery of the bulging cells
Cells were first grown as described above into early exponential phase (OD600nm ~ 0.05). After that, beta-lactam antibiotics and 100 mM Mg2+ was added into the liquid culture. Cells were then grown until OD600nm reached a plateau (OD600nm ~ 0.2), harvested immediately and washed twice with 100 mM Mg2+/LB 28 before transfer onto drug-free LB/agarose pads for live cell microscopy. Agarose pads were also supplemented with 100 mM Mg2+ to prevent premature cell lysis.
Supplementary Material
Highlights.
Live cell microscopy is used to monitor single cell shape dynamics.
Beta-lactam induced lysis proceeds through four distinct physical stages.
Dynamics of bulge formation and its stability vary for different beta-lactams.
Cells with stabilized bulges revert to rod shape upon drug removal.
Acknowledgments
This work was supported by National Institute of Health Grant 5R01GM081617-05 (to R.K.) and National Institute of Allergy and Infectious Disease Grant AI081059 (to D.K.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berry J, Summer EJ, Struck DK, Young R. The final step in the phage 39 infection cycle: the Rz and Rz1 lysis proteins link the inner and outer membranes. Mol Microbiol. 2008;70:341–351. doi: 10.1111/j.1365-2958.2008.06408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsell DC, Cota-Robles EH. Production and ultrastructure of lysozyme 43 and ethylenediaminetetraacetate-lysozyme spheroplasts of Escherichia coli. J Bacteriol. 1967;44(93):427–437. doi: 10.1128/jb.93.1.427-437.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HS, Yao Z, Goehring NW, Kishony R, Beckwith J, Kahne D. Rapid beta-lactam-induced lysis requires successful assembly of the cell division machinery. Proc Natl Acad Sci U S A. 2009;106:21872–21877. doi: 10.1073/pnas.0911674106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly KE, Huang KC, Wingreen NS, Mukhopadhyay R. Mechanics of membrane bulging during cell-wall disruption in Gram-negative bacteria. Phys Rev E Stat Nonlin Soft Matter Phys. 2011;83:041922. doi: 10.1103/PhysRevE.83.041922. [DOI] [PubMed] [Google Scholar]
- de Pedro MA, Holtje JV, Schwarz H. Fast lysis of Escherichia coli filament cells requires differentiation of potential division sites. Microbiology. 2002;148:79–86. doi: 10.1099/00221287-148-1-79. [DOI] [PubMed] [Google Scholar]
- Freinkman E, Chng SS, Kahne D. The complex that inserts lipopolysaccharide into the bacterial outer membrane forms a two-protein plug-andbarrel. Proc Natl Acad Sci U S A. 2011;108:2486–2491. doi: 10.1073/pnas.1015617108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahara Y, Nikaido H. Asymmetric localization of lipopolysaccharides on the outer membrane of Salmonella typhimurium. J Bacteriol. 1980;141:1463–1465. doi: 10.1128/jb.141.3.1463-1465.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerding MA, Ogata Y, Pecora ND, Niki H, de Boer PA. The trans-envelope Tol-Pal complex is part of the cell division machinery and required for proper outer-membrane invagination during cell constriction in E. coli. Mol Microbiol. 2007;63:10081025. doi: 10.1111/j.1365-2958.2006.05571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehring NW, Beckwith J. Diverse paths to midcell: assembly of the bacterial cell division machinery. Curr Biol. 2005;15:R514–526. doi: 10.1016/j.cub.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Guberman JM, Fay A, Dworkin J, Wingreen NS, Gitai Z. PSICIC: noise and asymmetry in bacterial division revealed by computational image analysis at sub-pixel resolution. PLoS Comput Biol. 2008;4:e1000233. doi: 10.1371/journal.pcbi.1000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamad B. The Antibiotic Market Market Indicators. Nat Rev Drug Discov. 2010;9:676–676. doi: 10.1038/nrd3267. [DOI] [PubMed] [Google Scholar]
- Holtje JV. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol Mol Biol Rev. 1998;62:181–203. doi: 10.1128/mmbr.62.1.181-203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KC, Mukhopadhyay R, Wen B, Gitai Z, Wingreen NS. Cell shape and cell-wall organization in Gram-negative bacteria. Proc Natl Acad Sci U S A. 2008;105:19282–19287. doi: 10.1073/pnas.0805309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby GH, Young KD. Cell Cycle-Independent Lysis of Escherichia coli by Cefsulodin, an Inhibitor of Penicillin-Binding Protein-1a and Protein-1b. Journal of Bacteriology. 1991;173:1–5. doi: 10.1128/jb.173.1.1-5.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseleau-Petit D, Liebart JC, Ayala JA, D’Ari R. Unstable Escherichia coli L forms revisited: growth requires peptidoglycan synthesis. J Bacteriol. 2007;189:65126520. doi: 10.1128/JB.00273-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y, Nikaido H. Outer membrane of Salmonella typhimurium: accessibility of phospholipid head groups to phospholipase c and cyanogen bromide activated dextran in the external medium. Biochemistry. 1976;15:2561–2570. doi: 10.1021/bi00657a012. [DOI] [PubMed] [Google Scholar]
- Lederberg J, St Clair J. Protoplasts and L-type growth of Escherichia coli. J Bacteriol. 1958;75:143–160. doi: 10.1128/jb.75.2.143-160.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages JM, James CE, Winterhalter M. The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nat Rev Microbiol. 2008;6:893–903. doi: 10.1038/nrmicro1994. [DOI] [PubMed] [Google Scholar]
- Paradis-Bleau C, Markovski M, Uehara T, Lupoli TJ, Walker S, Kahne DE, Bernhardt TG. Lipoprotein cofactors located in the outer membrane activate bacterial cell wall polymerases. Cell. 2010;143:1110–1120. doi: 10.1016/j.cell.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshes G, Vanounou S, Fishov I, Feingold M. Cell shape dynamics in Escherichia coli. Biophys J. 2008;94:251–264. doi: 10.1529/biophysj.107.104398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt BG. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975;72:2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper DJ, Strominger JL. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965;54:1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Typas A, Banzhaf M, van den Berg van Saparoea B, Verheul J, Biboy J, Nichols RJ, Zietek M, Beilharz K, Kannenberg K, von Rechenberg M, et al. Regulation of peptidoglycan synthesis by outer-membrane proteins. Cell. 2010;143:1097–1109. doi: 10.1016/j.cell.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise EM, Jr, Park JT. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci U S A. 1965;54:75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.