Abstract
Angiogenesis is a crucial process whereby new blood vessels are formed from pre-existing vessels and occurs under both normal and pathophysiological conditions. The process is precisely regulated through the balance between pro-angiogenic and anti-angiogenic mechanisms, and many of these mechanisms have been well-characterized through extensive research; however, little is known about how angiogenesis is regulated at the transcriptional level. We have recently shown that deletion of the Forkhead box (Fox) transcription factor Foxc1 in cells of neural crest (NC) lineage leads to aberrant vessel growth in the normally avascular corneas of mice, and that the effect is cell-type specific, because the corneas of mice lacking Foxc1 expression in vascular endothelial cells remained avascular. The NC-specific Foxc1 deletion was also associated with elevated levels of both pro-angiogenic factors, such as the matrix metalloproteases (MMPs) MMP-3, MMP-9, and MMP-19, and the angiogenic inhibitor soluble vascular endothelial growth factor receptor 1 (sVEGFR-1). Thus, FoxC1 appears to control angiogenesis by regulating two distinct and opposing mechanisms; if so, vascular development could be determined, at least in part, by a competitive balance between pro-angiogenic and anti-angiogenic FoxC1-regulated pathways. In this review, we describe the mechanisms by which FoxC1 regulates vessel growth and discuss how these observations could contribute to a more complete understanding of the role of FoxC1 in pathological angiogenesis.
Introduction
Under both physiological and pathological conditions, new blood vessels are formed from pre-existing vessels through a process called angiogenesis, which is precisely controlled by the balance between pro-angiogenic and anti-angiogenic factors. Vascular endothelial growth factor (VEGF)-A is perhaps the best known angiogenic factor described thus far, and alternative splicing of the VEGF-A gene transcript generates several VEGF-A isoforms (e.g., VEGF121, VEGF145, VEGF165, VEGF189, and VEGF206in humans) with different activities and bioavailabilities (Woolard et al., 2009). The translated VEGF-A proteins are stored in the extracellular matrix (ECM), and their bioavailability is enhanced by matrix metalloproteases (MMPs), which catalyze the proteolytic release or cleavage of VEGF-A from the ECM (Arroyo and Iruela-Arispe, 2010; Bergers et al., 2000; Ferrara, 2010). Free VEGF-A is responsible for controlling multiple processes of angiogenesis, whereas VEGF-A bioavailability is also negatively regulated by a soluble form of VEGF receptor 1 (see below).
VEGF-A promotes angiogenic activity in vascular endothelial cells by binding to either of two tyrosine kinase receptors, VEGF receptor 1 (VEGFR-1, also known as Flt-1) or VEGFR-2 (also known as KDR in humans and as Flk-1 in mice), which stimulates endothelial-cell proliferation, migration, and tube formation (de Vries et al., 1992; Terman et al., 1992). Soluble VEGFR-1 (sVEGFR-1 or sflt-1) (Wu et al., 2010) is a truncated splice variant that binds VEGF-A with high affinity but lacks the transmembrane region and intracellular tyrosine-kinase domain of VEGFR-1; consequently, sVEGFR-1 functions as an angiogenesis inhibitor by sequestering VEGF-A in the ECM. sVEGFR-1 is highly expressed in the human cornea (Ambati et al., 2007), where it is crucial for maintaining corneal avascularity (Ambati et al., 2006), and disruption of the balance between pro-angiogenic and anti-angiogenic factors such as sVEGFR-1 can lead to abnormal growth of vessels from the limbus of the eye into the cornea (i.e., corneal neovascularization), which affects millions of people and is a leading cause of blindness or impaired vision (Regenfuss et al., 2008; Sassa and Hata, 2010; Tolentino, 2009). Furthermore, because the cornea is uniquely avascular and easily accessible, many pro/anti-angiogenic agents (including sVEGFR-1) have been identified by evaluating their ability to influence the growth of corneal vessels (Montezuma et al., 2009) in animal models of alkali burn injury (Ambati et al., 2003a; Ambati et al., 2003b), corneal suture placement (Corrent et al., 1989; Williams and Coster, 1985), or the corneal micropocket assay ( Kenyon et al., 1996; Rogers et al., 2007).
Forkhead box (Fox) transcription factors in vessel formation
Members of the Fox transcription factor family, including FoxC and FoxO, have been implicated in vascular formation (Papanicolaou et al., 2008). During early stages of vascular development, FoxC transcription factor interacts with the Ets transcription factor Etv2 (Etsrp71, ER71) to regulate endothelial-specific gene expression such as Flk-1 and VE-cadherin (De Val et al., 2008). In fact, FoxC expression precedes Etv2 in the lateral plate mesoderm of the zebrafish embryo, and FoxC can bind to the Etv2 enhancer (Veldman and Lin, 2012). Morpholino knockdown of the zebrafish FoxC genes (foxc1a and foxc1b) leads to a reduction in Etv2 expression, suggesting that in addition to the shared role in endothelial gene regulation (De Val et al., 2008), FoxC functions upstream of Etv2 to control formation of endothelial cell precursors (angioblasts) from the mesoderm during vasculogenesis (Veldman and Lin, 2012). Interestingly, the primitive erythroid lineage is also affected in FoxC morphant zebrafish, although it might be due to defective paraxial mesoderm (Veldman and Lin, 2012). Lack of FoxC in zebrafish also results in defects in angiogenic vessel patterning, disruptions in vascular basement membrane integrity, and increased vascular permeability, as well as arteriovenous malformations (Skarie and Link, 2009). Similarly, mice deficient for the FoxC homologs (Foxc1 and Foxc2) show abnormal vessel morphogenesis/remodeling and impaired arterial cell determination (Kume et al., 2001; Seo et al., 2006). Taken together, FoxC acts as a key regulator for vascular development.
FoxC1 and corneal vessel growth
Axenfeld-Rieger Syndrome (ARS) is an autosomal-dominant genetic disorder characterized by a variety of malformations in the anterior ocular segment (Alward, 2000; Chang et al., 2012; Tumer and Bach-Holm, 2009), and patients whose ARS pedigree evolves from mutations in FOXC1 or from changes in FOXC1 gene copy number often display evidence of pathological corneal vessel growth (Seo et al., 2012). As described above, the involvement of FoxC1 in vascular formation during development has been demonstrated previously, and we have recently shown that Foxc1 is also a key inhibitor of corneal neovascularization in mice (Seo et al., 2012). Mice homozygous for either a global Foxc1-null mutation (i.e., Foxc1−/− mice) (Kume et al., 1998) or for a Foxc1-null mutation in neural crest (NC) cells (i.e., NC-Foxc1−/− mice) (Seo et al., 2012) die postnatally, but the embryos displayed prominent evidence of vessel growth throughout the cornea, whereas the corneas of mice that expressed a vascular endothelial-specific Foxc1-null mutation were avascular. Furthermore, ocular abnormalities similar to those associated with FOXC1 mutations in patients with ARS were observed in homozygous NC-Foxc1-null embryos or heterozygous NC-Foxc1-null (NC-Foxc1+/−) adult mice, and the limbal vessels of heterozygous mutant embryos were disrupted (Seo et al., 2012). Collectively, these results suggest that the abnormal corneal neovascularization observed in patients with FOXC1 mutations could be caused by the impairment of FOXC1 regulatory activity in NC-derived corneal stromal cells.
The growth of corneal vessels in response to alkali burn injury was also enhanced in adult NC-Foxc1+/− mice, and this enhancement was abolished by VEGF blockade, which suggests that FoxC1 regulates corneal angiogenesis through a VEGF-dependent pathway. However, corneal levels of VEGF expression in NC-Foxc1−/− embryos were normal, while the expression of MMP-3, MMP-9, and MMP-19, which are known to cleave and release VEGF-A from the ECM (Arroyo and Iruela-Arispe, 2010; Ferrara, 2010; Lee et al., 2005), were significantly upregulated. This apparent link between MMP proteins and FoxC1 or other Fox transcription factors has been reported previously (Table 1): MMP7 is a key component of the signaling pathway that mediates the FOXC1-induced invasion of breast cancer cells (Sizemore and Keri, 2012), MMP-9 and MMP-13 expression are regulated by FOXO3a (Storz et al., 2009), and the transcription of MMP-9 is activated in response to tumor necrosis factor (TNF)-α by interactions between FoxO4 and Sp1 (Li et al., 2007). Thus, FoxC1 appears to limit corneal angiogenesis by reducing MMP expression, which subsequently limits the bioavailability (but not the amount) of VEGF in the ECM.
Table 1.
Regulation of MMP Expression by Fox Transcription Factors
| Fox Protein | MMP | Tissue or Cell Type | Reference |
|---|---|---|---|
| FoxC1 | MMP7 | Breast cancer tissue and breast cancer cell lines | (Sizemore and Keri, 2012) |
| FoxM1 | MMP9 | Human colorectal tissue samples, pancreatic cancer cells, papillary thyroid carcinoma, and breast cancer cell lines (MDA-MB-231 and SUM149) | (Ahmad et al., 2010; Ahmed et al., 2012; Uddin et al., 2011; Wang et al., 2010) |
| MMP2 | Glioma cells, oral cavity squamous cell carcinoma, and pancreatic cancer cells | (Chen et al., 2009; Dai et al., 2007; Wang et al., 2007) | |
| FoxO3a | MMP9 MMP13 |
Cancer cell lines (HeLa and MDA-MB-435) | (Storz et al., 2009) |
| MMP3 | Endothelial cells (human umbilical vein endothelial cells) | (Lee et al., 2008) | |
| FoxO4 | MMP9 | Rat aortic smooth muscle cells, COS cells, and C2C12 cells | (Li et al., 2007) |
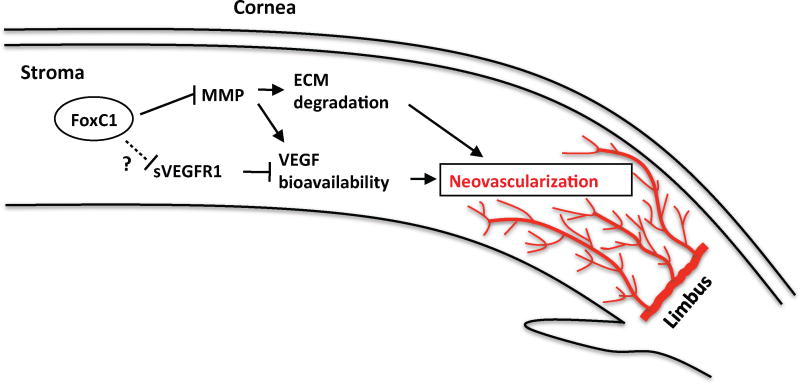
The Foxc1-null mutations in mice were also associated with elevated corneal levels of sVEGFR-1 (Seo et al., 2012), which is counterintuitive, because sVEGFR-1 is an angiogenesis inhibitor. Thus, FoxC1 appears to control corneal vessel growth by regulating at least two competing mechanisms: pro-angiogenic MMP-mediated increases in VEGF bioavailability and anti-angiogenic sVEGFR-1 upregulation (Fig. 1). MMP activity also stimulates endothelial cell migration by degrading the ECM, and Foxc1 deficiency in the corneal stroma resulted in disorganization of the ECM (Seo et al., 2012). The mechanisms by which FoxC1 influences sVEGFR-1 levels have yet to be identified and may only be induced during pathological corneal vessel growth; if so, sVEGFR-1 could be primarily responsible for limiting vessel growth in the cornea, and its anti-angiogenic activity may be impaired by the loss of FoxC1 expression.
Figure 1.
FoxC1 regulates the precisely controlled balance between pro-angiogenic pathways (e.g., MMP-mediated ECM degradation and release of VEGF from the ECM, which increases VEGF bioavailability) and anti-angiogenic pathways (e.g., sVEGFR-1-mediated VEGF sequestration) in the cornea [This figure is adapted from ( Seo et al., 2012)].
Like FoxC1, the SOX (SRY [sex determining region Y]-related HMG [high mobility group]-box) transcription factors are crucially involved in vascular development, and abnormal corneal vascularity has been observed in mice heterozygous for the Ragged Opossum (RaOp) mutation (Francois and Ramchandran, 2012), which is a dominant-negative Sox18 truncation that is believed to suppress the function of F-group SOX members (Sox7, Sox17, and Sox18) (Francois et al., 2008; James et al., 2003). Thus, the SoxF transcription factors may also function as angiogenic inhibitors in the developing cornea, but whether SoxF interacts synergistically with FoxC1 or must be expressed in the NC-derived corneal stroma to maintain corneal transparency has yet to be determined.
Future perspectives
Because FoxC1 is expressed in vascular endothelial cells, where it promotes angiogenic activity (De Val et al., 2008; Hayashi and Kume, 2008; Kume et al., 2001; Skarie and Link, 2009), and our recent findings indicate that FoxC1 expression in the corneal stroma prevents the growth of vessels from the limbus, the regulation of angiogenesis by FoxC1 appears to be cell-type dependent. In the kidneys, hearts, and lungs of adult mice, Foxc1 is expressed both by cells that are positive for CD31 expression, a marker for vascular endothelial cells, and by CD31 – cells, which include fibroblasts and other types of nonvascular cells (Sasman et al., 2012). Collectively, these observations suggest that the vasculature of these organs could be determined, at least in part, by a balance between pro-angiogenic FoxC1 activity in the vascular endothelium and anti-angiogenic FoxC1 activity in the surrounding fibroblasts and nonvascular stromal cells. FOXC1 has also been shown to regulate the invasiveness of basal-like breast cancers (Sizemore and Keri, 2012), and recent studies have linked the survival of patients with breast cancer to variations in FOXC1 expression (Bloushtain-Qimron et al., 2008; Muggerud et al., 2010). Thus, ablation of FoxC1 expression in specific cell types could yield new insights into the growth of vessels both during normal development and under pathological conditions.
Acknowledgments
The authors thank W. Kevin Meisner, PhD, ELS, for editorial support. This work was supported by the National Institutes of Health (HL074121 and EY019484).
Footnotes
Gene names are in all uppercase letters for human Fox genes (e.g., FOXC1). Only the first letter is capitalized for mouse Fox genes (e.g., Foxc1), and the first and subclass letters are capitalized for all chordates (e.g., FoxC1) (Kaestner et al., 2000). All letters are lowercase for zebrafish Fox genes (e.g., foxc1).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad A, Wang Z, Kong D, Ali S, Li Y, Banerjee S, Ali R, Sarkar FH. FoxM1 down-regulation leads to inhibition of proliferation, migration and invasion of breast cancer cells through the modulation of extra-cellular matrix degrading factors. Breast Cancer Res Treat. 2010;122:337–346. doi: 10.1007/s10549-009-0572-1. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Uddin S, Hussain AR, Alyan A, Jehan Z, Al-Dayel F, Al-Nuaim A, Al-Sobhi S, Amin T, Bavi P, Al-Kuraya KS. FoxM1 and its association with matrix metalloproteinases (MMP) signaling pathway in papillary thyroid carcinoma. The Journal of clinical endocrinology and metabolism. 2012;97:E1–E13. doi: 10.1210/jc.2011-1506. [DOI] [PubMed] [Google Scholar]
- Alward WL. Axenfeld-Rieger syndrome in the age of molecular genetics. Am J Ophthalmol. 2000;130:107–115. doi: 10.1016/s0002-9394(00)00525-0. [DOI] [PubMed] [Google Scholar]
- Ambati BK, Anand A, Joussen AM, Kuziel WA, Adamis AP, Ambati J. Sustained inhibition of corneal neovascularization by genetic ablation of CCR5. Investigative ophthalmology & visual science. 2003a;44:590–593. doi: 10.1167/iovs.02-0685. [DOI] [PubMed] [Google Scholar]
- Ambati BK, Joussen AM, Kuziel WA, Adamis AP, Ambati J. Inhibition of corneal neovascularization by genetic ablation of CCR2. Cornea. 2003b;22:465–467. doi: 10.1097/00003226-200307000-00013. [DOI] [PubMed] [Google Scholar]
- Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb MT, Kleinman ME, Caldwell RB, Lin Q, Ogura Y, Orecchia A, Samuelson DA, Agnew DW, St Leger J, Green WR, Mahasreshti PJ, Curiel DT, Kwan D, Marsh H, Ikeda S, Leiper LJ, Collinson JM, Bogdanovich S, Khurana TS, Shibuya M, Baldwin ME, Ferrara N, Gerber HP, De Falco S, Witta J, Baffi JZ, Raisler BJ, Ambati J. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati BK, Patterson E, Jani P, Jenkins C, Higgins E, Singh N, Suthar T, Vira N, Smith K, Caldwell R. Soluble vascular endothelial growth factor receptor-1 contributes to the corneal antiangiogenic barrier. The British journal of ophthalmology. 2007;91:505–508. doi: 10.1136/bjo.2006.107417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo AG, Iruela-Arispe ML. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc Res. 2010;86:226–235. doi: 10.1093/cvr/cvq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloushtain-Qimron N, Yao J, Snyder EL, Shipitsin M, Campbell LL, Mani SA, Hu M, Chen H, Ustyansky V, Antosiewicz JE, Argani P, Halushka MK, Thomson JA, Pharoah P, Porgador A, Sukumar S, Parsons R, Richardson AL, Stampfer MR, Gelman RS, Nikolskaya T, Nikolsky Y, Polyak K. Cell type-specific DNA methylation patterns in the human breast. Proc Natl Acad Sci U S A. 2008;105:14076–14081. doi: 10.1073/pnas.0805206105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TC, Summers CG, Schimmenti LA, Grajewski AL. Axenfeld-Rieger syndrome: new perspectives. The British journal of ophthalmology. 2012;96:318–322. doi: 10.1136/bjophthalmol-2011-300801. [DOI] [PubMed] [Google Scholar]
- Chen CH, Chien CY, Huang CC, Hwang CF, Chuang HC, Fang FM, Huang HY, Chen CM, Liu HL, Huang CY. Expression of FLJ10540 is correlated with aggressiveness of oral cavity squamous cell carcinoma by stimulating cell migration and invasion through increased FOXM1 and MMP-2 activity. Oncogene. 2009;28:2723–2737. doi: 10.1038/onc.2009.128. [DOI] [PubMed] [Google Scholar]
- Corrent G, Roussel TJ, Tseng SC, Watson BD. Promotion of graft survival by photothrombotic occlusion of corneal neovascularization. Arch Ophthalmol. 1989;107:1501–1506. doi: 10.1001/archopht.1989.01070020575043. [DOI] [PubMed] [Google Scholar]
- Dai B, Kang SH, Gong W, Liu M, Aldape KD, Sawaya R, Huang S. Aberrant FoxM1B expression increases matrix metalloproteinase-2 transcription and enhances the invasion of glioma cells. Oncogene. 2007;26:6212–6219. doi: 10.1038/sj.onc.1210443. [DOI] [PubMed] [Google Scholar]
- De Val S, Chi NC, Meadows SM, Minovitsky S, Anderson JP, Harris IS, Ehlers ML, Agarwal P, Visel A, Xu SM, Pennacchio LA, Dubchak I, Krieg PA, Stainier DY, Black BL. Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell. 2008;135:1053–1064. doi: 10.1016/j.cell.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Binding to the extracellular matrix and proteolytic processing: two key mechanisms regulating vascular endothelial growth factor action. Mol Biol Cell. 2010;21:687–690. doi: 10.1091/mbc.E09-07-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, Davidson T, Tutt D, Cheah KS, Stacker SA, Muscat GE, Achen MG, Dejana E, Koopman P. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–647. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- Francois M, Ramchandran R. Studies on Axenfeld-Rieger syndrome patients and mice reveal Foxc1’s role in corneal neovascularization. Proc Natl Acad Sci U S A. 2012;109:1818–1819. doi: 10.1073/pnas.1119291109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Kume T. Forkhead transcription factors regulate expression of the chemokine receptor CXCR4 in endothelial cells and CXCL12-induced cell migration. Biochem Biophys Res Commun. 2008;367:584–589. doi: 10.1016/j.bbrc.2007.12.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James K, Hosking B, Gardner J, Muscat GE, Koopman P. Sox18 mutations in the ragged mouse alleles ragged-like and opossum. Genesis. 2003;36:1–6. doi: 10.1002/gene.10190. [DOI] [PubMed] [Google Scholar]
- Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D’Amato RJ. A model of angiogenesis in the mouse cornea. Investigative ophthalmology & visual science. 1996;37:1625–1632. [PubMed] [Google Scholar]
- Kume T, Deng KY, Winfrey V, Gould DB, Walter MA, Hogan BL. The forkhead/winged helix gene Mf1 is disrupted in the pleiotropic mouse mutation congenital hydrocephalus. Cell. 1998;93:985–996. doi: 10.1016/s0092-8674(00)81204-0. [DOI] [PubMed] [Google Scholar]
- Kume T, Jiang H, Topczewska JM, Hogan BL. The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes Dev. 2001;15:2470–2482. doi: 10.1101/gad.907301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, You HJ, Won JY, Youn SW, Cho HJ, Park KW, Park WY, Seo JS, Park YB, Walsh K, Oh BH, Kim HS. Forkhead factor, FOXO3a, induces apoptosis of endothelial cells through activation of matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2008;28:302–308. doi: 10.1161/ATVBAHA.107.150664. [DOI] [PubMed] [Google Scholar]
- Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liang J, Castrillon DH, DePinho RA, Olson EN, Liu ZP. FoxO4 regulates tumor necrosis factor alpha-directed smooth muscle cell migration by activating matrix metalloproteinase 9 gene transcription. Mol Cell Biol. 2007;27:2676–2686. doi: 10.1128/MCB.01748-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montezuma SR, Vavvas D, Miller JW. Review of the ocular angiogenesis animal models. Semin Ophthalmol. 2009;24:52–61. doi: 10.1080/08820530902800017. [DOI] [PubMed] [Google Scholar]
- Muggerud AA, Ronneberg JA, Warnberg F, Botling J, Busato F, Jovanovic J, Solvang H, Bukholm I, Borresen-Dale AL, Kristensen VN, Sorlie T, Tost J. Frequent aberrant DNA methylation of ABCB1, FOXC1, PPP2R2B and PTEN in ductal carcinoma in situ and early invasive breast cancer. Breast Cancer Res. 2010;12:R3. doi: 10.1186/bcr2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou KN, Izumiya Y, Walsh K. Forkhead transcription factors and cardiovascular biology. Circulation research. 2008;102:16–31. doi: 10.1161/CIRCRESAHA.107.164186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenfuss B, Bock F, Parthasarathy A, Cursiefen C. Corneal (lymph)angiogenesis--from bedside to bench and back: a tribute to Judah Folkman. Lymphat Res Biol. 2008;6:191–201. doi: 10.1089/lrb.2008.6348. [DOI] [PubMed] [Google Scholar]
- Rogers MS, Birsner AE, D’Amato RJ. The mouse cornea micropocket angiogenesis assay. Nat Protoc. 2007;2:2545–2550. doi: 10.1038/nprot.2007.368. [DOI] [PubMed] [Google Scholar]
- Sasman A, Nassano-Miller C, Shim KS, Koo HY, Liu T, Schultz KM, Millay M, Nanano A, Kang M, Suzuki T, Kume T. Generation of conditional alleles for Foxc1 and Foxc2 in mice. Genesis. 2012 doi: 10.1002/dvg.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa Y, Hata Y. Antiangiogenic drugs in the management of ocular diseases: Focus on antivascular endothelial growth factor. Clin Ophthalmol. 2010;4:275–283. doi: 10.2147/opth.s6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Fujita H, Nakano A, Kang M, Duarte A, Kume T. The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev Biol. 2006;294:458–470. doi: 10.1016/j.ydbio.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Seo S, Singh HP, Lacal PM, Sasman A, Fatima A, Liu T, Schultz KM, Losordo DW, Lehmann OJ, Kume T. Forkhead box transcription factor FoxC1 preserves corneal transparency by regulating vascular growth. Proc Natl Acad Sci U S A. 2012;109:2015–2020. doi: 10.1073/pnas.1109540109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore ST, Keri RA. The Forkhead Box Transcription Factor FOXC1 Promotes Breast Cancer Invasion by Inducing Matrix Metalloprotease 7 (MMP7) Expression. J Biol Chem. 2012 doi: 10.1074/jbc.M112.375865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarie JM, Link BA. FoxC1 is essential for vascular basement membrane integrity and hyaloid vessel morphogenesis. Investigative ophthalmology & visual science. 2009;50:5026–5034. doi: 10.1167/iovs.09-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz P, Doppler H, Copland JA, Simpson KJ, Toker A. FOXO3a promotes tumor cell invasion through the induction of matrix metalloproteinases. Mol Cell Biol. 2009;29:4906–4917. doi: 10.1128/MCB.00077-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, Bohlen P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- Tolentino MJ. Current molecular understanding and future treatment strategies for pathologic ocular neovascularization. Curr Mol Med. 2009;9:973–981. doi: 10.2174/156652409789712783. [DOI] [PubMed] [Google Scholar]
- Tumer Z, Bach-Holm D. Axenfeld-Rieger syndrome and spectrum of PITX2 and FOXC1 mutations. Eur J Hum Genet. 2009;17:1527–1539. doi: 10.1038/ejhg.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin S, Ahmed M, Hussain A, Abubaker J, Al-Sanea N, AbdulJabbar A, Ashari LH, Alhomoud S, Al-Dayel F, Jehan Z, Bavi P, Siraj AK, Al-Kuraya KS. Genome-wide expression analysis of Middle Eastern colorectal cancer reveals FOXM1 as a novel target for cancer therapy. Am J Pathol. 2011;178:537–547. doi: 10.1016/j.ajpath.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman MB, Lin S. Etsrp/Etv2 is directly regulated by Foxc1a/b in the zebrafish angioblast. Circulation research. 2012;110:220–229. doi: 10.1161/CIRCRESAHA.111.251298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ahmad A, Banerjee S, Azmi A, Kong D, Li Y, Sarkar FH. FoxM1 is a novel target of a natural agent in pancreatic cancer. Pharmaceutical research. 2010;27:1159–1168. doi: 10.1007/s11095-010-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Banerjee S, Kong D, Li Y, Sarkar FH. Down-regulation of Forkhead Box M1 transcription factor leads to the inhibition of invasion and angiogenesis of pancreatic cancer cells. Cancer Res. 2007;67:8293–8300. doi: 10.1158/0008-5472.CAN-07-1265. [DOI] [PubMed] [Google Scholar]
- Williams KA, Coster DJ. Penetrating corneal transplantation in the inbred rat: a new model. Investigative ophthalmology & visual science. 1985;26:23–30. [PubMed] [Google Scholar]
- Woolard J, Bevan HS, Harper SJ, Bates DO. Molecular diversity of VEGF-A as a regulator of its biological activity. Microcirculation. 2009;16:572–592. doi: 10.1080/10739680902997333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FT, Stefanini MO, Mac Gabhann F, Kontos CD, Annex BH, Popel AS. A systems biology perspective on sVEGFR1: its biological function, pathogenic role and therapeutic use. J Cell Mol Med. 2010;14:528–552. doi: 10.1111/j.1582-4934.2009.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]