Abstract
Generating antitumor responses through the inhibition of tumor-derived immune suppression represents a promising strategy in the development of cancer immunotherapeutics. Here we present a strategy incorporating delivery of the bacterium Salmonella typhimurium (ST), naturally tropic for the hypoxic tumor environment, transformed with an shRNA plasmid against the immunosuppressive molecule indoleamine 2,3-dioxygenase 1 (shIDO). When systemically delivered into mice, shIDO silences host IDO expression and leads to massive intratumoral cell death that is associated with significant tumor infiltration by polymorphonuclear neutrophils (PMNs). shIDO-ST treatment causes tumor cell death independently of host IDO and adaptive immunity, which may have important implications for use in immunosuppressed cancer patients. Further, shIDO-ST treatment increases reactive oxygen species (ROS) produced by infiltrating PMNs and conversely, PMN immunodepletion abrogates tumor control. Silencing of host IDO significantly enhances ST colonization, suggesting that IDO expression within the tumor controls the immune response to ST. In summary, we present a novel approach to cancer treatment that involves the specific silencing of tumor-derived IDO that allows for the recruitment of ROS-producing PMNs, which may act primarily to clear ST infection, but in the process, also induces apoptosis of surrounding tumor tissue resulting in a vigorous anti-tumor effect.
Keywords: Salmonella typhimurium, indoleamine 2, 3-dioxygenase 1 (IDO), shRNA, B16F10 melanoma, neutrophils
INTRODUCTION
One major immunoregulatory function of tumors is the aberrant and abundant expression of IDO, a tryptophan-catabolizing enzyme that acts as a potent suppressor of adaptive immunity. Several studies uncovered that IDO is chronically activated in many cancer patients, as evidenced by intratumoral IDO expression, elevated serum levels of the end product kynurenine as well as tryptophan depletion, and that IDO activation correlated with more extensive disease (1–4). Due to its association with cancer progression, IDO serves as an ideal target to augment antitumor responses, and as such, clinical trials designed to inhibit IDO alone, or in combination with other targets, have already been performed while others are ongoing.
More recently, IDO has been implicated in inducing apoptosis of PMNs, which hold considerable promise as efficient mediators of anticancer activity (5–8). They are the most abundant leukocyte subset and are regenerated in vast numbers daily. Several anticancer therapeutics have been shown to elicit PMNs as the key component of their antitumor efficacy (9–10). Nevertheless, such systemic therapies can be limited by toxicity, dependence on adaptive immunity, which is often suppressed in cancer patients, and, above all, immunoregulatory constituents of the tumor microenvironment that inhibit PMN killing of cancer cells (11–12).
Chemical IDO inhibitors, such as D-1-methyl tryptophan (D-1MT), can partially block IDO function in dendritic cells leading to antitumor immune activation and tumor suppression (13). Although additional chemical inhibitors have been discovered that preferentially target IDO, deleterious off-target effects and the induction of additional immunosuppressive molecules have been observed (14). While IDO drug targeted approaches appear promising for inducing anti-tumor immune responses, it is not currently known how to best target the catabolic function of IDO using chemical inhibitors or the preferred combinatorial approach with chemotherapy or immune modulators that would optimally improve immune responsiveness and provide the highest anti-tumor effects in the clinic.
In addition to IDO inhibitors, other targeted therapies such as imatinib have been shown to act through IDO to successfully treat tumors in murine models of gastrointestinal cancer (15). The use of inhibitory RNA technology (RNAi) such as silencing RNA (siRNA) can, in theory, overcome molecular off-target effects, but, as with chemical IDO-inhibitors, siRNA therapy cannot differentiate between cancerous or healthy tissue when administered systemically. Given the cost of chemical synthesis of siRNA, and the fact that exogenous administration of siRNA therapy acts transiently, alternate strategies incorporating RNAi have emerged. Small hairpin RNA (shRNA) technology is a practical alternative to siRNA as it is more cost effective for long-term treatment and can be administered using various delivery vectors to enhance stability in vivo. Several groups have turned to using the bacterium Salmonella typhimurium (ST) as a therapeutic delivery vector due to its natural tropism for hypoxic regions of tumor tissue that are likely immune privileged and prevent clearance (16–17). Engineered plasmids carried by recombinant ST, once present within the tumor site, can be released into tumor cells or phagocytic cells when bacteria are destroyed within lysosomes (18). Although previous studies in mice have observed modest tumor control with administration of attenuated ST alone, it has proven to be less effective in more aggressive tumor models (19–20). Moreover, in clinical trials using the ST strain VNP20009 for treatment of metastatic melanoma, minimal tumor ST colonization was observed, which ultimately resulted in no measurable regression. Thus, improving ST tumor colonization will likely enhance its therapeutic efficacy (21–22).
In the present study, we show that systemic injection of the clinically relevant ST strain, VNP20009, carrying an IDO-specific shRNA plasmid (shIDO-ST) results in substantial control of aggressive B16F10 melanomas. We found that the novel combination of IDO-silencing with tumor colonization by ST results in the intratumoral recruitment of ROS-producing PMNs, which are involved in ST clearance and may promote the induction of apoptosis of surrounding tumor cells. Upon depletion of PMN, greater intratumoral numbers of shIDO-ST bacteria were seen compared to ST control treatment, suggesting that host IDO silencing may allow for greater colonization of ST in the tumor occurring prior to the recruitment of PMN. Overall, this work describes a novel strategy to focus the cytotoxic activity of PMNs strictly within cancerous tissue.
MATERIALS AND METHODS
Animals and cell lines
C57BL/6, IDO-KO, and Rag1-KO mice (Jackson, 6–8 weeks) were obtained from breeding colonies housed at the City of Hope (COH) Animal Research Center. Animals were handled according to Institutional Animal Care and Use Committee guidelines under protocol #08048. The B16F10 melanoma line was a kind gift from Drs. Hua Yu and Marcin Kortylewski (COH) and maintained in DMEM containing 10% FBS. The H35 (CD8) and GK1.5 (CD4) hybridomas were purchased from ATCC and the RB6-8C5 (Gr1) hybridoma (originally produced by Robert L. Coffman) was a kind gift from Dr. Hans Schreiber. All hybridomas were maintained in RPMI containing 10% FBS.
Generation of shIDO-ST
shRNA constructs against IDO (Sigma, #SHCLNG-NM008324 and pEQshIDO (Welgen)) were tested for silencing by co-transfection of HEK293 cells (ATCC) with an IDO-expressing plasmid (Origene) at a ratio of 5:1 followed by western blot analysis using mouse antibody clone 10.1 (Millipore). The pLKO.1-puro lentiviral vector containing the 21-mer shRNA sense sequence CGTCTCTCTATTGGTGGAAAT (shIDO#9), pEQshIDO or scrambled shRNA (shScr) sequence (Sigma) was electroporated into ST strain VNP20009 (ATCC#202165) with a BTX600 electroporator (BTX) at 2.5 kV, 186 ohms. Transformed ST were grown to late log phase (optical density (O.D.) ~0.70–0.80) and diluted in PBS before administration to mice. To calculate CFU/ml, an O.D. of 1 equal to 109 CFU/ml was used.
Tumor challenge and therapy
B16F10 cells (2.5 × 105) were injected subcutaneously into the upper left abdomen of C57BL/6 mice [corresponding to Time (d) = 0]. Treatment consisted of either PBS or 2.5×106 cfu of shIDO-or shScr-ST injected intravenously (i.v.) twice, 4 days apart, into mice with tumor diameters ≥7 mm. Preparation and treatment of tumor-bearing mice with D-1MT with cyclophosphamide (CY) was done as previously described (13). Briefly, 2.5 mg of D-1MT (Sigma), prepared fresh in 0.5% Tween 80/0.5% Methylcellulose (v/v in water), was administered to mice (when tumors reached ≥ 7 mm in diameter) by oral gavage, twice daily (total of 5 mg/day) for the duration of the experiment. D-1MT was protected from light whenever possible. Three days following the start of D-1MT treatment, a single dose of cyclophosphamide (CY) was given intraperitoneally at 150 mg/kg.
Quantitative PCR for detection of IDO levels
Mice bearing B16F10 tumors (≥7 mm in diameter) were i.v. injected with 2.5 × 106 cfu of shIDO-ST or shScr-ST. After 48 hours, mice were sacrificed and RNA was extracted from tumor homogenates for generation of cDNA (Fermentas). SYBR®-Green qPCR analysis (BD Biosciences) was carried out for 40 cycles using primers specific for IDO and using GAPDH for normalization. IDO primers: Forward: 5′-ggaaccgaggggatgacgatgttc-3′; Reverse: 5′-agactggtagctatgtcgtgcagtgc-3′. GAPDH primers: Forward: 5′-caaggtcatccatgacaactttg-3′; Reverse: 5′-gtccaccaccctgttgctgtag-3′. QPCR analysis for in vitro shRNA studies in HEK293 cells co-transfected with an IDO-expressing plasmid or B16F10 endogenously expressing IDO were also done as mentioned above. Transfection efficiency with Lipofectamine 2000 (Invitrogen), determined using a GFP-expressing plasmid control and flow cytometry, was ~80% for HEK293 cells and ~40% for B16F10 cells. Murine IFN-γ (Peprotech) was added to B16F10 cultures for 48–72 hours, to induce IDO expression, at a concentration of 600 U/ml.
Immunofluorescence staining
B16F10 cells seeded on coverslips were infected for 2 hours at a ratio of 10 bacterium/cell, washed, and then incubated overnight in DMEM-10 containing 10 μg/mL gentamicin. Cells were fixed/permeabilized with 1:1 acetone:methanol and stained with FITC-LPS antibody (Santa Cruz Biotech) overnight at 4°C followed by DAPI staining. Cells were imaged at 100X magnification on an Axiovert 200 using live imaging software (Axiovision). Image shown is representative of cells observed within multiple fields. Staining for flow cytometry can be found in supplementary methods.
Depletion of PMN
Selective depletion of PMNs was achieved by repetitive i.p. injections of Gr-1 depleting antibody (30 μg/injection). Antibody was administered 2 days after first treatment with ST and then given every 3 days throughout the duration of the experiment.
RESULTS
Systemic shIDO-ST treatment silences tumor-derived IDO
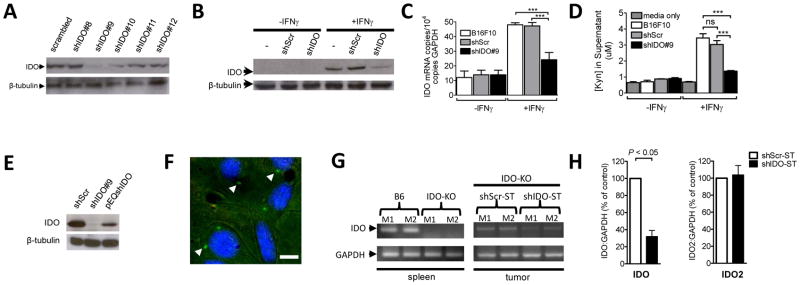
Prior studies have demonstrated IDO expression in human melanomas and in the murine B16F10 melanoma line (23–24). Silencing of IDO in these cells, prior to challenging mice, showed attenuation of tumor growth, suggesting that IDO expressed by the tumor can induce immunosuppression (23). Our first objective was to find an shRNA specific to IDO that could significantly reduce its expression. Therefore, five shRNA constructs were tested for their ability to silence IDO protein expressed from a separate plasmid in HEK293 cells. We observed significant reduction of IDO expression from construct #9 (Fig. 1A) with >75% knockdown compared to scrambled control. We also observed significant suppression of endogenous IDO protein (Fig. 1B) and mRNA (Fig. 1C) in IFNγ-treated B16F10 cells that had been transfected with shIDO#9, compared to untransfected or shScr-transfected cells. The suppression of endogenous IDO in B16F10 cells correlated with a dramatic reduction in kynurenine produced in the culture supernatant (Fig. 1D). In other studies, HEK293 cells were co-transfected with IDO and a published alternate shRNA sequence against IDO, pEQshIDO (23). We found that shIDO#9 indeed was more effective in reducing IDO protein in HEK293-IDO transfectants than the published shRNA sequence (Fig. 1E), which is consistent with a specific inhibition of IDO by the shRNAi construct. We then transformed shIDO#9 into VNP20009 by electroporation (21, 25–26). Assessing the newly generated recombinant ST (shIDO-ST), we determined that >75% of cultured B16F10 melanoma cells could be infected as determined by the frequency of intracellular LPS expression (Fig. 1F) and that infectivity was similar to GFP-expressing and shScr-ST controls (Supplementary Fig. 1). These data indicated that the transformation of shIDO#9 plasmid did not alter infectivity of VNP20009.
Fig. 1. Systemic shIDO-ST treatment silences tumor-derived IDO.
A, HEK293 cells were co-transfected with an IDO-expressing plasmid and the indicated shRNA constructs. Western blot analysis was done 48h post-transfection. β-tubulin, loading control. B–D, Untransfected B16F10 or B16F10 cells transfected with shIDO or shScr plasmids were incubated in the absence or presence of 600 U/ml IFNγ. Seventy-two hours post-IFNγ treatment, protein and RNA were extracted from cells for IDO detection by western blot, B, and quantitative PCR, C, and supernatants were analyzed for kynurenine concentration [Kyn] by HPLC, D. E, HEK293 cells were co-transfected with an IDO-expressing plasmid and the indicated shRNA constructs at a ratio of 1:5. IDO WB analysis was carried out 48h post-transfection. β-tubulin, loading control. F, In vitro infection of B16F10 cells with ST carrying shIDO#9 (shIDO-ST). ST is labeled with a FITC-LPS-specific antibody (arrows) and B16F10 nuclei are stained with DAPI. Magnification: 100X. Scale bar, 5 um. G, left panel, Spleens from C57BL/6 (B6) or IDO-KO mice (n=2) were examined for presence of IDO by PCR. right panel, B16F10 tumor-bearing IDO-KO mice (n=2) were treated with ST carrying scrambled shRNA plasmid (shScr-ST) or shIDO-ST. cDNA from tumor was generated 24h post-treatment for detection of IDO by PCR. GAPDH, loading control. H, Tumors from IDO-KO mice (n=4) treated and processed as in F were used to produce cDNA for detection of IDO and IDO2 by quantitative RT-PCR. ***P<0.001 by Student’s t-test. M=mouse.
Next, we confirmed IDO expression in B16F10 tumors in mice, since conflicting studies have shown either an abundance or absence of expression (23, 27). To measure tumor-specific IDO expression in vivo, we used mice deleted of the Indo gene (IDO-KO), and thus deficient in cellular IDO (Fig. 1G, left panel). We first treated IDO-KO mice, bearing subcutaneous (s.c.) B16F10 tumors, twice intravenously (i.v.), 4 days apart, with shIDO-ST or the negative control shScr-ST, which is identical to shIDO-ST with the exception that it encodes a non-specific shRNA sequence. Forty-eight hours after treatment, we could detect tumor IDO mRNA expression by PCR, with decreased levels in shIDO-ST-treated mice (Fig. 1G, right panel). Further analysis by quantitative RT-PCR revealed a significant reduction in tumor IDO expression from shIDO-ST-treated compared to shScr-ST-treated IDO-KO mice (Fig. 1H). We observed no changes to IDO2, which has similar function and structure to IDO, thus reiterating the specificity of shIDO-ST. These data confirm that IDO is expressed in B16F10 tumors and that its expression is reduced by ~70% in mice by shIDO-ST.
Systemic shIDO-ST treatment inhibits tumor growth independent of host IDO
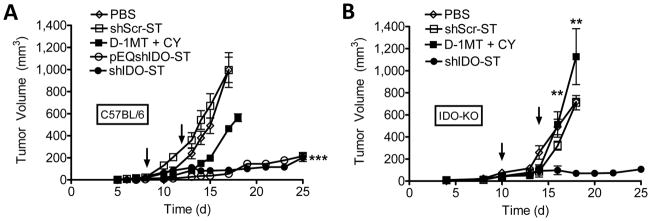
To assess its antitumor properties, we next carried out longitudinal measurements of subcutaneous B16F10 tumor growth in C57BL/6 mice treated therapeutically with shIDO-ST. ShIDO-ST was successful in attenuating B16F10 growth in contrast to groups treated with shScr-ST or PBS (Fig. 2A). Further, we found that recombinant VNP20009 carrying the alternate IDO shRNA (pEQshIDO-ST) could attenuate tumor growth to the same degree as shIDO-ST (Fig. 2A). Both strains inhibited tumor growth to a significantly greater extent than the D-1MT+CY, which itself was able to significantly reduce tumor cell growth, although it is possible that further optimization of the D-1MT+CY treatment may be required for an optimal anti-tumor effect in our aggressive model. Tumor growth in mice treated with shScr-ST was indistinguishable from untransformed VNP20009 (data not shown). Moreover, the number of melanoma lung metastases were significantly decreased in shIDO-ST as compared to PBS or shScr-ST (Supplementary Fig. 2), although shScr-ST was also partially successful in suppressing lung metastases, likely as a result of its tumor-homing properties.
Fig. 2. Systemic shIDO-ST treatment inhibits tumor growth independent of host IDO.
A, B16F10 tumor-bearing mice (n=4) were treated with PBS, shScr-ST, shIDO-ST, pEQshIDO-ST (arrows), or D-1MT+cyclophosphamide (CY) as described in Materials and Methods. Error bars indicate standard error of the mean (SEM). B, IDO-KO tumor-bearing mice (n=4) were treated with PBS, shScr-ST, shIDO-ST, (arrows) or D-1MT+CY. ***P<0.001 by 1-way ANOVA test.
Small molecule inhibitors, such as D-1MT, target IDO activity in host APCs though not tumor cells and, as single agents, are not curative against IDO-expressing tumor cells (13, 28). Consequently, we investigated whether shIDO-ST could have a direct anti-tumor effect by controlling IDO expressing B16F10 tumor growth in the absence of host IDO. We found that shIDO-ST suppressed B16F10 growth in IDO-KO mice significantly better than shScr-ST- and D-1MT+CY-treated groups (Fig. 2B). These results confirm the mode of action of shIDO-ST is mediated primarily through the silencing of tumor-derived IDO since shScr-ST did not significantly reduce tumor growth compared to PBS treatment. They also demonstrate that IDO expression by B16F10 is sufficient to block antitumor immune responses regardless of host IDO expression and highlight the effectiveness of shIDO-ST in controlling tumor growth as a result of IDO silencing.
Systemic shIDO-ST treatment suppresses tumor growth in the absence of functional adaptive immunity
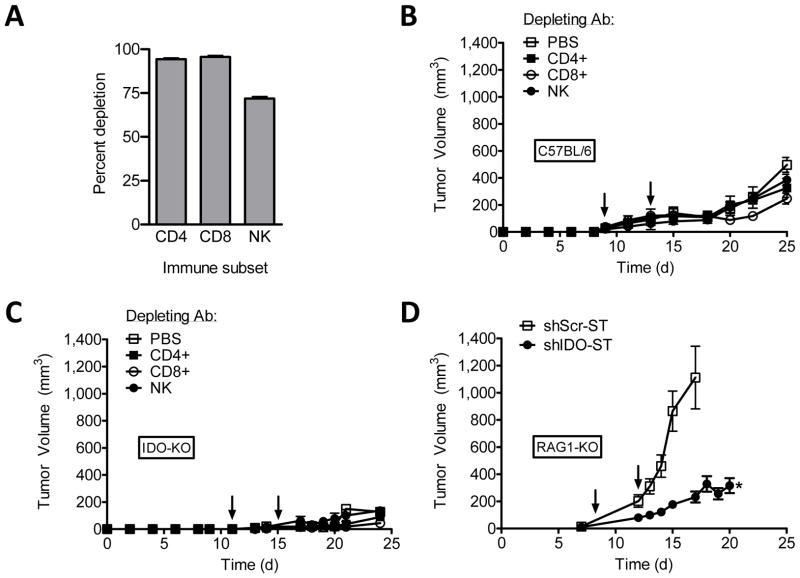
To assess the dependence of shIDO-ST therapy on adaptive T cell immunity, we tested whether shIDO-ST could attenuate tumor growth in C57BL/6 or IDO-KO mice depleted of specific T- or NK- cell immune subsets (Fig. 3A) (29). In stark contrast to IDO inhibitors that require CD8+ or CD4+ T cells for antitumor activity, we observed no discernable changes in tumor growth control by shIDO-ST in mice depleted of CD8+, CD4+, or NK cell immune subsets (Fig. 3, B and C). Further evaluation of the treatment in tumor-bearing Rag1-KO mice, which are naturally devoid of mature T and B cells, revealed that shIDO-ST remains active in suppressing B16F10 growth (Fig. 3D), albeit for an apparently shorter period of time before tumor escape. These results present a novel mechanism of tumor control by shIDO-ST that is not fully dependent on cellular IDO or adaptive T cell immunity generally required by chemical and RNAi inhibitors. Furthermore, the data represent an alternate mechanism of tumor evasion by IDO that does not involve adaptive T cell immunity, which may be defective especially in chemotherapy-treated cancer patients.
Fig. 3. Systemic shIDO-ST treatment suppresses tumor growth in the absence of functional adaptive immunity.
A, Depleting antibody specific for CD4+, CD8+, or NK subsets were administered i.p. into B16F10 tumor-bearing mice (n=3). Data represents cell populations in blood 24 hours after i.p. injection. B and C, B16F10 tumor-bearing C57BL/6 mice in B or IDO-KO mice in C (n=4) were treated (arrows) with shIDO-ST. Antibody depletion of CD8+, CD4+, and NK immune subsets began 2d after the first shIDO-ST inoculation, with maintenance depletions every 3d. D, B16F10 tumor-bearing RAG1-KO mice (n=4) were treated (arrows) with shScr-ST or shIDO-ST and tumor volume was measured longitudinally. *P<0.05 by Student’s t test.
Treatment with shIDO-ST increases tumor influx of PMNs and induces significant intratumoral cell death
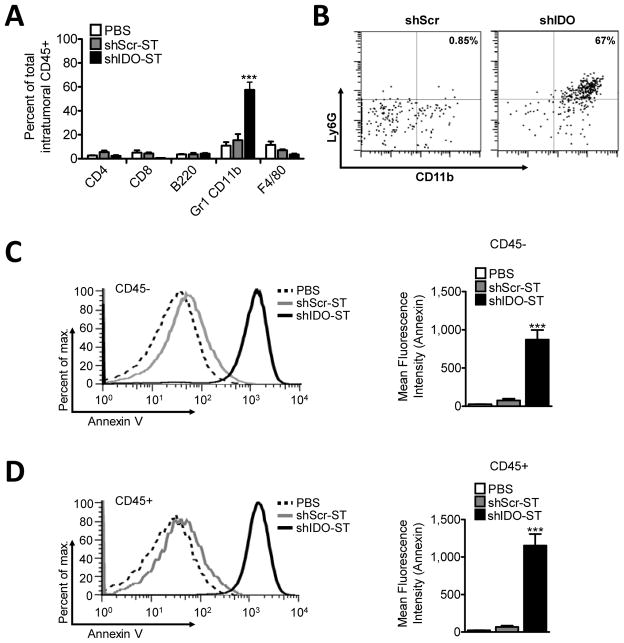
Given the surprising differences in activity profile of shIDO-ST in T- and B- cell deficient recipients, we used flow cytometry to analyze the cell populations that localize to the tumor site in shIDO-ST treated mice. Among investigated lymphocyte and monocyte subsets, we found only a significant increase in intratumoral Gr1+CD11b+ cells of shIDO-ST- versus either shScr-ST or PBS-treated mice (Fig. 4A, P<0.001), which was not observed in the spleen or tumor-draining lymph nodes (Supplementary Fig. 3). Because the Gr-1 antibody recognizes both Ly6G and Ly6C subpopulations (30), the increased frequency of Gr1+CD11b+ cells might represent myeloid derived suppressor cells (MDSCs), PMNs, or other subsets of monocytes. Since ST colonization of spleen has been shown to significantly increase splenic PMN frequency (31), we predicted that tumor colonization by shIDO-ST would attract PMNs into the tumor to account for a large percentage of Gr1+CD11b+ cells. Using an antibody against the PMN-specific marker Ly6G, we determined the percentage of Gr1+CD11b+ cells that were of neutrophil lineage. As shown in Fig. 4B, 67% of CD45+ cells (>90% of Gr1+CD11b+) were Ly6G+, indicating that shIDO-ST treatment leads to increased intratumoral recruitment of PMNs or granulocytic MDSCs, implicating Ly6G+CD11b+ cells as a key immune subset involved in tumor growth control.
Fig. 4. Treatment with shIDO-ST increases tumor influx of PMN and induces total intratumoral cell death.
C57BL/6 mice bearing B16F10 tumors (≥7–8 mm diameter) were treated twice, 4d apart, with 2.5×106 shScr-ST, shIDO-ST, or PBS. Tumors were excised ~1 week after the 2nd treatment and single-cell suspensions were generated. A, Percentage of T, B, MDSC, and macrophage subsets from total intratumoral CD45+ cells using flow cytometry. B, Percentage of Ly6G+CD11b+ PMN gated from intratumoral CD45+ cells. C, left, Histogram from representative mouse of intratumoral CD45− Annexin V staining. right, Grouped (n=4) intratumoral Annexin V staining within CD45− population. D, left, Histogram from representative mouse of intratumoral CD45+ Annexin V staining. right, Grouped (n=4) intratumoral Annexin V staining within CD45+ population. ***P<0.001, by Student’s t test.
Recruitment of cytotoxic PMNs into the tumor can increase local oxidative stress leading to intratumoral cell death indicative of both tumor cells and the vascularized tumor stroma being destroyed, significantly lowering the potential for tumor re-establishment. Therefore, we measured the extent of intratumoral cellular apoptosis in leukocyte (CD45+) and tumor/stroma (CD45−) subsets by Annexin V staining of tumor single-cell suspensions from mice treated systemically with shIDO-ST or shScr-ST. In line with PMN-mediated oxidative stress, we observed significant Annexin V staining of both CD45+ and CD45− populations in shIDO-ST-treated mice, suggesting total intratumoral apoptosis (Fig. 4, C and D). Further analysis revealed that both CD8+ and CD4+ populations had a significantly higher frequency of Annexin V positive cells (Supplementary Fig. 4). Increased Annexin V staining could indicate activation induced cell death (AICD) of T cells following shIDO-ST treatment or cell death secondary to oxidative stress caused by the presence of cytotoxic PMN. Altogether, these results demonstrate that silencing IDO in the tumor using shIDO-ST leads to increased recruitment of PMNs, which may increase oxidative stress within the tumor microenvironment. These data also are the first to implicate IDO in the regulation of PMN activity in vivo and represents a novel strategy to recruit and focus the cytotoxic properties of PMNs using tumor-colonizing ST (31).
Treatment with shIDO-ST results in enhanced ST colonization and augments intratumoral PMN recruitment and activation, which is required for antitumor efficacy
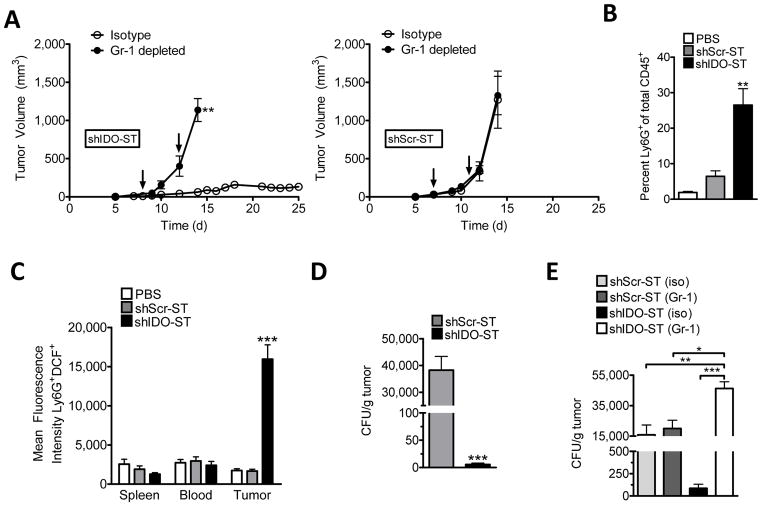
To determine if PMNs were required for tumor growth suppression by shIDO-ST, we initiated depletion studies in shIDO-ST- or shScr-ST-treated mice bearing B16F10 melanomas. As shown in Fig. 5A (left panel), PMN depletion using Gr-1 antibody resulted in uncontrolled tumor growth in shIDO-ST-treated mice compared to mice given control isotype antibody (P<0.01). Abrogation of growth control resulting from Gr-1 depletion confirms that the therapeutic efficacy of shIDO-ST is dependent on host PMNs. Further, IDO silencing alone (i.e. in the absence of PMNs) is insufficient to induce tumor apoptosis, even under hypoxic conditions (Supplementary Fig. 5). In mice treated with shScr-ST, no significant difference in tumor growth kinetics was seen comparing Gr-1 or control isotype depleted groups (Fig. 5A, right panel) supporting previous observations that shScr-ST does not recruit intratumoral PMNs and therefore cannot control tumor growth to any measurable extent. These data provide evidence that PMN contribute to shIDO-ST tumor growth control in vivo and re-emphasizes a unique property of shIDO-ST treatment.
Fig. 5. Treatment with shIDO-ST results in enhanced ST colonization and augments intratumoral PMN recruitment and activation, which is required for antitumor efficacy.
A, B16F10 tumor-bearing C57BL/6 mice were treated (arrows) with either shIDO-ST, left, or shScr-ST, right, when tumors reached ≥7–8 mm in diameter. Two days following the first ST injection, mice were depleted of PMN with maintenance depletions every 3 days. B, Two days after treatment of tumor-bearing mice with PBS, shScr-ST, or shIDO-ST, single-cell suspensions of B16F10 tumors (n=4) were analyzed for CD11b+Ly6G+ cells by flow cytometry. Data represents PMN frequency from total CD45+ cells. C, Mean fluorescence intensity (MFI) of Ly6G+DCF+ cells present in total CD45+ cells from blood, spleen and tumor of treated mice. D, Tumor homogenates in B were lysed and plated onto LB-ampicillin plates. Colony forming units (CFU) per gram tumor were calculated after 24 hrs. E, C57BL/6 mice (n=4) bearing B16F10 tumors were treated as in A. 48h post-treatment, mice were sacrificed and tumor homogenates lysed and plated onto LB-ampicillin plates. *P<0.05, **P<0.01, ***P<0.001 by Student’s t test.
Although we established that PMNs were obligatory for the therapeutic efficacy of shIDO-ST, the mechanism by which the PMNs caused regression of B16F10 tumors remained undefined. Therefore, we initiated studies to measure ROS levels, a major product generated by PMNs during microbial infection and a potent mediator of tumor-killing (32–33). To quantify PMN ROS activity, we used the nonfluorescent probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA), which diffuses into cells and can be converted by ROS to the highly fluorescent 2′, 7′-dichlorofluorescein (DCF) detectable by flow cytometry (34). Tumor-bearing mice receiving either PBS, shScr-ST or shIDO-ST therapy were sacrificed 48 hours after treatment and intratumoral PMNs were assessed for ROS activity. Within only 48 hours, significantly increased intratumoral PMN frequencies in mice treated with shIDO-ST were observed in comparison to PBS- and shScr-ST-treated groups (Fig. 5B, P<0.01). Furthermore, when PMNs from shIDO-ST-treated mice were analyzed for ROS activity using the DCFH-DA fluorescence assay, we found that only PMNs present in tumor, but not in spleen or blood, exhibited significant increases in fluorescence compared to PBS or shScr-ST-treated groups (Fig. 5C, P<0.001). These results indicate that shIDO-ST treatment generates and recruits ROS-producing PMNs exclusively into the tumor and highlights IDO silencing as a key step in this process since shScr-ST therapy alone is insufficient to duplicate this effect.
The presence of ROS-producing PMNs in shIDO-ST-colonized tumors is an observation consistent with resolution of bacterial infection. To determine whether shIDO-ST persistence is affected by the increased presence of PMNs, we enumerated ST in the tumors of mice receiving either shScr-ST or shIDO-ST. In mice receiving shScr-ST, we found considerable numbers of bacteria 48 hrs following treatment, while we found significantly less bacteria in tumors of mice receiving shIDO-ST (Fig. 5D, P<0.001), which may be attributable to increased presence of ROS-producing PMN. We then proceeded to deplete PMNs in tumor-bearing mice treated with shScr-ST or shIDO-ST. As expected, we observed no change in shScr-ST colony forming units (CFUs) in isotype or Gr-1 depleted mice (Fig. 5E). In contrast, we observed a significant increase in shIDO-ST in tumors of Gr-1 depleted mice (P<0.001), suggesting that PMNs recruited into the tumor play a direct role in ST clearance. Interestingly, we also observed significantly more shIDO-ST colonization compared to shScr-ST in PMN-depleted mice (P<0.05). Thus, silencing tumor-derived IDO may enhance the ability of ST to colonize tumor tissue preceding the influx of PMN that ultimately clear the infection (22). Enhanced colonization of tumor through IDO silencing is also supported by previous studies that have implicated IDO in anti-microbial activity (35). Taken together, these observations provide evidence that tumor-targeted delivery of IDO-specific shRNA by ST leads to enhanced bacterial vector colonization and recruitment of cytotoxic PMNs that are involved in ST clearance, creating a microenvironment that is not conducive for tumor growth.
DISCUSSION
IDO expression antagonizes antitumor immune responses and facilitates tumor outgrowth in a variety of cancers, thus emphasizing the need for effective IDO inhibition strategies. As shown in this study and in clinical trials of metastatic melanoma, the ST therapeutic VNP20009 alone is ineffective in controlling tumor growth (36). However, using the unique combination of shIDO delivered by VNP20009 to attenuate growth of the highly aggressive, nonimmunogenic B16F10 tumor line, we have developed and characterized a promising therapeutic strategy that highlights newly described roles for IDO and Salmonella in innate immunity and tumor regression. We found that shIDO-ST given therapeutically silences tumor-derived IDO, in contrast to current IDO inhibitors, which are relatively ineffective as monotherapy against IDO-expressing tumors (13, 28). We have also observed therapeutic efficacy of shIDO-ST in other IDO-expressing tumors such as Pan02, a pancreatic tumor line syngeneic to C57BL/6 mice (Supplementary Fig. 6). Additionally, while another group was successful in silencing tumor-derived IDO expression and in attenuating tumor growth, a limitation of their study was a requirement of using tumor cells stably transfected with shIDO plasmid as a therapeutic treatment (23). Unlike other therapeutic strategies used in immunodeficient mouse models, shIDO-ST was also successful in suppressing tumor growth in the absence of a functional adaptive immune system, which can be advantageous in immune compromised cancer patients, especially those heavily pretreated with chemotherapy.
The limited contribution of adaptive immune cells to the anti-tumor function of shIDO-ST suggested that hypoxia-driven accumulation of shIDO-ST into tumors may subvert vital metabolic events requiring IDO, leading to cell death, with immune cells being neither necessary nor sufficient mechanistically. However, the significant influx of PMNs into tumor following shIDO-ST treatment indicated that innate, not adaptive, immunity was responsible for the observed antitumor effect. PMNs were characterized by significantly increased ROS production, which can potentially generate oxidative stress within the microenvironment and induce apoptosis of intratumoral cells (37–38). Depletion of PMNs (Fig. 5A) allowed for two major observations: 1) significant loss of tumor control by shIDO-ST and 2) an increased colonization by shIDO-ST, which may precede the influx of PMNs. Thus, shIDO-ST is superior to unmodified VNP20009 because it stimulates both enhanced ST tumor colonization and focused PMN cytotoxicity. Furthermore, we assert that in addition to acting as a tumor-specific delivery vehicle for shIDO, ST serves an additional role in attracting PMNs into IDO-attenuated tumors. Ultimately, both IDO silencing and ST work synergistically to suppress tumor growth.
Although the enhanced production of ROS and loss of therapeutic function following Gr-1 depletion may give some indication that PMN are involved in tumor suppression by shIDO-ST, it is still not known whether they kill tumor cells directly. Thus, functional assays to determine the tumor killing capacity of isolated PMN or ROS-expressing granulocytic MDSCs are still required. Furthermore, although i.p. administration of Gr-1 is a common method for depleting PMN (39), the antibody is specific for both Ly6G+ and Ly6C+ populations, thus leaving the possibility of depleting granulocytic or monocytic MDSCs that mediate tumor suppression. However, the cytotoxic effects of PMNs are more likely to contribute to the anti-tumor effect of shIDO-ST than the overall suppressive nature of granulocytic MDSCs. Thus, while we can not unequivocally assert that PMNs are the key mediators of tumor suppression, our studies strongly suggest that shIDO-ST increases the frequency of ROS-producing PMNs in the tumor and, furthermore, Gr-1 depletion of PMNs abrogates the function of the therapy.
To our knowledge, no previous work using IDO inhibition has described PMNs as potential effectors for tumor killing. As shown in Fig. 4A, we observed massive infiltration of PMNs, while CD4+ and CD8+ T cells remained unchanged at relatively low frequencies. The common observation that effector T cells are found at relatively low numbers in tumor tissue and that T cell proliferation is extremely inefficient within the tumor microenvironment may, in part, account for the failure of many T cell-based immunotherapies (40–41) and further highlights the advantages of harnessing PMNs. Another major advantage of PMNs over adaptive T cell immunity is the ability to destroy tumor cells, intratumoral suppressor cells, and tumor stroma such as stromal cells, lymphatics, and blood vessels to significantly decrease the potential for tumor regrowth (42). Since tumor cell-specific T cells will not necessarily reduce surrounding stroma or suppressor cell subset frequency, there is increased potential for tumor cells to re-emerge and proliferate (43). Furthermore, because PMNs are constantly and rapidly regenerated, and are not subject to exhaustion, anergy or tolerance, there is potential to continuously treat with shIDO-ST to activate PMN responses within growing tumors. Although single shIDO-ST treatment was insufficient to suppress tumor rechallenge, indicating an absence of acquired resistance, we have observed extended control of primary tumor growth after repeated administrations of shIDO-ST (data not shown) demonstrating long-term effectiveness of the treatment.
The clinical IDO inhibitor D-1MT, originally thought to only target IDO activity, has now been shown to exert its inhibitory effects on an additional target known as indoleamine 2,3-dioxygenase 2 (IDO2), which is thought to be functionally inactive in human tumors (44–45). Whether D-1MT or the 1MT stereoisomer L-1MT has greater clinical efficacy is controversial (46–47). Because our strategy does not require a functional adaptive immune system, further investigation into the translational efficacy of shIDO-ST can be accomplished using IDO-expressing human tumor lines in xenogeneic mouse models (24). These models can also be used to optimize shIDO-ST tumor colonization further, thereby increasing efficacy.
Ultimately, this work presents a novel approach to the treatment of cancer that takes advantage of the specific shRNA-targeting of tumor IDO and the tumor-homing capacity of ST to generate a focused cytotoxic PMN response capable of causing massive intratumoral apoptosis. The ability to colonize tumor more efficiently, and its independence from adaptive immunity, which is prone to a variety of immunosuppressive pathways and escape by numerous mechanisms (48–50), makes shIDO-ST a practical alternative to current IDO inhibitors. Autoimmune effects such as vitiligo were not observed during shIDO-ST treatment, suggesting minimal toxicity, albeit a more rigorous test of prolonged shIDO-ST administration in B16F10 melanoma mice was not performed. The capacity for ST to colonize most solid tumors increases the potential of shIDO-ST as a therapy to control a variety of cancers via targeted PMN cytotoxicity.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by 5P01-CA030206-28-Prj3, NIH-RAID Administrative Supplement to 3P01-CA030206-28S2, R01 CA72669, ThinkCure, the Nesvig Foundation (DJD) and a Minority Supplement to 3P01-CA030206-28S3 (ERM). The COH Cancer Center is supported by 5P30-CA033572-27.
We thank Marcin Kortylewski for helpful discussions and Hans Schreiber for the RB6-8C5 hybridoma.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Huang A, Fuchs D, Widner B, Glover C, Henderson DC, Allen-Mersh TG. Serum tryptophan decrease correlates with immune activation and impaired quality of life in colorectal cancer. Br J Cancer. 2002;86:1691–6. doi: 10.1038/sj.bjc.6600336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okamoto A, Nikaido T, Ochiai K, Takakura S, Saito M, Aoki Y, et al. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res. 2005;11:6030–9. doi: 10.1158/1078-0432.CCR-04-2671. [DOI] [PubMed] [Google Scholar]
- 3.Weinlich G, Murr C, Richardsen L, Winkler C, Fuchs D. Decreased serum tryptophan concentration predicts poor prognosis in malignant melanoma patients. Dermatology. 2007;214:8–14. doi: 10.1159/000096906. [DOI] [PubMed] [Google Scholar]
- 4.Godin-Ethier J, Hanafi LA, Piccirillo CA, Lapointe R. Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin Cancer Res. 17:6985–91. doi: 10.1158/1078-0432.CCR-11-1331. [DOI] [PubMed] [Google Scholar]
- 5.Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97:339–45. doi: 10.1182/blood.v97.2.339. [DOI] [PubMed] [Google Scholar]
- 6.Seino K, Kayagaki N, Okumura K, Yagita H. Antitumor effect of locally produced CD95 ligand. Nat Med. 1997;3:165–70. doi: 10.1038/nm0297-165. [DOI] [PubMed] [Google Scholar]
- 7.Arai H, Gordon D, Nabel EG, Nabel GJ. Gene transfer of Fas ligand induces tumor regression in vivo. Proc Natl Acad Sci U S A. 1997;94:13862–7. doi: 10.1073/pnas.94.25.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van der Sluijs K, Singh R, Dijkhuis A, Snoek M, Lutter R. Indoleamine 2,3-dioxygenase activity induces neutrophil apoptosis. Critical Care. 2011;15:208. [Google Scholar]
- 9.Stockmeyer B, Beyer T, Neuhuber W, Repp R, Kalden JR, Valerius T, et al. Polymorphonuclear granulocytes induce antibody-dependent apoptosis in human breast cancer cells. J Immunol. 2003;171:5124–9. doi: 10.4049/jimmunol.171.10.5124. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez-Ilizaliturri FJ, Jupudy V, Ostberg J, Oflazoglu E, Huberman A, Repasky E, et al. Neutrophils contribute to the biological antitumor activity of rituximab in a non-Hodgkin’s lymphoma severe combined immunodeficiency mouse model. Clin Cancer Res. 2003;9:5866–73. [PubMed] [Google Scholar]
- 11.Chen YL, Chen SH, Wang JY, Yang BC. Fas ligand on tumor cells mediates inactivation of neutrophils. J Immunol. 2003;171:1183–91. doi: 10.4049/jimmunol.171.3.1183. [DOI] [PubMed] [Google Scholar]
- 12.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67:792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 14.Lob S, Konigsrainer A, Rammensee HG, Opelz G, Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer. 2009;9:445–52. doi: 10.1038/nrc2639. [DOI] [PubMed] [Google Scholar]
- 15.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 17:1094–100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pawelek JM, Low KB, Bermudes D. Bacteria as tumour-targeting vectors. Lancet Oncol. 2003;4:548–56. doi: 10.1016/s1470-2045(03)01194-x. [DOI] [PubMed] [Google Scholar]
- 17.Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997;57:4537–44. [PubMed] [Google Scholar]
- 18.Grillot-Courvalin C, Goussard S, Courvalin P. Bacteria as gene delivery vectors for mammalian cells. Current opinion in biotechnology. 1999;10:477–81. doi: 10.1016/s0958-1669(99)00013-0. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman RM. Tumor-targeting amino acid auxotrophic Salmonella typhimurium. Amino Acids. 2009;37:509–21. doi: 10.1007/s00726-009-0261-8. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, et al. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium. J Cell Biochem. 2009;106:992–8. doi: 10.1002/jcb.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Low KB, Ittensohn M, Luo X, Zheng LM, King I, Pawelek JM, et al. Construction of VNP20009: a novel, genetically stable antibiotic-sensitive strain of tumor-targeting Salmonella for parenteral administration in humans. Methods Mol Med. 2004;90:47–60. [PubMed] [Google Scholar]
- 22.Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20:142–52. doi: 10.1200/JCO.2002.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng X, Koropatnick J, Li M, Zhang X, Ling F, Ren X, et al. Reinstalling antitumor immunity by inhibiting tumor-derived immunosuppressive molecule IDO through RNA interference. J Immunol. 2006;177:5639–46. doi: 10.4049/jimmunol.177.8.5639. [DOI] [PubMed] [Google Scholar]
- 24.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 25.King I, Itterson M, Bermudes D. Tumor-targeted Salmonella typhimurium overexpressing cytosine deaminase: a novel, tumor-selective therapy. Methods Mol Biol. 2009;542:649–59. doi: 10.1007/978-1-59745-561-9_33. [DOI] [PubMed] [Google Scholar]
- 26.Chen G, Wei DP, Jia LJ, Tang B, Shu L, Zhang K, et al. Oral delivery of tumor-targeting Salmonella exhibits promising therapeutic efficacy and low toxicity. Cancer Sci. 2009;100:2437–43. doi: 10.1111/j.1349-7006.2009.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banerjee T, Duhadaway JB, Gaspari P, Sutanto-Ward E, Munn DH, Mellor AL, et al. A key in vivo antitumor mechanism of action of natural product-based brassinins is inhibition of indoleamine 2,3-dioxygenase. Oncogene. 2008;27:2851–7. doi: 10.1038/sj.onc.1210939. [DOI] [PubMed] [Google Scholar]
- 28.Kumar S, Malachowski WP, DuHadaway JB, LaLonde JM, Carroll PJ, Jaller D, et al. Indoleamine 2,3-dioxygenase is the anticancer target for a novel series of potent naphthoquinone-based inhibitors. J Med Chem. 2008;51:1706–18. doi: 10.1021/jm7014155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manuel ER, Blache CA, Paquette R, Kaltcheva TI, Ishizaki H, Ellenhorn JD, et al. Enhancement of cancer vaccine therapy by systemic delivery of a tumor-targeting Salmonella-based STAT3 shRNA suppresses the growth of established melanoma tumors. Cancer Res. 71:4183–91. doi: 10.1158/0008-5472.CAN-10-4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–408. [PubMed] [Google Scholar]
- 31.Kirby AC, Yrlid U, Wick MJ. The innate immune response differs in primary and secondary Salmonella infection. J Immunol. 2002;169:4450–9. doi: 10.4049/jimmunol.169.8.4450. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Yi J. Cancer cell killing via ROS: to increase or decrease, that is the question. Cancer Biol Ther. 2008;7:1875–84. doi: 10.4161/cbt.7.12.7067. [DOI] [PubMed] [Google Scholar]
- 33.Zivkovic M, Poljak-Blazi M, Egger G, Sunjic SB, Schaur RJ, Zarkovic N. Oxidative burst and anticancer activities of rat neutrophils. Biofactors. 2005;24:305–12. doi: 10.1002/biof.5520240136. [DOI] [PubMed] [Google Scholar]
- 34.Rothe G, Valet G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2′,7′-dichlorofluorescin. J Leukoc Biol. 1990;47:440–8. [PubMed] [Google Scholar]
- 35.Carlin JM, Ozaki Y, Byrne GI, Brown RR, Borden EC. Interferons and indoleamine 2,3-dioxygenase: role in antimicrobial and antitumor effects. Experientia. 1989;45:535–41. doi: 10.1007/BF01990503. [DOI] [PubMed] [Google Scholar]
- 36.Dolgin E. From spinach scare to cancer care. Nat Med. 17:273–5. doi: 10.1038/nm0311-273. [DOI] [PubMed] [Google Scholar]
- 37.Lichtenstein A, Seelig M, Berek J, Zighelboim J. Human neutrophil-mediated lysis of ovarian cancer cells. Blood. 1989;74:805–9. [PubMed] [Google Scholar]
- 38.di Carlo E, Iezzi M, Pannellini T, Zaccardi F, Modesti A, Forni G, et al. Neutrophils in anti-cancer immunological strategies: old players in new games. J Hematother Stem Cell Res. 2001;10:739–48. doi: 10.1089/152581601317210836. [DOI] [PubMed] [Google Scholar]
- 39.Westphal K, Leschner S, Jablonska J, Loessner H, Weiss S. Containment of tumor-colonizing bacteria by host neutrophils. Cancer Res. 2008;68:2952–60. doi: 10.1158/0008-5472.CAN-07-2984. [DOI] [PubMed] [Google Scholar]
- 40.Powell JD. The induction and maintenance of T cell anergy. Clin Immunol. 2006;120:239–46. doi: 10.1016/j.clim.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Gajewski TF. Identifying and overcoming immune resistance mechanisms in the melanoma tumor microenvironment. Clin Cancer Res. 2006;12:2326s–30s. doi: 10.1158/1078-0432.CCR-05-2517. [DOI] [PubMed] [Google Scholar]
- 42.Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng. 1999;1:241–63. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- 43.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 330:827–30. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 44.Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082–7. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 45.Lob S, Konigsrainer A, Zieker D, Brucher BL, Rammensee HG, Opelz G, et al. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother. 2009;58:153–7. doi: 10.1007/s00262-008-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qian F, Villella J, Wallace PK, Mhawech-Fauceglia P, Tario JD, Jr, Andrews C, et al. Efficacy of levo-1-methyl tryptophan and dextro-1-methyl tryptophan in reversing indoleamine-2,3-dioxygenase-mediated arrest of T-cell proliferation in human epithelial ovarian cancer. Cancer Res. 2009;69:5498–504. doi: 10.1158/0008-5472.CAN-08-2106. [DOI] [PubMed] [Google Scholar]
- 47.Lob S, Konigsrainer A, Schafer R, Rammensee HG, Opelz G, Terness P. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood. 2008;111:2152–4. doi: 10.1182/blood-2007-10-116111. [DOI] [PubMed] [Google Scholar]
- 48.Maeurer MJ, Gollin SM, Martin D, Swaney W, Bryant J, Castelli C, et al. Tumor escape from immune recognition: lethal recurrent melanoma in a patient associated with downregulation of the peptide transporter protein TAP-1 and loss of expression of the immunodominant MART-1/Melan-A antigen. J Clin Invest. 1996;98:1633–41. doi: 10.1172/JCI118958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Igney FH, Krammer PH. Immune escape of tumors: apoptosis resistance and tumor counterattack. J Leukoc Biol. 2002;71:907–20. [PubMed] [Google Scholar]
- 50.Paschen A, Mendez RM, Jimenez P, Sucker A, Ruiz-Cabello F, Song M, et al. Complete loss of HLA class I antigen expression on melanoma cells: a result of successive mutational events. Int J Cancer. 2003;103:759–67. doi: 10.1002/ijc.10906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.