Abstract
Background
Fears that are maladaptive or inappropriate can be reduced through extinction training. However, extinction is highly context-sensitive, resulting in the renewal of fear following shifts in context, and limiting the clinical efficacy of extinction training. Lesion and inactivation studies have shown that the contextualization of extinction depends on the hippocampus. Parallel studies have found that intrahippocampal scopolamine blocks contextual fear conditioning. Importantly, this effect was replicated using a non-invasive technique in which a low dose of scopolamine was administered systemically. We aimed to transfer the effects of this non-invasive approach to block the contextualization of fear extinction.
Methods
Rats were tone fear conditioned and extinguished under various systemic doses of scopolamine or the saline vehicle. They were subsequently tested (off drug) for tone fear in a context that was the same (controls) or shifted (renewal group) with respect to the extinction context.
Results
The lowest dose of scopolamine produced a significant attenuation of fear renewal when renewal was tested either in the original training context or a novel context. The drug also slowed the rate of long-term extinction memory formation, which was readily overcome by extending extinction training. Scopolamine only gave this effect when it was administered during, but not after extinction training. Higher doses of scopolamine severely disrupted extinction learning.
Conclusions
We discovered that disrupting contextual processing during extinction with the cholinergic antagonist scopolamine blocked subsequent fear renewal. Low doses of scopolamine may be a clinically promising adjunct to exposure therapy by making extinction more relapse-resistant.
Keywords: fear conditioning, extinction, renewal, hippocampus, scopolamine, exposure therapy, anxiety
Introduction
Fear orchestrates and organizes the way in which each species defends itself in a dangerous situation (1). However, when the mechanisms underlying fear go awry, the consequence is as detrimental as a functional fear system is beneficial. Fear can be maladaptive when elicited in non-threatening situations or in excess, and the persistence of such inappropriate fears contributes to the formation of anxiety disorders and phobias (2).
Fear extinction, wherein a fear-provoking stimulus is repeatedly presented in the absence of an aversive consequence, provides one of the most effective ways of reducing fear (3). However, extinction does not erase the original fear memory formed during acquisition (4). Instead, extinction produces a new inhibitory memory that can serve to suppress, or compete with the original fear (5). Importantly, extinction memories are more tenuous and sensitive to disruption in contrast to the resilient and enduring nature of fear memories. Indeed, following extinction, several factors cause fear to recover (6). In fear renewal, a rodent presented with an extinguished stimulus in a context that is different than the extinction context will show a renewal of the original fear response (7, 8), demonstrating the context-dependency of extinction. This effect has been extended to human studies as well (9–11). Fear renewal poses a serious problem for the treatment of anxiety disorders in the clinic, as patients are often extinguished in a context (e.g. therapist’s office) that is different then the context in which they are likely to re-encounter extinguished fear stimuli (3).
Interestingly, the majority of experimental research on fear extinction and the development of techniques for the treatment of phobias and anxiety disorders have focused on methods of enhancing extinction or “erasing” the original fear memory, rather then on reducing relapse or preventing fear renewal. Specifically, pharmacological studies tend to use methods to enhance learning during extinction (e.g. cognitive enhancers). One such example is the use of the partial NMDA agonist d-cycloserine (DCS) to enhance extinction learning (12). While some, but not all, investigations of this drug have shown it increases the rate of extinction (12–16), there is considerable evidence that it does not change the nature of extinction (15). That is, extinction under DCS remains context bound and renewal is not attenuated (15, 16). Thus, while such approaches may enhance the rate of extinction, they importantly do not attenuate relapse effects such as renewal, which are perhaps a much more clinically important consideration for the long-term effectiveness of exposure treatment.
Taking a different approach, other groups of researchers have attempted to erase the original fear formed, as opposed to enhancing extinction. While fear extinction is thought to be a form of new inhibitory learning rather then an unlearning of the original fear (4, 6), researchers have suggested that extinction immediately following a traumatic event (10 minutes) may serve to “erase” or cause the “unlearning” of fear because extinction occurs before the trauma memory has consolidated (17). However, the translatability of this research is limited because exposure therapy must be given very soon after the trauma, which would be difficult to achieve in many situations. For example, in individuals that seek treatment for an anxiety disorder the interval between symptom onset and treatment seeking ranges between 9 and 23 years (18).
Other approaches have used a brief extinction (“reminder”) session followed by either propranolol (19–22) or a longer extinction session (23, 24) to cause a “deconsolidation” of the original fear memory. These methods have proved sometimes successful and sometimes not (25). Another limitation to this approach is that the mechanisms mediating this type of deconsolidation still appear to be time-limited, requiring therapy to occur shortly after trauma (26–28).
Behavioral strategies for overcoming the contextualization of extinction have attempted to extinguish in multiple contexts. These studies have also met with mixed results in human samples, with one research group showing a significant reduction of context renewal (29) and another group failing to find such benefits (30). Importantly, Bouton has shown that extinction in 4 different contexts did not prevent renewal when rats were shifted either back to the original fear acquisition context or an entirely novel context (31). Another behavioral approach uses a cue as a reminder for extinction. While this approach appears successful in highly controlled laboratory settings (32, 33) it has produced only weak and non-replicated results in a human anxious sample (34).
Recent investigations into the neural underpinnings of fear renewal offer important insights into the brain regions that must be targeted to specifically eliminate renewal. These studies have implicated the hippocampus (HPC) in the contextual regulation of extinction (35–40). These data show that lesions or temporary and reversible inactivation of the HPC prior to the acquisition and/or expression of fear extinction are able to disrupt fear renewal. Such findings are in line with a more general role for the hippocampus in the encoding and processing of integrated contextual representations (41, 42). Collectively, these findings demonstrate that contextual processing during fear-related learning may be attenuated by manipulations that render the HPC dysfunctional.
While interfering with hippocampal function during or shortly after extinction prevents fear renewal, the invasiveness of these experimental procedures makes them clinically unfeasible for the treatment of anxiety disorders. Therefore, we sought a readily translatable pharmacological approach to impair the hippocampus during extinction. Ideally such a treatment would have 3 characteristics: 1) it would be systemically deliverable; 2) it would have a history of clinical use in humans; and 3) it would preferentially target contextual learning. For insight, we turned to studies in contextual fear conditioning that used the muscarinic cholinergic antagonist scopolamine (SCOP) to temporarily and selectively disrupt contextual fear conditioning in a dose-dependent, hippocampus-mediated manner (43, 44).
Our prior work testing rats with a wide range of doses (.01 to 100 mg/kg) showed that low doses of the drug had selective effects on contextual learning and closely paralleled the behavioral effects of intrahippocampal SCOP (45). Higher doses had a more general impact on learning and behavior. For example, the ED50 for hippocampus-independent tone fear conditioning was about 14 times higher than the ED50 for hippocampus-dependent context conditioning, even though this comparison is made off-drug and tone and context learning occurred simultaneously. Additionally, SCOP has been used in humans to treat a variety of conditions including seasickness (46), Parkinson’s disease (47) and depression (48–50). Thus, SCOP met the three characteristics we noted above, indicating it was a promising candidate for the decontextualization of extinction.
To examine the effects of scopolamine in reducing fear renewal after extinction, rats were tone fear conditioned, extinguished under scopolamine or the vehicle saline, and subsequently tested for fear renewal. We used a design for which we have previously demonstrated a role for the hippocampus in renewal (35). Control experiments included the application of higher doses of scopolamine, post-injection administration of scopolamine and variations in the experimental design.
Methods and Materials
Subjects
A total of 90 naïve, adult male Long-Evans rats, initially weighing 300 g, purchased from Harlan (Indianapolis, IN) were used. Housing, feeding and handling involved standard procedures described in Supplemental Methods.
Drugs
Scopolamine hydrobromide (Sigma-Aldrich, St. Louis, MO) was dissolved in 0.9% saline (vehicle) to obtain a concentration of 0.1 mg/ml. Doses of 0.5, 1.35, and 2.7 were also tested (Supplemental Figure S1). Injections were made into the intraperitoneal (i.p.) cavity. See Supplemental Methods for additional information.
Behavioral Testing Apparatus, Fear Conditioning and Analyses
The behavioral testing apparatus consisted of three distinct physical “contexts” (counterbalanced) and modes of transport to these contexts (4 experimental chambers/context). A detailed description of fear conditioning procedures and automated analysis of freezing behavior are provided in the Supplemental Methods (also see Figure 1A).
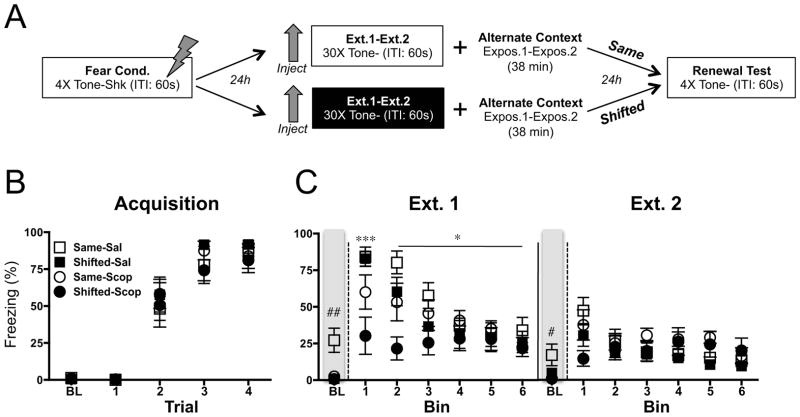
Figure 1.
Effects of scopolamine on fear extinction (two sessions). (A) Experimental design. Tone: 80 dB, 30 sec. Shock: .9 mA, 2 sec. Inter-trial Interval (ITI): 60 sec. (B) Fear acquisition. Mean (±sem) percent baseline (BL) freezing and tone freezing during each tone-footshock trial. (C) Extinction. Mean (±sem) percent freezing during baseline and each bin of 5 tone-alone presentations for extinction (ext.) sessions 1–2. Rats were extinguished in the same context as acquisition/test (Same) or in a novel context (Shifted), under scopolamine (Scop) or the vehicle saline (Sal) (n=8). Freezing during the first and second bins in the novel context was reduced by scopolamine compared to saline (***p <.001; *p <.05). Pre-extinction baseline fear was reduced for scopolamine-treated animals ((##p <.01; #p <.05).
Statistical Analyses
Data were statistically analyzed using analyses of variance (ANOVAs). Following significant findings, pairwise comparisons using a Bonferroni correction were performed. See Supplemental Methods for additional details.
Results
Scopolamine alters freezing during extinction
Tone fear-conditioned rats were extinguished under the influence of scopolamine to investigate the effects of scopolamine-mediated extinction on subsequent fear renewal. In our first experiment, rodents were fear conditioned and given two sessions of extinction under a low, systemic dose of scopolamine (i.p., 0.1 mg/kg) (design depicted in Figure 1A).
Figure 1B shows the fear acquisition data. All rats acquired significant tone fear across acquisition trials (F(3, 84) = 140.9, p < .0001). Animals were equally split into groups (Same/Shifted and Saline/Scopolamine) based on their final levels of fear and rate of acquisition, ensuring that groups were balanced prior to extinction. Freezing during extinction session one is displayed in Figure 1C (left panel). There was an overall significant effect of drug condition (F(1, 150) = 4.59, p < .05), which interacted with the rate of behavior change during extinction, such that animals extinguished under scopolamine showed a slower loss of freezing across trials compared to saline controls (F(5, 150) = 7.56, p < .0001). This was partially driven by an initial reduction in freezing in scopolamine compared to saline-treated animals extinguished in a novel context (shifted groups: Bin 1: p < .001; Bin 2: p < .05), and disappeared as saline controls extinguished to the same low levels of freezing as scopolamine rats (Bin: 6: p > .05). Caution should be exercised in interpreting extinction data while rats are on drug, as scopolamine is known to affect behavioral performance (45). Drug, context, and interaction effects disappeared by the second session of extinction (Figure 1C) (F’s < 1). Interestingly baseline context fear prior to each extinction session (collapsed across contexts) was also reduced by scopolamine (Ext.1: F(1, 28) = 8.50, p < .01; Ext.2: F(1, 28) = 6.33, p < .05), further suggesting that scopolamine may interfere with the processing of contextual cues (“BL” shown in left panel of Figures 1B and C).
Extinction under scopolamine attenuates renewal
Following extinction, rats were tested for tone fear renewal in the same context as extinction (“AAA” design) or shifted out of the extinction context (“ABA” renewal). Baseline freezing to the context prior to tone onset (Figure 2A) was similarly negligible between groups (F’s <1), ensuring that tone fear expression was not due to an interaction between tone and context fear (51). In the critical tone test after extinction (Figure 2B) the saline-treated shifted rats froze more than the saline-treated same context rats, demonstrating standard fear renewal in saline controls (p < .01). Scopolamine-treated rats froze similarly in the same and shifted conditions indicating that the drug abolished renewal (p > .05), providing evidence that intact cholinergic transmission during extinction is required for later fear renewal. This finding is consistent with those obtained when the hippocampus is inactivated during extinction (40) suggesting that cholinergic antagonism disrupts the ability for the HPC to function normally with respect to contextual encoding during extinction and subsequent contextual modulation of extinction memory retrieval. It should be noted that this effect was unique to this low dose of scopolamine, as extinction under higher of the drug had the effect of hindering extinction recall in a dose-dependent fashion (Supplemental Figure S1).
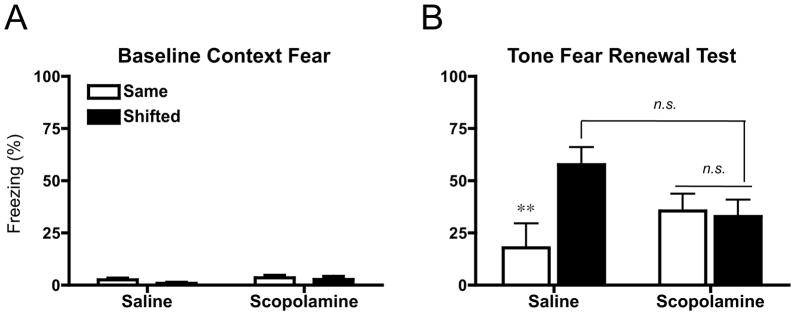
Figure 2.
Fear renewal following 2 extinction sessions. (A) Mean (±sem) percent freezing during 180 s baseline period preceding tone onset. (B) Mean (±sem) percent freezing to four, 30 sec tone presentations (ITI: 60 sec) in either the Same or Shifted context during test. Saline controls showed significant renewal (Same vs Shifted: **p<.01), which was blocked in animals that had been extinguished under the influence of scopolamine (n.s., p > .05).
While scopolamine completely blocked renewal, the difference between the saline-shifted and scopolamine-shifted groups fell short of statistical significance (p<.05). This may have been caused by a modest attenuation of extinction in both scopolamine treated groups. We hypothesized that because animals might have learned extinction in a changed, hippocampus-independent-like manner, extinction memories may have taken longer to acquire. Thus, we asked whether additional sessions of extinction under scopolamine could produce a greater reduction in context fear for shifted rats.
Extinction under scopolamine strengthens with additional sessions
To try to enhance the degree of extinction under scopolamine we conducted a longer, four-session, extinction phase to strengthen extinction memory recall in both the same and shifted conditions. Rats were fear conditioned, extinguished and tested using a procedure identical to that used in the two-session extinction experiment, with the exception that they received four sessions of extinction and context exposure (see Figure 3A for design).
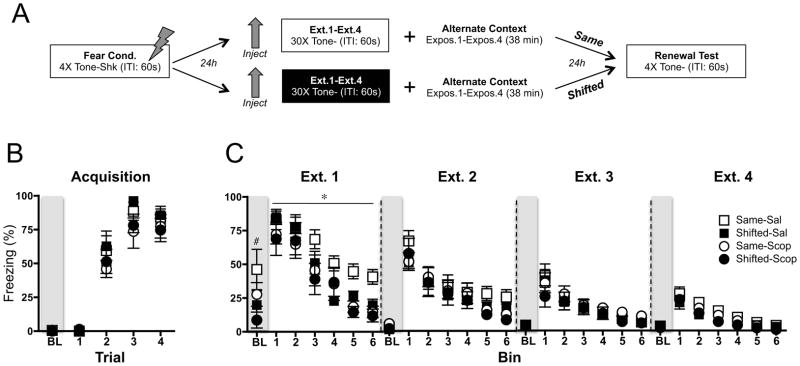
Figure 3.
Effects of scopolamine on four sessions of fear extinction. (A) Experimental design. Tone: 80 dB, 30 sec. Shock: .9 mA, 2 sec. Inter-trial Interval (ITI): 60 sec. (B) Fear acquisition. Mean (±sem) percent baseline (BL) freezing and tone freezing during each tone-footshock trial. (C) Extinction. Mean (±sem) percent freezing during baseline and each bin of 5 tone-alone presentations for extinction (ext.) sessions 1–4. Rats were extinguished in the same context as acquisition/test (Same) or in a novel context (Shifted), under scopolamine (Scop) or the vehicle saline (Sal) (n=7–8). Freezing during extinction 1 was reduced by scopolamine (*p <.05). Pre-extinction baseline fear was reduced for shifted animals (#p <.05).
All rats showed significant fear acquisition (F(3, 78) = 146.3, p < .0001) (Figure 3B). Similar to the previous results, the data for extinction session 1 (Figure 3C) reveal an overall significant amount of extinction across tone bins (F (5, 140) = 73.64, p < .0001), with a significant effect of drug such that scopolamine-extinguished animals showed lower fear expression during extinction compared to saline controls (F(1, 140) = 4.73, p < .05). Baseline freezing to the context prior to each extinction session revealed an overall increase in freezing for rodents extinguished in the acquisition context (extinction 1: F(1, 26)=4.27, p<.05) that did not interact with drug condition (F<1). Freezing differences during baseline and extinction disappeared by extinction 2.
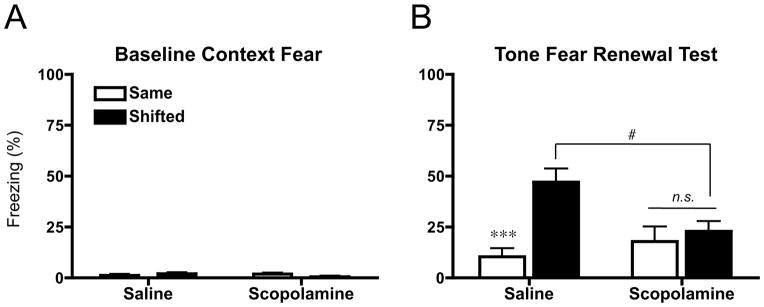
When animals were tested for tone fear renewal, we found again, minimal levels of baseline freezing (Figure 4A). Average freezing to the tone at test (Figure 4B) demonstrated fear renewal in saline controls (p < .001), which was blocked by scopolamine (p > .05). This replicated our previous finding, providing further support for the idea that intact cholinergic transmission during extinction is required for fear renewal. Moreover, we saw a significant attenuation of tone freezing in the shifted condition for scopolamine contrasted to saline rats (p < .05). Thus, while the formation of extinction memories under cholinergic antagonism may require more extinction training, what is learned seems to be more resilient to fear relapse effects such as renewal.
Figure 4.
Fear renewal following four sessions of extinction. (A) Mean (±sem) percent freezing during 180 s baseline period preceding tone onset. (B) Mean (±sem) percent freezing to four, 30 sec tone presentations (ITI: 60 sec) in either the Same or Shifted context at test. Saline controls showed significant renewal (***p<.001), which was blocked by pre-extinction scopolamine (n.s., p>.05). Scopolamine-extinguished rats showed a significant decrease in freezing in the shifted condition (#p<.05).
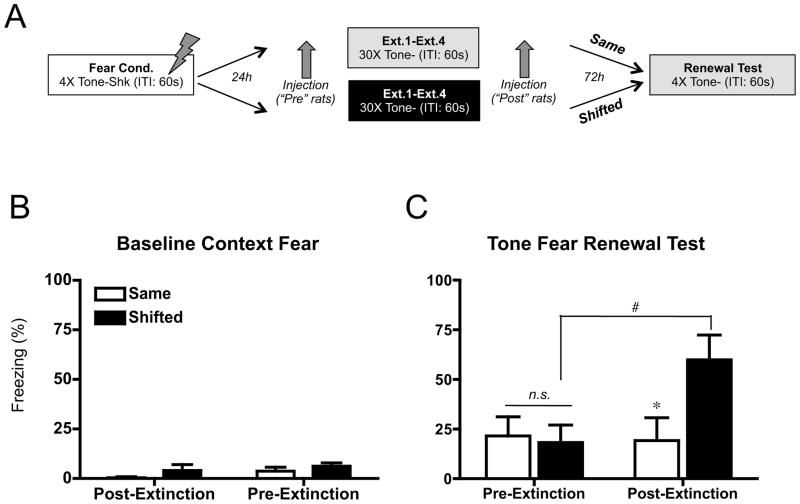
Scopolamine before, but not after, extinction prevents renewal in a novel context
In the first 3 experiments (Figures 1, 3 and S1) we examined the renewal that occurs when testing is in the original fear acquisition context (ABA renewal). Renewal also occurs when testing is in a novel context (ABC renewal (5)). ABC renewal may be more clinically relevant than ABA renewal as return to the same context as fear acquisition is unlikely. Therefore, to test the generality of our findings the final experiment used an ABC renewal design. The renewal condition used a different context for acquisition (A), extinction (B) and renewal (C). The controls had acquisition in one context (A) while both extinction and testing occurred in the same context (C).
Our interpretation of scopolamine’s influence on renewal is that extinction training becomes more general when context processing is disrupted during extinction. This interpretation predicts that scopolamine must be present during extinction to influence later renewal of fear. However, the prior experiments did not rule out the possibility that simply receiving scopolamine influenced later performance, as controls never received the drug. Therefore, rather than a vehicle-only control, the present experiment injected the drug 15 min after extinction in the control condition. In this way all animals were equated for drug exposure, the difference was whether or not the drug was given in conjunction with extinction.
While scopolamine made extinction more generalizable, it is possible that it diminished the robustness of the extinction. For example, extinction memory acquired under scopolamine might not last as long as drug-free extinction. Therefore, we tested renewal 3 days rather than one day after extinction.
Our ABA extinction design equated exposure to both the extinction and alternate contexts (see figures 1a, 3a, S1a). This procedure equates all groups for exposure to the various contexts. It is also a necessary feature of an ABA design as it reduces baseline context fear prior to testing, which is required for auditory fear conditioning data to be interpretable (51). By testing renewal in a novel context, the ABC design obviates the need for extinguishing fear of the test context. During exposure therapy clients are unlikely to have anything equivalent to our “alternate” context exposure, so the ABC design again makes this procedure more analogous to a typical exposure treatment.
Fear acquisition and extinction proceeded similar to our previous experiments (Figures 1 and 3). Seventy-two hours after the final extinction session, animals were tested for tone fear in either a novel context (“shifted” rats) or the context in which they were extinguished (“same” rats). Baseline freezing (Figure 5B) prior to presentation of the tone was low and similar across groups (F < 1).
Figure 5.
Effects of pre or post extinction scopolamine on fear renewal in a novel context and recovery following the passage of time. (A) Experimental design. Tone: 80 dB, 30 sec. Shock: .9 mA, 2 sec. Inter-trial Interval (ITI): 60 sec. Note that the tone test occurred in a third, novel context. (B) Mean (±sem) percent freezing during 180 s baseline period preceding tone onset. (C) Mean (±sem) percent freezing to four, 30 sec tone presentations (ITI: 60 sec) in either the Same or Shifted context at test. Pre-extinction scopolamine injected animals showed no fear renewal, as freezing levels between the same and shifted groups were not significantly different (n.s.,p >.05). Conversely, animals injected with scopolamine subsequent to extinction sessions showed significant renewal (*p<.05). Pre-extinction scopolamine-extinguished rats showed a significant decrease in freezing in the shifted condition (#p<.05) compared to shifted rats injected post-extinction (n=7–8).
Post-extinction injected animals displayed significant fear renewal; the shifted rats froze more than non-shifted rats (Figure 5C), p < .05). Pre-extinction injected rats did not show renewal; average freezing to the tone at test demonstrated no differences in freezing between shifted and same animals treated with pre-training scopolamine (Figure 5C, p > .05). This blockade replicated our previous findings, extending our attenuation of renewal effect to renewal in a novel context and to fear recovery after the passage of time. Importantly, pre-extinction scopolamine-extinguished rats showed a significant decrease in freezing in the shifted condition (p < .05) compared to shifted rats injected post-extinction. Thus scopolamine must be present during extinction to have its effects; simply receiving the drug does not impact later performance. In addition, the tone-fear extinction memory was still robust 3 days after extinction.
Discussion
In the data reported here, we examined the effects of administering scopolamine prior or subsequent to extinction training on fear renewal. We found that when extinction training occurred under scopolamine, a significant attenuation in fear renewal could be observed. Indeed, extinction memories formed under scopolamine were long lasting and more resilient to shifts in context. This reduced sensitivity to context shift made the rats less susceptible to relapse effects such as renewal. Furthermore, we showed that the effect on renewal is an interaction between extinction training and drug; the drug only works this way when given in conjunction with extinction. Collectively, these findings suggest that cholinergic blockade during extinction produces learning that is changed in nature: the safety memory is more slowly acquired and resilient to shifts in context. Importantly, the slowing of log-term memory formation is readily overcome by additional extinction training (compare Figures 2B and 4B).
Extinction memories formed under scopolamine are slower to acquire
The rate at which a fear behavior is reduced across repeated exposures to a feared stimulus is often taken as an indication of successful fear extinction. Importantly, facilitating or increasing the rate of extinction performance has been used as an indirect marker of enhanced extinction. The data presented here suggests that not only is extinction rate a poor indicator of whether a fear is actually being extinguished, but that slower extinction may in some cases be indicative of extinction memories that are resilient to relapse.
That is, while the presence of scopolamine during extinction makes freezing behavior during extinction or “within-session extinction” difficult to interpret, the requirement for additional sessions of extinction (four as opposed to two session) in order to reduce tone fear at test for scopolamine treated rats demonstrates that extinction learning under scopolamine requires more sessions to be learned. Indeed, when scopolamine is administered during or after a single session of extinction, animals fail to retain those extinction memories (52). In this study, Santini et al., reported that a systemic scopolamine dose 15 times greater than our 0.1 mg/kg dose reduced extinction when only a single extinction session was used. They attributed this effect to an action on infralimbic cortex as a direct cortical infusion had similar effects. Thus, it is possible that in our experiments the drug’s ability to slow the formation of the extinction memory was mediated by the infralimbic cortex, while the drug’s ability to enhance generalization of the extinction memory involved the hippocampus (see below). It is also possible that Santini et al. would have seen similar effects had they used a more robust extinction training procedure as was used here.
Thus, scopolamine may disrupt some of the processes underlying extinction such that additional sessions are required to retain extinction memories, however, the memories that are eventually formed posses qualities that contribute to its resiliency. This is consistent with the idea that the rate of within-session extinction is a poor indicator of whether an extinction memory will be retained at test (53–56).
De-contextualizing extinction
Another key characteristic of extinction is its sensitivity to the context in which it occurs (6). This contextualization of extinction lies at the heart of relapse, as extinction – no matter how strong or how weak – remains dependent on context specificity for expression. The experiments presented here directly address this issue in that they specifically target the contextual encoding that occurs during extinction utilizing a pharmacological agent previously shown to block contextual processing mediated by the dorsal hippocampus (43, 45).
The approach taken here is not the first to specifically target the context-dependency of renewal, however, the method implemented to do so is. Previous researchers have focused on attenuating renewal by making extinction learning more generalizable across contexts. Notably, experiments in both rodents and human subjects alike have attempted to make extinction more generalizable and less susceptible to renewal by extinguishing in multiple contexts or using reminder cues from the extinction session to “bridge the gap” between extinction and test contexts (9, 31, 34, 57). These studies have resulted in mixed findings with some obtaining benefits of such behavioral protocols and others not.
Our approach similarly aimed to reduce renewal, but instead aimed to remove the contextual encoding that occurs during extinction itself by using a low dose of scopolamine, a cholinergic antagonist which has been shown to interfere with contextual processing and exert its effect via the dorsal hippocampus (44). This approach took advantage of the known research regarding the role of the hippocampal formation in both context fear and fear renewal after extinction. Indeed, the hippocampus has been repeatedly implicated in the formation of contextual representations (41, 42) and the contextual encoding and/or retrieval required to drive renewal (35–40, 58, 59). By administering scopolamine prior to extinction, we were able to mimic the attenuation of renewal observed when the hippocampus is directly inactivated or lesioned during extinction. Thus, our data extend the notion that the hippocampus is normally involved in the context-bound nature of extinction, and reveals a non-invasive, temporary and translatable pharmacological manipulation that serves to “de-contextualize” extinction learning.
Cholinergic contributions to contextual and cued learning
Cholinergic transmission has been repeatedly implicated in fear conditioning paradigms thought to involve the hippocampus, such as contextual and trace fear conditioning (43–45, 60–62). A number of microinfusion studies further localize the role of muscarinic acetylcholine receptors to areas including the dorsal hippocampus, entorhinal cortex and perirhinal cortex (44, 63–65). The data presented here extend the role of cholinergic processing from these contextual fear effects to context-sensitive fear extinction. This suggests that the role of the dorsal hippocampus in contextual fear conditioning and fear extinction is similar (35) with both effects dependent on intact cholinergic transmission.
Systemic administration of scopolamine has been shown to also effect delay auditory fear conditioning, provided high doses are administered (45, 62). Interestingly, we found that at higher doses of scopolamine, animals failed to appropriately extinguish their tone fear at all, consistent with the blockade of processing auditory associations. Moreover, it has been suggested that high doses of scopolamine (8 mg/kg), administered prior to testing for fear after extinction might serve to reverse extinction that has already accrued to the CS (66). Collectively, these findings are inline with the idea that low doses of scopolamine effect hippocampal, but not amygdala processing, while higher doses may additionally disrupt functioning within the amygdala.
Clinical Implications
These findings support the idea that disrupting hippocampal functioning during extinction produces a changed, “context-free” extinction. These results have implications for the treatment of phobias and anxiety disorders in the clinic. Currently championed pharmacological adjuncts to exposure therapy have focused on cognitive enhancers (12, 14), which while speeding up extinction do not change its fundamental, context-dependent nature. In contrast, we used a compound that is known to impair cognition and while this manipulation slowed the rate of extinction memory formation (requiring more training sessions) it made extinction more robust. Moreover, scopolamine is a drug that is easily translatable to the clinic, as it is already approved for several uses in humans (see Introduction).
The clinical translatability of scopolamine, combined with the finding that extinction memories formed under this compound, while slower to acquire, demonstrate a changed nature – resilient against shifts in the environment – suggests that clinical-based treatments may benefit by incorporating scopolamine into exposure therapy.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01 MH62122 (M.S.F.), a NARSAD Distinguished Investigator Award # 18667 (MSF) and a Charles F. Scott Endowed Fellowship (M.Z.).
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bolles RC, Fanselow MS. A Perceptual-Defensive-Recuperative Model of Fear and Pain. Behav Brain Sci. 1980;3(2):291–301. [Google Scholar]
- 2.Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychol Rev. 2001;108(1):4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- 3.Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behav Res Ther. 2008;46(1):5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Pavlov IP. Conditioned Reflexes. Oxford University Press; 1927. [Google Scholar]
- 5.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52(10):976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 6.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11(5):485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 7.Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process. 1979;5(4):368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- 8.Bouton ME, King DA. Contextual control of the extinction of conditioned fear: tests for the associative value of the context. J Exp Psychol Anim Behav Process. 1983;9(3):248–265. [PubMed] [Google Scholar]
- 9.Mystkowski JL, Craske MG, Echiverri AM, Labus JS. Mental reinstatement of context and return of fear in spider-fearful participants. Behav Ther. 2006;37(1):49–60. doi: 10.1016/j.beth.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Mystkowski JL, Mineka S, Vernon LL, Zinbarg RE. Changes in caffeine states enhance return of fear in spider phobia. J Consult Clin Psychol. 2003;71(2):243–250. doi: 10.1037/0022-006x.71.2.243. [DOI] [PubMed] [Google Scholar]
- 11.Mineka S, Mystkowski JL, Hladek D, Rodriguez BI. The effects of changing contexts on return of fear following exposure therapy for spider fear. J Consult Clin Psychol. 1999;67(4):599–604. doi: 10.1037//0022-006x.67.4.599. [DOI] [PubMed] [Google Scholar]
- 12.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22(6):2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60(4):369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 14.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61(11):1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 15.Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behav Neurosci. 2006;120(5):1159–1162. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]
- 16.Bouton ME, Vurbic D, Woods AM. D-cycloserine facilitates context-specific fear extinction learning. Neurobiol Learn Mem. 2008;90(3):504–510. doi: 10.1016/j.nlm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers KM, Ressler KJ, Davis M. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learn Mem. 2006;13(2):216–223. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang PS, Berglund P, Olfson M, Pincus HA, Wells KB, Kessler RC. Failure and delay in initial treatment contact after first onset of mental disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):603–613. doi: 10.1001/archpsyc.62.6.603. [DOI] [PubMed] [Google Scholar]
- 19.Debiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129(2):267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12(3):256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- 21.Soeter M, Kindt M. Dissociating response systems: erasing fear from memory. Neurobiol Learn Mem. 2010;94(1):30–41. doi: 10.1016/j.nlm.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42(6):503–506. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324(5929):951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463(7277):49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang CH, Berke JD, Maren S. Single-unit activity in the medial prefrontal cortex during immediate and delayed extinction of fear in rats. PLoS One. 2010;5(8):e11971. doi: 10.1371/journal.pone.0011971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330(6007):1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inda MC, Muravieva EV, Alberini CM. Memory retrieval and the passage of time: from reconsolidation and strengthening to extinction. J Neurosci. 2011;31(5):1635–1643. doi: 10.1523/JNEUROSCI.4736-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36(3):521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- 29.Vansteenwegen D, Vervliet B, Iberico C, Baeyens F, Van den Bergh O, Hermans D. The repeated confrontation with videotapes of spiders in multiple contexts attenuates renewal of fear in spider-anxious students. Behav Res Ther. 2007;45(6):1169–1179. doi: 10.1016/j.brat.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 30.Neumann DL, Waters AM, Westbury HR. The use of an unpleasant sound as the unconditional stimulus in aversive Pavlovian conditioning experiments that involve children and adolescent participants. Behav Res Methods. 2008;40(2):622–625. doi: 10.3758/brm.40.2.622. [DOI] [PubMed] [Google Scholar]
- 31.Bouton ME, Garcia-Gutierrez A, Zilski J, Moody EW. Extinction in multiple contexts does not necessarily make extinction less vulnerable to relapse. Behav Res Ther. 2006;44(7):983–994. doi: 10.1016/j.brat.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Brooks DC, Bouton ME. A retrieval cue for extinction attenuates spontaneous recovery. J Exp Psychol Anim Behav Process. 1993;19(1):77–89. doi: 10.1037//0097-7403.19.1.77. [DOI] [PubMed] [Google Scholar]
- 33.Vansteenwegen D, Vervliet B, Hermans D, Beckers T, Baeyens F, Eelen P. Stronger renewal in human fear conditioning when tested with an acquisition retrieval cue than with an extinction retrieval cue. Behav Res Ther. 2006;44(12):1717–1725. doi: 10.1016/j.brat.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Culver NC, Stoyanova M, Craske MG. Clinical relevance of retrieval cues for attenuating context renewal of fear. J Anxiety Disord. 2011;25(2):284–292. doi: 10.1016/j.janxdis.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Zelikowsky M, Pham DL, Fanselow MS. Temporal factors control hippocampal contributions to fear renewal after extinction. Hippocampus. 2011 doi: 10.1002/hipo.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17(9):749–758. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- 37.Ji J, Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn Mem. 2005;12(3):270–276. doi: 10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci. 2001;21(5):1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corcoran KA, Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn Mem. 2004;11(5):598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25(39):8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110(1–2):73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- 42.Fanselow MS. From contextual fear to a dynamic view of memory systems. Trends Cogn Sci. 2010;14(1):7–15. doi: 10.1016/j.tics.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anagnostaras SG, Maren S, Fanselow MS. Scopolamine selectively disrupts the acquisition of contextual fear conditioning in rats. Neurobiol Learn Mem. 1995;64(3):191–194. doi: 10.1006/nlme.1995.0001. [DOI] [PubMed] [Google Scholar]
- 44.Gale GD, Anagnostaras SG, Fanselow MS. Cholinergic modulation of pavlovian fear conditioning: effects of intrahippocampal scopolamine infusion. Hippocampus. 2001;11(4):371–376. doi: 10.1002/hipo.1051. [DOI] [PubMed] [Google Scholar]
- 45.Anagnostaras SG, Maren S, Sage JR, Goodrich S, Fanselow MS. Scopolamine and Pavlovian fear conditioning in rats: dose-effect analysis. Neuropsychopharmacology. 1999;21(6):731–744. doi: 10.1016/S0893-133X(99)00083-4. [DOI] [PubMed] [Google Scholar]
- 46.Bar R, Gil A, Tal D. Safety of double-dose transdermal scopolamine. Pharmacotherapy. 2009;29(9):1082–1088. doi: 10.1592/phco.29.9.1082. [DOI] [PubMed] [Google Scholar]
- 47.Perez LM, Farriols C, Puente V, Planas J, Ruiz I. The use of subcutaneous scopolamine as a palliative treatment in Parkinson’s disease. Palliat Med. 2011;25(1):92–93. doi: 10.1177/0269216310381662. [DOI] [PubMed] [Google Scholar]
- 48.Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63(10):1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furey ML, Khanna A, Hoffman EM, Drevets WC. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479–2488. doi: 10.1038/npp.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janowsky DS. Serendipity strikes again: scopolamine as an antidepressant agent in bipolar depressed patients. Curr Psychiatry Rep. 2011;13(6):443–445. doi: 10.1007/s11920-011-0239-6. [DOI] [PubMed] [Google Scholar]
- 51.Jacobs NS, Cushman JD, Fanselow MS. The accurate measurement of fear memory in Pavlovian conditioning: Resolving the baseline issue. J Neurosci Methods. 2010;190(2):235–239. doi: 10.1016/j.jneumeth.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santini E, Sepulveda-Orengo M, Porter JT. Muscarinic Receptors Modulate the Intrinsic Excitability of Infralimbic Neurons and Consolidation of Fear Extinction. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pitman RK, Orr SP, Altman B, Longpre RE, Poire RE, Macklin ML. Emotional processing during eye movement desensitization and reprocessing therapy of Vietnam veterans with chronic posttraumatic stress disorder. Compr Psychiatry. 1996;37(6):419–429. doi: 10.1016/s0010-440x(96)90025-5. [DOI] [PubMed] [Google Scholar]
- 54.Pitman RK, Orr SP, Altman B, Longpre RE, Poire RE, Macklin ML, Michaels MJ, Steketee GS. Emotional processing and outcome of imaginal flooding therapy in Vietnam veterans with chronic posttraumatic stress disorder. Compr Psychiatry. 1996;37(6):409–418. doi: 10.1016/s0010-440x(96)90024-3. [DOI] [PubMed] [Google Scholar]
- 55.Plendl W, Wotjak CT. Dissociation of within- and between-session extinction of conditioned fear. J Neurosci. 2010;30(14):4990–4998. doi: 10.1523/JNEUROSCI.6038-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy. 2008;46(1):5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60(4):352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 58.Ji J, Maren S. Lesions of the entorhinal cortex or fornix disrupt the context-dependence of fear extinction in rats. Behav Brain Res. 2008;194(2):201–206. doi: 10.1016/j.bbr.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ji J, Maren S. Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learn Mem. 2008;15(4):244–251. doi: 10.1101/lm.794808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pang MH, Kim NS, Kim IH, Kim H, Kim HT, Choi JS. Cholinergic transmission in the dorsal hippocampus modulates trace but not delay fear conditioning. Neurobiol Learn Mem. 2010;94(2):206–213. doi: 10.1016/j.nlm.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 61.Hunt PS, Richardson R. Pharmacological dissociation of trace and long-delay fear conditioning in young rats. Neurobiol Learn Mem. 2007;87(1):86–92. doi: 10.1016/j.nlm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 62.Feiro O, Gould TJ. The interactive effects of nicotinic and muscarinic cholinergic receptor inhibition on fear conditioning in young and aged C57BL/6 mice. Pharmacol Biochem Behav. 2005;80(2):251–262. doi: 10.1016/j.pbb.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 63.Bang SJ, Brown TH. Muscarinic receptors in perirhinal cortex control trace conditioning. J Neurosci. 2009;29(14):4346–4350. doi: 10.1523/JNEUROSCI.0069-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rogers JL, Kesner RP. Cholinergic modulation of the hippocampus during encoding and retrieval of tone/shock-induced fear conditioning. Learn Mem. 2004;11(1):102–107. doi: 10.1101/lm.64604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esclassan F, Coutureau E, Di Scala G, Marchand AR. A cholinergic-dependent role for the entorhinal cortex in trace fear conditioning. J Neurosci. 2009;29(25):8087–8093. doi: 10.1523/JNEUROSCI.0543-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prado-Alcala RA, Haiek M, Rivas S, Roldan-Roldan G, Quirarte GL. Reversal of extinction by scopolamine. Physiol Behav. 1994;56(1):27–30. doi: 10.1016/0031-9384(94)90257-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.