Abstract
Factors that result in augmented reinstatement, including increased withdrawal period duration and high levels of cocaine consumption, may provide insight into relapse vulnerability. The neural basis of augmented reinstatement may arise from more pronounced changes in plasticity required for reinstatement and/or the emergence of plasticity expressed only during a specific withdrawal period or under specific intake conditions. In this study, we examined the impact of withdrawal period duration and cocaine intake on the magnitude of cocaine-primed reinstatement and extracellular glutamate in the nucleus accumbens, which has been shown to be required for cocaine-primed reinstatement. Rats were assigned to self-administer under conditions resulting in low (2 hr/day; 0.5 mg/kg/infusion, IV) or high (6 hr/day; 1.0 mg/kg/infusion, IV) levels of cocaine intake. After 1, 21, or 60 days of withdrawal, drug seeking and extracellular glutamate levels in the nucleus accumbens were measured before and after a cocaine injection. Cocaine reinstated lever pressing and elevated extracellular glutamate at every withdrawal time point tested, which is consistent with the conclusion that increased glutamatergic signaling in the nucleus accumbens is required for cocaine-induced reinstatement. Interestingly, high-intake rats exhibited augmented reinstatement at every time point tested, yet failed to exhibit higher levels of cocaine-induced increases in extracellular glutamate relative to low-intake rats. Our current data indicate that augmented reinstatement in high-intake rats is not due to relative differences in extracellular levels of glutamate in the nucleus accumbens, but rather may stem from intake-dependent plasticity.
Keywords: cocaine, glutamate, nucleus accumbens, reinstatement, relapse, self-administration
Drug addiction in humans is a chronic relapsing disorder whereby the development of drug-induced changes in brain function gives rise to compulsive cocaine seeking and heightened relapse vulnerability. Compulsive drug seeking is often modeled using the reinstatement paradigm whereby one assesses the capacity of specific stimuli to reinstate extinguished responding on a lever that had previously been used to self-administer cocaine (Epstein et al., 2006; Schmidt et al., 2005). Interestingly, the amount of cocaine consumed by rodents during self-administration training strongly correlates with the magnitude of reinstated drug seeking produced by a cocaine challenge (Baker et al., 2001; Sutton et al., 2003). Moreover, rats with a history of high levels of cocaine consumption display increased levels, and a more compulsive pattern, of drug seeking relative to low-intake rats (Ahmed and Koob, 1998; Deroche et al., 1999; Mantsch et al., 2004; Vanderschuren and Everitt, 2004; Walker et al., 2000). Thus, manipulation of self-administration parameters can be used to identify drug-induced plasticity that contributes to compulsive drug seeking and relapse vulnerability.
Attempts to identify the neural basis of addiction have demonstrated a critical role for adaptations resulting in abnormal levels of extracellular glutamate in the nucleus accumbens in the development of drug seeking. Relative to drug-naïve subjects, rats withdrawn from repeated cocaine exhibit reduced basal and increased cocaine-evoked levels of extracellular glutamate in the nucleus accumbens (Miguens et al., 2008; Pierce et al., 1996; Reid and Berger, 1996; Szumlinski et al., 2005). Manipulations that prevent or mask these changes have been shown to blunt cocaine-induced reinstatement (Baker et al., 2003; Berglind et al., 2009; Knackstedt et al., 2010; Madayag et al., 2007; McFarland et al., 2003). Thus, abnormal activity of the cellular mechanisms regulating levels of extracellular glutamate may give rise to drug seeking in rodents. Because the nature of drug-induced changes underlying compulsive drug seeking is often distinct during different periods of withdrawal (Bachtell and Self, 2008; Sorge and Stewart, 2005; Tran-Nguyen et al., 1998; White et al., 1995; Zhang et al., 1997), it is critical to reveal the time course of plasticity. In regards to extracellular glutamate, most studies have sampled levels after 2–3 weeks of withdrawal (Knackstedt et al., 2010; Madayag et al., 2010; Pierce et al., 1996; Reid and Berger, 1996; Szumlinski et al., 2005), although see (Miguens et al., 2008); thus, there is no clear consensus of the time profile of these changes (ie, how long they persist). Although required for the expression of cocaine-induced reinstatement (Baker et al., 2003; Berglind et al., 2009; Knackstedt et al., 2010; Madayag et al., 2007; McFarland et al., 2003), it is also important to determine if relative differences in the magnitude of abnormal extracellular glutamate levels in the nucleus accumbens results in parallel changes in the magnitude of reinstatement (Madayag et al., 2010). In order to more fully understand the neural basis of addiction, as well as to validate putative targets for the treatment of addiction, it is imperative to more fully explore the impact of glutamate dysfunction in the nucleus accumbens.
In the current study, we examined the impact of intake conditions on extracellular glutamate in the nucleus accumbens and reinstatement magnitude after 1, 21, or 60 days of withdrawal from the last session of cocaine self-administration. The primary objective was to create conditions that result in different levels of reinstatement in order to determine whether there would be parallel changes in the magnitude of glutamate dysfunction as reflected by extracellular levels. Importantly, experimenter-controlled manipulations were used to create lowand high-intake subjects in order to isolate cocaine-induced plasticity from other risk factors, such as genetic differences that may contribute to individual differences in reinstatement magnitude. In addition, the design allowed us to more fully characterize both the impact of intake conditions and withdrawal period on drug seeking by co-varying these variables.
Materials and methods
Subjects
These experiments utilized sixty eight male Sprague Dawley rats (Harlan, Indianapolis, IN) weighing 275–325 grams upon arrival. Rats were individually housed in a temperature-controlled colony room with a 12-h reversed light/dark cycle. Housing conditions and experimental protocols were approved by the Marquette University Institutional Animal Care and Use Committee and carried out according to the NIH Guide for the Care and Use of Laboratory Animals (revised 1996).
Surgeries
Rats were implanted with indwelling catheters under ketamine HCl (100 mg/kg, IP, Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (2 mg/kg, IP, Lloyd Laboratories, Shenandoah, IA, USA) anesthesia in order to minimize pain and discomfort. A silicon tubing catheter (Dow Corning Co., Midland, MI; 0.64 mm ID; 1.19 mm OD) was implanted such that it entered the jugular vein through the right posterior facial vein and terminated at the right atrium. The catheter was sutured to the vein at the entry point. The distal aspect of the catheter, which consisted of a 22-gauge guide cannula (Plastics One Inc., Roanoke, VA) attached with dental acrylic to a piece of polypropylene monofilament surgical mesh (Atrium Medical, Co., Hudson, NH), exited 2 cm posterior to the scapulae. Throughout the experiment, catheters were filled daily with a heparin solution (83 i.u./ml; Elkins-Sinn, Inc., Cherry Hill, NJ) and capped when disconnected from the leash/delivery line assembly. Rats were also implanted with indwelling bilateral guide cannulae (20 gauge, 14 mm; Plastics One, Roanoke VA) using the following coordinates derived from Paxinos and Watson (1986): + 0.9 mm anterior, ± 2.5 mm mediolateral to Bregma, and −4.4 mm from the surface of the skull at a 6° angle from vertical. The placement of the active region of the microdialysis probe, which began 1 mm beyond the ventral tip of the guide cannula, was primarily in the nucleus accumbens (see figure 5) although regions immediately dorsal and ventral to this were also likely sampled. Following surgery, rats were given at least five days to recover prior to testing. During this time, rats were provided acetaminophen (480 mg/L) in their drinking water and injected daily with a sterile cefazolin antibiotic solution (15 mg, IV; West-Ward Pharmaceutical Co., Eatontown, NJ) in order to minimize pain and discomfort.
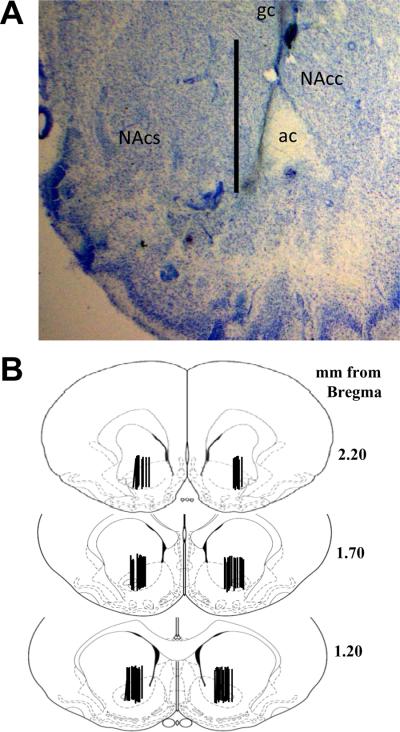
Figure 5.
The placement of the microdialysis probes resulted in sampling primarily from the nucleus accumbens core, although aspects of ventral nucleus accumbens shell and the striatum dorsal to the nucleus accumbens were sampled as well. (a) A representative coronal section illustrating the placement of microdialysis probes. The tract created extends approximately 3 mm from the guide cannula with sampling only occurring from the most ventral 2 mm because this is the portion of the probe containing the dialysis membrane. Scale bar = 2 mm, ac (anterior commissure), NAcc (nucleus accumbens core), NAcs (nucleus accumbens shell). (b) Schematics depicting the distribution of the active membrane portion of the microdialysis probes illustrate the regions sampled in this study.
Self-Administration Apparatus
Self-administration occurred in operant chambers (ENV-008CT, MED-Associates Inc., St Albans, VT, USA) that were housed encased in sound attenuating cubicles (ENV-016M, MED-Associates Inc.) and equipped with two retractable levers, two stimulus lights, and a water bottle. The cubicle had an exhaust fan to provide ventilation and background white noise. Each chamber had a polyurethane delivery line (0.51 mm i.d. × 1.52 mm o.d.), encased in a stainless steel spring leash (Plastics One Inc., Roanoke, VA) and connected to a 30-ml syringe inserted into an infusion pump (Razel, Stamford, CT) via a leak-proof fluid swivel (Instech Lab. Inc., Plymouth Meeting, PA). The swivel and leash assembly was counter-balanced to reduce restrained movement.
Cocaine Self-Administration Training
At least five days after surgery, rats were food restricted with water available ad libitum; food restriction continued for the duration of the experiment (e.g., through reinstatement testing) and involved daily administration of 15 grams of rat chow in the late afternoon or immediately following an operant session. Food restriction was included since it has been shown to promote acquisition of cocaine self-administration and to duplicate methods used in earlier reports examining cocaine-induced disruptions in extracellular glutamate levels in the nucleus accumbens (Carroll et al., 1981; LaLumiere and Kalivas, 2008; Madayag et al., 2010; Zheng et al., 2012). Rats were placed into the operant conditioning chambers overnight and responses on the lever designated as active resulted in the delivery of food pellets under a fixed ratio 1 schedule of reinforcement. Daily food training continued until subjects received at least 150 food rewards in a session, which typically occurred following the first session. During the acquisition phase of the experiment, all rats underwent drug self-administration training during daily 2-hr sessions in which operant responses on the active lever were reinforced with an infusion of cocaine (0.5 mg/kg/200 μl IV, National Institute on Drug Abuse, Bethesda, MD, USA) under a fixed ratio 1 schedule of reinforcement. Each reinforced lever response resulted in the illumination of the stimulus light located above the active lever and was followed by a 25-s time-out period. Responding on a second, inactive lever located on the back wall was recorded but had no programmed consequences. Acquisition of cocaine self-administration was operationally defined as < 10% variation in daily responding over at least three consecutive sessions. During the maintenance phase of the experiment, rats were assigned to self-administer cocaine under low- (0.5 mg/kg/200 μl IV; 2-hr/day for 11 days) or high-intake conditions (1.0 mg/kg/200 μl IV; 6-hr/day for 11 days). The saline controls experienced the same procedures as described for the low-intake subjects except they self-administered saline rather than cocaine.
Test Day
The test day occurred after 1, 21 or 60 days following the last self-administration session and involved measuring extracellular glutamate in the nucleus accumbens using in vivo microdialysis and operant responding. Rats were implanted with removable microdialysis probes and placed in the self-administration chamber with the levers extracted the evening before their assigned test day as previously described (Madayag et al., 2007). The next day, dialysis buffer (5 mM glucose, 140 mM NaCl, 1.4 mM CaCl2, 1.2 mM MgCl2, and 0.5 mM phosphate, pH 7.4) was pumped through the microdialysis probes at a rate of 2 μl/min for at least 3 hours prior to sampling. Five 20-min baseline samples were collected from drug naïve, low- and high-ntake rats. Afterwards, cocaine-withdrawn rats underwent additional testing in which the levers were inserted into the chambers, and extracellular glutamate levels and operant responses were assessed under extinction conditions for two hrs before (extinction phase) and after a cocaine injection (10 mg/kg, IP; reinstatement phase). The cocaine injection precluded the use of the more sensitive no-net flux approach to measuring basal neurotransmitter levels, however, the method used in the current experiments has successfully been used previously to detect changes in extracellular glutamate levels in the nucleus accumbens following repeated cocaine administration (Kau et al., 2008; Madayag et al., 2007; Miguens et al., 2008; Pierce et al., 1996; Reid and Berger, 1996). The dialysate was collected into 10μL of 15% perchloric acid and 0.25mM EDTA.
Quantification of Glutamate
The concentration of glutamate in microdialysis samples was determined using HPLC coupled to fluorescence detection. Precolumn derivatization of glutamate with o-pthaldehyde was performed using a Shimadzu LC10AD VP autosampler. The mobile phase consisted of 13% acetonitrile, 100 mM Na2HPO4 and 0.1 mM EDTA, pH of 5.90. Glutamate was separated using a reversed-phase column (4 μM; 140 × 6.0 mm; Phenomenex, Torrance, CA), and detected using a Shimadzu 10RF-AXL fluorescence detector with an excitation and emission wavelength of 320 and 400 nm, respectively (Baker et al., 2002; Baker et al., 2003). The concentration of glutamate in dialysis samples was quantified by comparing peak heights from samples and external standards. The concentrations of glutamate in the external standards were 0.1, 0.3, 1.0, 3.0, and 10 μM.
Histology
Rats were given an overdose of pentobarbital (60–75 mg/kg, IP), and the brains fixed by intracardiac infusion of 0.9% saline followed by 2.5% formalin solution. Brains were then removed and stored in 2.5% formalin for at least 7 days prior to sectioning. The tissue was then blocked and coronal sections (100 μm) were cut and stained with cresyl violet to verify probe placements.
Statistical analyses
SPSS software (version 19) was used to perform the statistical analyses. Data were analyzed using analysis of variance (ANOVA) with self-administration access conditions and withdrawal length as potential between-subject factors and time (self-administration day or 20-min interval) as a repeated factor. Significance required an alpha value of 0.05 or less for comparisons using ANOVA. Post hoc comparisons were conducted using Fisher LSD with Bonferroni corrections (to control for alpha inflation) applied to one-tailed tests; the required alpha value is reported in the results for each analysis.
RESULTS
Impact of self-administration conditions on cocaine intake
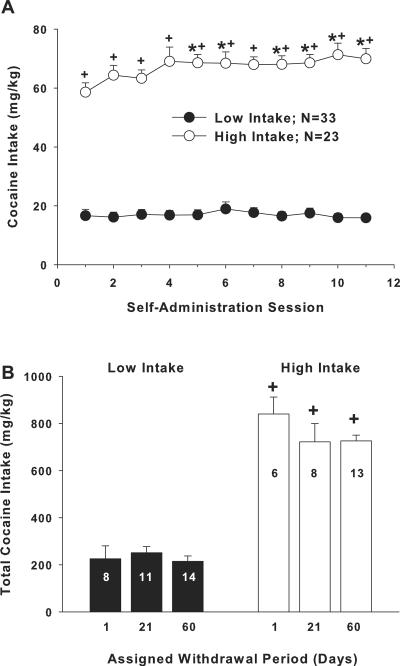
Figure 1 illustrates the mean (± SEM) cocaine intake (mg/kg) for rats self-administering under low- or high-intake conditions. Analysis of variance (ANOVA) with intake condition as a between-subjects factor and daily self-administration session as a within subjects factor produced a significant interaction (F10,580=3.18, p<.01). Analysis of simple main effects demonstrated that high-intake (F10,260=3.13, p<.01), but not low-intake rats (F10,320=1.04, p>.05), exhibited a significant main effect of time. Post hoc analyses revealed that high-intake rats exhibited a significant increase in cocaine intake on days 5–11 relative to the first self-administration session, and a significant increase in intake on all days relative to low-intake rats (figure 1a, Fisher LSD with Bonferroni correction, p<.0047).
Figure 1.
Increased access conditions and dose of cocaine available during self-administration training result in a significant increase in cocaine intake (mg/kg, IV) across eleven maintenance self-administration sessions. (a) Cocaine intake across eleven daily self-administration sessions from rats that were assigned to self-administer cocaine under low- (0.5 mg/kg/200 μl IV; 2-hr/day) or high-intake (1.0 mg/kg/200 μl IV; 2-hr/day 6 hr/day) conditions. * indicates a significant difference from day 1; Fisher LSD with Bonferroni correction, p<.0047. + indicates a significant difference from low-intake rats, Fisher LSD with Bonferroni correction, p<.0047. (b) Cocaine intake for low- and high-intake rats assigned to withdrawal periods of 1, 21, or 60 days. The N/group is listed in each bar. + indicates a significant difference from low-intake rats, ANOVA main effect, p<.001.
Figure 1b illustrates the levels of cocaine intake for the low- and high-intake assigned to the three withdrawal time points of 1, 21, and 60 days. An ANOVA with intake condition and withdrawal as between subjects factor produced a significant main effect of intake (F1,54=226, p<.001), without a significant main effect of withdrawal time (F2,54=1.04, p>.05) or an interaction between these variables (F2,54=1.23, p>.05).
Heightened relapse susceptibility in high-intake rats
Figure 2 illustrates mean (± SEM) lever pressing before (extinction phase) and after a cocaine injection (reinstatement phase) in low- and high-intake rats. An ANOVA with time as a repeated measure (20 min), and intake condition and withdrawal period as between subjects factor produced only a significant interaction between time and access (F11,594=4.68, p<.001) and a main effect of access (F1,54=8.662, p<.01). Further analysis of the interaction by comparing the time variable at each level of the access variable produced a simple main effect of time in low- (F11,352=4.26, p<.001) and high-intake groups (F11,286=9.46, p<.001). Post hoc analyses revealed that low-intake rats exhibited a significant increase in responding during the 2nd, 3rd, and 4th 20-min time bins of the reinstatement phase (i.e., following the cocaine injection) relative to the last time bin of the extinction phase (i.e., preceding the injection, Fisher LSD with Bonferroni correction, p<.00625). High-intake rats exhibited a significant increase in responding during the first three 20-min time bins in the reinstatement phase relative to the last time bin in the extinction phase (figure 2a, Fisher LSD with Bonferroni correction, p<.00625). High-intake rats also exhibited a significant increase in responding during the first reinstatement time bin relative to low-intake rats (Fisher LSD with Bonferroni correction, p<.00625).
Figure 2.
High-intake conditions but not length of withdrawal resulted in a significant increase in cocaine-induced reinstatement magnitude. (a) Operant responses during twenty-min intervals before (extinction) and after a cocaine injection (10 mg/kg, IP; reinstatement) from rats that had self-administered cocaine under low- or high-intake conditions. * indicates a significant difference from the last interval of the extinction phase of the experiment; Fisher LSD with Bonferroni correction, p<.00625. + indicates a significant difference from low-intake rats, Fisher LSD with Bonferroni correction, p<.00625. (b) Total responses following a cocaine injection for low- and high-intake rats that underwent withdrawal periods of 1, 21, or 60 days. The N/group is listed in each bar. + indicates a significant difference from low-intake rats, ANOVA main effect, p<.01.
Although a significant effect of withdrawal was not obtained in the above ANOVA, we specifically compared reinstatement magnitude across the withdrawal assignments since this was a primary measure in the current study (figure 2b). An ANOVA comparing total response rats following the cocaine injection with intake and withdrawal as between subjects variables produced only a main effect of intake conditions (F1,54=11.19, p<.01).
Low- and High-intake rats display reduced levels of extracellular glutamate in the nucleus accumbens
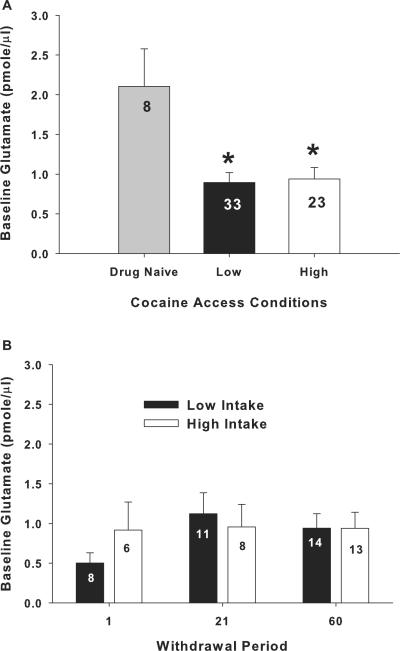
Figure 3 illustrates mean (± SEM) extracellular glutamate levels during baseline in both low- and high-intake groups. An ANOVA comparing extracellular glutamate levels in the nucleus accumbens between drug-naïve, low-intake, and high-intake rats produced a significant main effect (F2,65=7.46, p<.001). Post hoc analyses revealed that low- and high-intake rats exhibited a significant decrease in extracellular glutamate levels relative to drug-naïve controls. There was no difference between low- and high-intake rats (figure 3a, Fisher LSD with Bonferroni correction, p>.033). Figure 5 illustrates that the probes were primarily located in the nucleus accumbens core, although aspects of ventral nucleus accumbens shell and the striatum dorsal to the nucleus accumbens were likely sampled as well
Figure 3.
Rats withdrawn from cocaine self-administration exhibited reduced basal levels of glutamate in the nucleus accumbens regardless of intake conditions or length of withdrawal. (a) Basal extracellular glutamate levels (pmole/μl) sampled using microdialysis in the nucleus accumbens of drug-naïve or rats that had self-administered cocaine under low- or high-intake conditions. * indicates a significant difference from drug-naïve controls; Fisher LSD with Bonferroni correction, p<.033). (b) Extracellular glutamate levels (pmole/μl) in the nucleus accumbens from rats withdrawn from low- or high-cocaine intake for 1, 21, or 60 days. The N/group is listed in each bar.
Figure 3b illustrates the lack of an impact of withdrawal on the mean (± SEM) extracellular glutamate levels during baseline in both low- and high-intake groups. An ANOVA with intake condition and withdrawal period as between subjects variables failed to produce an interaction (F2,54=,634, p>.05) or a main effect of either intake condition (F1,54=,170, p>.05) or length of withdrawal (F2,54=,812, p>.05).
Low- and High-intake rats display cocaine-induced increases in extracellular glutamate in the nucleus accumbens
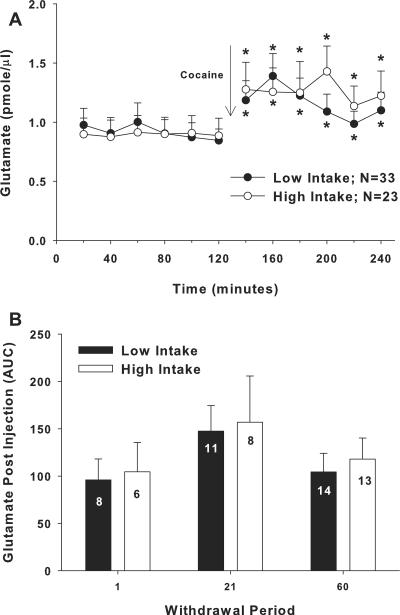
Figure 4a illustrates mean (± SEM) extracellular glutamate levels for two hours before and after a cocaine injection (10 mg/kg, IP). An ANOVA with time (20-min bin) as a repeated measure variable and intake condition and withdrawal as between subjects factors produced only a significant effect of time (F11,594=7.37, p<.001). Post hoc analyses revealed that extracellular glutamate levels were significantly elevated at every time bin following the cocaine injection relative to the last time bin preceding the injection (figure 4a, Fisher LSD with Bonferroni correction, p<.008).
Figure 4.
Rats withdrawn from cocaine self-administration displayed equivalent increases in extracellular levels of glutamate in the nucleus accumbens following a cocaine injection (10 mg/kg, IP) regardless of intake conditions or length of withdrawal. (a) Extracellular glutamate levels (pmole/μl) sampled during 20-min intervals in the nucleus accumbens from rats that had self-administered cocaine under low- or high-intake conditions. * indicates a significant difference from the last sample prior to the cocaine injection; Fisher LSD with Bonferroni correction, p<.0167). (b) Extracellular glutamate levels (pmole/μl) in the nucleus accumbens from rats withdrawn from low- or high-cocaine intake for 1, 21, or 60 days. The N/group is listed in each bar.
Although a significant effect of withdrawal was not obtained in the above ANOVA, figure 4b depicts the levels of extracellular glutamate (area under the curve; AUC) following the cocaine injection in low- and high-intake rats across the three time points. An ANOVA comparing glutamate levels following the cocaine injection with intake condition and withdrawal as between subjects factors failed to produce an interaction (F2,54=.005, p>.05) or a main effect of intake (F1,54=.197, p>.05) or withdrawal (F2,54=1.712, p>.05).
DISCUSSION
Identifying plasticity that gives rise to relapse is critical to understanding the neural basis of addiction, and will aid in the assessment and development of novel therapeutics. Toward this end, the reinstatement paradigm is widely used to establish the importance of identified adaptations to relapse behavior. Earlier experiments indicate that abnormal levels of extracellular glutamate in the nucleus accumbens present during baseline or following a cocaine injection are required for cocaine-induced reinstatement. Specifically, manipulations that prevent or mask cocaine-induced increases in extracellular glutamate in the nucleus accumbens also block cocaine-induced reinstatement (Baker et al., 2003; Berglind et al., 2009; Knackstedt et al., 2010; Li et al., 2010; Madayag et al., 2007; McFarland et al., 2003). The purpose of the current study was to determine whether glutamate dysfunction in the nucleus accumbens underlies relapse vulnerability. To study this, we varied both cocaine intake and length of withdrawal in part to better characterize the impact of these variables on drug seeking, but also to create conditions whereby rats trained to self-administer cocaine exhibit significant differences in reinstatement magnitude.
In the current study, the level of cocaine-induced reinstatement was augmented in rats that self-administered cocaine under high-intake conditions, which extends earlier reports by demonstrating that this phenomenon exists at every phase of withdrawal (Ahmed and Cador, 2006; Ahmed and Koob, 1998; Baker et al., 2001; Kippin et al., 2006; Lenoir and Ahmed, 2007; Mantsch et al., 2004; Rogers et al., 2008; Sutton et al., 2000; Vanderschuren and Everitt, 2004). The addition of the time course profile provides further support that this form of behavioral plasticity represents a persistent, sensitized increase in the motivational effects of cocaine (Ahmed and Cador, 2006; Deroche et al., 1999). While this conclusion appears to be obfuscated by the finding that high levels of cocaine intake are associated with reduced behavioral plasticity in the form of behavioral sensitization (Ben-Shahar et al., 2004; Knackstedt and Kalivas, 2007), this may be due to the emergence of stereotypic behavior interfering with locomotor activity (Ferrario et al., 2005). In addition to cocaine-primed behavior, reinstatement by an intermittent electric footshock is augmented in rats with a history of daily high-intake cocaine self-administration (Mantsch et al., 2008). Collectively, these studies indicate that high levels of cocaine consumed generate persistent plasticity resulting in enhanced relapse vulnerability.
The magnitude of reinstatement has also been shown to be sensitive to the length of withdrawal with longer periods resulting in enhanced drug seeking (Lu et al., 2004; Neisewander et al., 2000; Tran-Nguyen et al., 1998), an effect that has been termed incubation and linked to changes in excitatory signaling (Conrad et al., 2008; Grimm et al., 2001; Lu et al., 2005; Lu et al., 2007). Unlike earlier reports, we did not observe an impact of withdrawal length on the magnitude of reinstatement in either low- or high-cocaine intake rats, however this is likely due to our use of cocaine to elicit drug seeking. While incubation has been widely reported in studies using drug-paired context or discrete cues to reinstatement drug seeking (Grimm et al., 2001; Lu et al., 2004; Lu et al., 2005), cocaine-primed reinstatement has yielded mixed findings (Deroche-Gamonet et al., 2003; Lu et al., 2004; Marinelli et al., 2003; Tran-Nguyen et al., 1998). Despite our negative results, the capacity of withdrawal length to influence other forms of behavioral or cellular plasticity warrants its inclusion in attempts to identify plasticity that may underlie augmented reinstatement in high-intake rats (Bachtell and Self, 2008; Sorge and Stewart, 2005; Tran-Nguyen et al., 1998; White et al., 1995; Zhang et al., 1997).
In the current study, we observed abnormal extracellular glutamate levels during baseline in both low- and high-cocaine intake groups irrespective of withdrawal period or cocaine intake conditions. The addition of the time profile of this form of neurochemical plasticity extends earlier findings by demonstrating the existence of abnormal glutamate during early and late withdrawal periods (Madayag et al., 2010; Madayag et al., 2007; Miguens et al., 2008; Pierce et al., 1996; Reid and Berger, 1996). Our observation that cocaine-induced dysregulation of extracellular glutamate was present as soon as 24 hr after the last self-administration session are consistent with an earlier report (Miguens et al., 2008), and suggests that changes in cellular mechanisms regulating glutamate homeostasis develop during the course of cocaine self-administration and not as a result of cocaine withdrawal. The latter was a possibility given that the majority of studies detecting abnormal levels extracellular glutamate were conducted after 2–3 weeks of withdrawal (Madayag et al., 2010; Madayag et al., 2007; Pierce et al., 1996; Reid and Berger, 1996; Szumlinski et al., 2005). That abnormal glutamate levels in the nucleus accumbens were detected sixty days after the last self-administration session indicates the persistent nature of this form of cocaine-induced plasticity. Although not directly examined in the current studies, cellular mechanisms previously implicated include GLT-1 and system xc-, which contribute to the clearance and release of glutamate, respectively (Baker et al., 2003; Knackstedt et al., 2010; Madayag et al., 2007). Interestingly, both system xc- and GLT-1 are primarily expressed on astrocytes (Lehre et al., 1995; Pow, 2001; Rothstein et al., 1994), which raise the provocative scenario that drug-induced plasticity impacting glia are fundamental to the development of compulsive drug seeking.
The co-existence of reinstatement and glutamate dysfunction in both access groups during each stage of withdrawal is supportive of the conclusion that adaptations in the cellular mechanisms that regulate extracellular glutamate in the nucleus accumbens are required for the expression of cocaine-induced reinstatement (Baker et al., 2003; Knackstedt et al., 2010; Li et al., 2010; Madayag et al., 2007). Despite this, our current data indicate that augmented reinstatement in high-intake rats is not a product of relative differences in the magnitude of glutamate dysfunction as reflected by extracellular glutamate levels in the nucleus accumbens. These findings extend our earlier report by demonstrating a lack of intake-dependent differences at any withdrawal period (Madayag et al., 2010). The inability of differential changes in extracellular glutamate to account for heightened reinstatement in high-intake rats does not preclude drug-induced plasticity impacting other aspects of excitatory signaling. Indeed, withdrawal- and/or intake-dependent changes in AMPA receptor expression and intrinisic excitability have been detected (Boudreau et al., 2007; Conrad et al., 2008; Kourrich and Thomas, 2009; McCutcheon et al., 2011; Mu et al., 2010). In particular, extended access cocaine produces an increase in the surface expression of GluA2-lacking/Ca2+-permeable AMPA receptors in the nucleus accumbens. It is unknown whether this form of plasticity is intake dependent since it has yet to be examined in rats self-administering under low-intake conditions (Wolf, 2011), however, rats receiving low levels of experimenter-delivered cocaine do not display an increase in surface expression of these receptors (McCutcheon et al., 2011). To the extent that low-intake rats are found to mirror this later group receiving experimenter-delivered cocaine, heightened excitatory drive stemming from GluA2-lacking/Ca2+-permeable AMPA receptors may be a key example of intake-dependent plasticity that gives rise to relapse vulnerability. Collectively, these data indicate the need for further study in order to better understand the neural determinants of craving intensity.
The lack of intake-dependent differences in extracellular glutamate indicate that abnormal levels of extracellular glutamate are not sufficient to escalate daily cocaine intake, since this form of behavioral plasticity is only observed in high-intake rats (Ahmed and Cador, 2006; Ahmed and Koob, 1998; Kippin et al., 2006; Mantsch et al., 2004). An indirect link between extracellular glutamate levels and escalation of cocaine intake was proposed in our earlier study as a result of our finding that the cysteine prodrug N-acetylcysteine, which is used to increase glutamate release from system xc− (Baker et al., 2003), prevented both escalation of cocaine intake and cocaine-induced disruptions in extracellular glutamate in the nucleus accumbens (Madayag et al., 2007). There are several interpretations of the combined data sets, including the possibility that extracellular glutamate levels do not contribute to the escalation of cocaine intake. This would indicate that N-acetylcysteine exerts effects in addition to the presumed mechanism of action involving increased glutamate release from system xc−, which is a prime determinant of extracellular glutamate levels in the nucleus accumbens (Baker et al., 2002). While possible, the effects of N-acetylcysteine on multiple behaviors, including cocaine-induced reinstatement, are blocked by inhibiting system xc− (Baker et al., 2008; Kau et al., 2008). Alternatively, the collective data set may indicate that cocaine-induced disruptions in extracellular glutamate in the nucleus accumbens are necessary but not sufficient to generate escalation of cocaine intake. This would indicate the presence of neuroplasticity that is evident in high-, but not low-intake rats (e.g., expression patterns of GluA2-lacking/Ca2+-permeable AMPA receptors). The existence of drug-induced plasticity that require high levels of cocaine needs to be closely examined and would have broad implications ranging from the predictive validity of the reinstatement paradigm to contemporary views of the neural basis of addiction. Thus, expanding the use of the reinstatement and related behavioral paradigms to study multiple features of compulsive drug seeking under varied conditions may advance our current views of the neural basis of addiction, which in turn may positively impact our ability to utilize behavioral paradigms to predict the therapeutic value of putative pharmacotherapies.
Acknowledgements
The studies in the current report were funded by NIDA grants DA017328 (DB) and DA015758 (JM). The authors do not have any conflict of interest to report.
Footnotes
Authors Contribution: The experiments were conducted in part by VL, LK, KK, and AM. The experiments were designed primarily by JM and DB. The manuscript was written primarily by VL, JM, and DB.
References
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Self DW. Renewed cocaine exposure produces transient alterations in nucleus accumbens AMPA receptor-mediated behavior. J Neurosci. 2008;28:12808–12814. doi: 10.1523/JNEUROSCI.2060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baker DA, Tran-Nguyen TL, Fuchs RA, Neisewander JL. Influence of individual differences and chronic fluoxetine treatment on cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2001;155:18–26. doi: 10.1007/s002130000676. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW, Jr., LaLumiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29:3715–3719. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Intravenous self-administration of etonitazene, cocaine and phencyclidine in rats during food deprivation and satiation. J Pharmacol Exp Ther. 1981;217:241–247. [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Martinez A, Le Moal M, Piazza PV. Relationships between individual sensitivity to CS- and cocaine-induced reinstatement in the rat. Psychopharmacology (Berl) 2003;168:201–207. doi: 10.1007/s00213-002-1306-9. [DOI] [PubMed] [Google Scholar]
- Deroche V, Le Moal M, Piazza PV. Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur J Neurosci. 1999;11:2731–2736. doi: 10.1046/j.1460-9568.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau KS, Madayag A, Mantsch JR, Grier MD, Abdulhameed O, Baker DA. Blunted cystine-glutamate antiporter function in the nucleus accumbens promotes cocaine-induced drug seeking. Neuroscience. 2008;155:530–537. doi: 10.1016/j.neuroscience.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2006;187:60–67. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther. 2007;322:1103–1109. doi: 10.1124/jpet.107.122861. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Thomas MJ. Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. J Neurosci. 2009;29:12275–12283. doi: 10.1523/JNEUROSCI.3028-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Ahmed SH. Heroin-induced reinstatement is specific to compulsive heroin use and dissociable from heroin reward and sensitization. Neuropsychopharmacology. 2007;32:616–624. doi: 10.1038/sj.npp.1301083. [DOI] [PubMed] [Google Scholar]
- Li X, Li J, Gardner EL, Xi ZX. Activation of mGluR7s inhibits cocaine-induced reinstatement of drug-seeking behavior by a nucleus accumbens glutamate-mGluR2/3 mechanism in rats. J Neurochem. 2010;114:1368–1380. doi: 10.1111/j.1471-4159.2010.06851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology (Berl) 2004;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Madayag A, Kau KS, Lobner D, Mantsch JR, Wisniewski S, Baker DA. Drug-induced plasticity contributing to heightened relapse susceptibility: neurochemical changes and augmented reinstatement in high-intake rats. J Neurosci. 2010;30:210–217. doi: 10.1523/JNEUROSCI.1342-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, Grier MD, Baker DA. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27:13968–13976. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Francis DM, Katz ES, Hoks MA, Serge JP. Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology (Berl) 2008;195:591–603. doi: 10.1007/s00213-007-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Cooper DC, Baker LK, White FJ. Impulse activity of midbrain dopamine neurons modulates drug-seeking behavior. Psychopharmacology (Berl) 2003;168:84–98. doi: 10.1007/s00213-003-1491-1. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguens M, Del Olmo N, Higuera-Matas A, Torres I, Garcia-Lecumberri C, Ambrosio E. Glutamate and aspartate levels in the nucleus accumbens during cocaine self-administration and extinction: a time course microdialysis study. Psychopharmacology (Berl) 2008;196:303–313. doi: 10.1007/s00213-007-0958-x. [DOI] [PubMed] [Google Scholar]
- Mu P, Moyer JT, Ishikawa M, Zhang Y, Panksepp J, Sorg BA, Schluter OM, Dong Y. Exposure to cocaine dynamically regulates the intrinsic membrane excitability of nucleus accumbens neurons. J Neurosci. 2010;30:3689–3699. doi: 10.1523/JNEUROSCI.4063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2 ed. Academic Press; New York: 1986. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pow DV. Visualising the activity of the cystine-glutamate antiporter in glial cells using antibodies to aminoadipic acid, a selectively transported substrate. Glia. 2001;34:27–38. doi: 10.1002/glia.1037. [DOI] [PubMed] [Google Scholar]
- Reid MS, Berger SP. Evidence for sensitization of cocaine-induced nucleus accumbens glutamate release. Neuroreport. 1996;7:1325–1329. doi: 10.1097/00001756-199605170-00022. [DOI] [PubMed] [Google Scholar]
- Rogers JL, De Santis S, See RE. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology (Berl) 2008;199:615–624. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Stewart J. The contribution of drug history and time since termination of drug taking to footshock stress-induced cocaine seeking in rats. Psychopharmacology (Berl) 2005;183:210–217. doi: 10.1007/s00213-005-0160-y. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Karanian DA, Self DW. Factors that determine a propensity for cocaine-seeking behavior during abstinence in rats. Neuropsychopharmacology. 2000;22:626–641. doi: 10.1016/S0893-133X(99)00160-8. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Abernathy KE, Oleson EB, Klugmann M, Lominac KD, He DY, Ron D, During M, Kalivas PW. Homer Isoforms Differentially Regulate Cocaine-Induced Neuroplasticity. Neuropsychopharmacology. 2005 doi: 10.1038/sj.npp.1300890. [DOI] [PubMed] [Google Scholar]
- Tran-Nguyen LTL, Fuchs RA, Coffey GP, Baker DA, O'Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Walker J, Ahmed A, Gracy K, Koob G. Microinjections of an opiate receptor antagonist into the bed nucleus of the stria terminalis suppress heroin self-administration in dependent rats. Brain Research. 2000;854:85–92. doi: 10.1016/s0006-8993(99)02288-x. [DOI] [PubMed] [Google Scholar]
- White FJ, Hu XT, Zhang XF, Wolf ME. Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J Pharmacol Exp Ther. 1995;273:445–454. [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ, Wolf ME. Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. J Pharmacol Exp Ther. 1997;281:699–706. [PubMed] [Google Scholar]
- Zheng D, Cabeza de Vaca S, Carr KD. Food restriction increases acquisition, persistence and drug prime-induced expression of a cocaine-conditioned place preference in rats. Pharmacol Biochem Behav. 2012;100:538–544. doi: 10.1016/j.pbb.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]