Abstract
The prevalence of chronic kidney disease (CKD) has risen and will continue to rise in the United States and worldwide. This is alarming considering that CKD remains an irreversible condition and patients who progress to chronic kidney failure suffer reduced quality of life and high mortality rates. As such, it is imperative to identify modifiable risk factors to develop strategies to slow CKD progression. One such factor is hyperuricemia. Recent observational studies have associated hyperuricemia with kidney disease. In addition, hyperuricemia is largely prevalent in patients with CKD. Data from experimental studies have revealed several potential mechanisms by which hyperuricemia may contribute to the development and progression of CKD. In this manuscript we offer a critical review of the experimental evidence linking hyperuricemia to CKD, we highlight the gaps in our knowledge on the topic as it stands today, and we review the observational and interventional studies that have examined the potential nephro-protective effect of lowering uric acid in CKD patients . While uric acid may also be linked to cardiovascular disease and mortality in patients with CKD, this review will focus only on uric acid as a potential therapeutic target to prevent kidney disease onset and progression.
Index words: kidney disease progression, uric acid
In the last few decades, CKD has emerged as a global health problem of epidemic proportions 1. The prevalence of stage 3 CKD has increased in the United States with current estimates placing it at 11.5% 2. While in many persons CKD remains an asymptomatic pathologic condition that progresses slowly, for many others, CKD represents a progressive irreversible process that ultimately leads to the requirement for renal replacement therapy 3–5. In addition to the reduced quality of life, mortality rates among patients with ESRD remain extremely high. For example, in the 2011 US Renal Data System (USRDS) report, adjusted mortality rates for maintenance dialysis patients aged 45–64 years and ≥ 65 years were 154 and 313 deaths per 1,000 patient-years at risk, respectively. Both rates are some seven times greater than those seen in their counterparts in the general population6. Hence, and in the absence of curative therapy for patients with progressive CKD, it is important to pursue therapeutic interventions that may effectively slow CKD progression.
Carl Scheele, a Swedish pharmacist, discovered uric acid in 1776 in a bladder calculus and named it “acid of calculus”. Once the stone was moistened with nitric acid and dried, Scheele noted that the addition of dilute ammonium hydroxide converted it to the purple-red color characteristic of the ammonium salt of purpuric acid 7. Subsequently, Fourcroy, a French chemist, observed that chlorine water changed uric acid to urea and that distilling uric acid produced hydrocyanic acid, prompting him to name it “acid urique” 8.
Uric acid was thought to play a role in human diseases other than kidney stones as early as 1848 when Garrod, an English physician, discovered that uric acid is present in the blood of individuals suffering an acute gout attack; this observation prompted him to conclude that uric acid plays a role in gout 8. At the time, skepticism against a role for hyperuricemia in gout was roused by subsequent studies in which injecting uric acid into healthy animals and humans failed to induce gout. The skepticism was further fueled by studies revealing that uric acid was present in the blood of patients with other diseases, such as leukemia, and did not inevitably lead to the development of gout 9. Today, while we recognize that hyperuricemia alone is insufficient to cause gout and that other factors likely predispose to crystal formation, we also acknowledge that lowering uric acid levels is an effective strategy to prevent gout attacks 10. In contrast to gout, and even though the association between uric acid, gout, and kidney disease had been noted early on, a role for uric acid—lowering therapies in preventing and slowing kidney disease progression has not been established.
Uric acid homeostasis
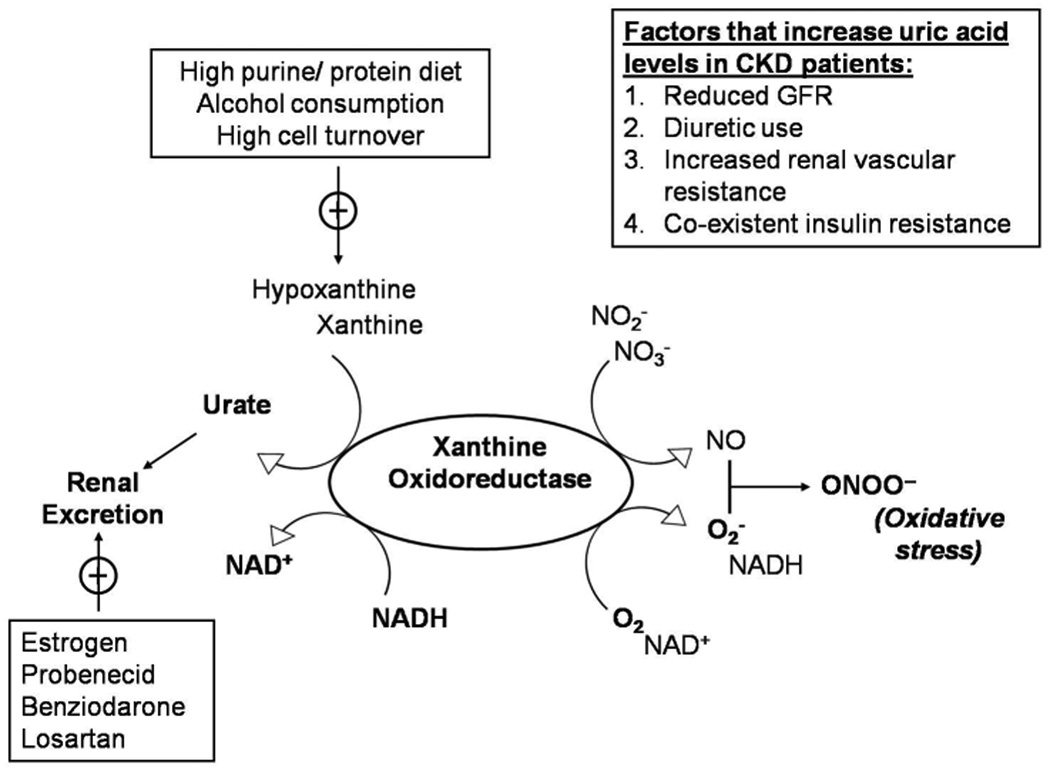
Uric acid is an end- product of purine metabolism that is produced mainly by the liver and the intestines but also by other peripheral tissues, such as muscles, the endothelium, and the kidney. Under normal conditions, two thirds of the produced uric acid is eliminated in the urine and one third is removed by the biliary tree. Although uric acid occurs predominantly as a urate anion under physiological pH, more uric acid than urate is present in the urine (pH 5–6) 11. In the kidney, urate is readily filtered by the glomerulus and subsequently reabsorbed by the proximal tubular cells of the kidney; the normal fractional excretion of uric acid is approximately 10% 12. The cell membrane is impermeable to the urate anion in the absence of specific transporters. Although urate transport is a complex and incompletely understood process 11, the efficiency with which the human kidney reabsorbs urate may contribute to the higher levels of serum uric acid in humans as compared to other species; this in addition to an uricase mutation preventing further uric acid degradation in humans13. It is generally accepted that the human urate transporter, URAT1 (encoded by the SLC22A12 gene), facilitates uric acid reabsorption in the proximal convoluted tubule 14. More recently, GLUT9 (encoded by SLC2A9), a member of the glucose transporter family, has been proposed to be a major regulator of uric acid homeostasis 15. In humans, it is mainly expressed in the proximal convoluted tubule on the basolateral membrane 11. Hyperuricemia is defined as the accumulation of serum uric acid beyond its solubility point in water (6.8 mg/dL), and develops due to uric acid over-production, under-secretion, or both 12. Uric acid homeostasis and main factors that lead to increased serum uric acid levels in CKD are schematically shown in Figure 1.
Figure 1.
Schematic representation of uric acid homeostasis.
Abbreviations: CKD, chornic kidney disease; GFR, glomerular filtration rate
A Plausible Role for uric acid in kidney disease
Traditionally, hyperuricemia associated with hyperuricosuria has been postulated to cause kidney disease by depositing intra-luminal crystal in the collecting duct of the nephron in a manner reminiscent of gouty arthropathy 16–17. Individuals with increased serum uric acid levels secondary to high dietary purine intake may also have a lower than normal urinary pH, favoring even more uric acid in the urine than urate. Considering that uric acid is less soluble than urate, this milieu would favor uric acid crystal formation 18. Uric acid crystals have the capacity to adhere to the surface of renal epithelial cells 19 and to induce an acute inflammatory response in such cell lines 20. In addition to an increased risk of kidney stone formation, such effects have also been shown to reduce glomerular filtration rate (GFR) 17.
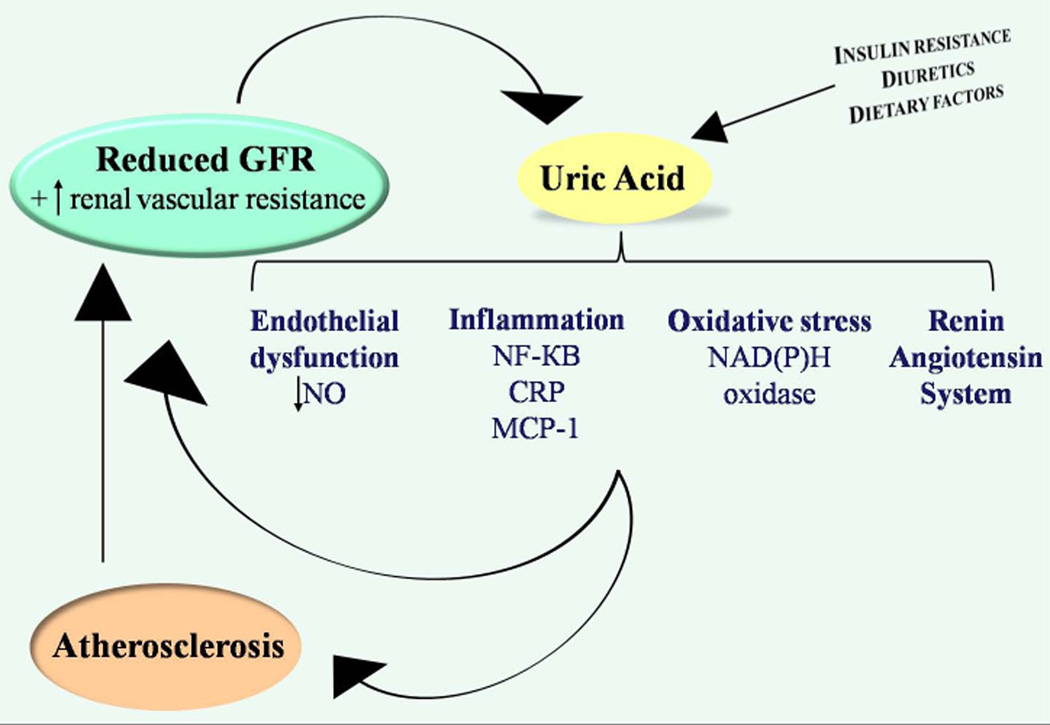
Contrary to the role of uric acid crystals in kidney disease, the non-crystal effects of uric acid remain contentious as, under physiological concentrations, urate is a powerful antioxidant that can scavenge superoxide, hydroxyl radicals, and singlet oxygen 21. Nevertheless, recent data may implicate mild hyperuricemia in kidney disease onset and progression. Experimentally induced hyperuricemia in rats leads to reduced urinary nitrites and to systemic and glomerular hypertension 22–23. The latter two can be prevented with the supplementation of L-arginine, suggesting that uric acid may cause endothelial dysfunction. This conclusion, while controversial, is supported by in vitro experimental studies showing that uric acid decreases nitric oxide (NO) production 24 and may also lead to NO depletion 25. In addition to a potential role in endothelial dysfunction, experimental hyperuricemia has been reported to cause an afferent renal arteriolopathy and tubulo-interstitial fibrosis in the kidney by activating the renin-angiotensin-aldosterone system (RAAS) 26. Uric acid has also been shown to activate the cytoplasmic phospholipase A2 and the inflammatory transcription factor nuclear factor κ B (NF-κB), leading to the inhibition of proximal tubular cellular proliferation in vitro 27. Other reported sequelae of raising serum uric acid levels include systemic cytokine production such as tumor necrosis factor α (TNF-α) 28 and the local expression of chemokines such as monocyte chemotactic protein 1 (MCP-1) in the kidney 29–30, and cyclooxygenase 2 (COX-2) in the blood vessels 30. Consistent with such experimental data, further animal studies suggest that lowering uric acid may slow CKD progression. Notably, lowering uric acid has been reported to reduce tubulointerstitial fibrosis both in the 5/6th nephrectomy model 31 and in diabetic nephropathy 32. Additionally, in humans, withdrawing uric acid—lowering therapy was found to increase urinary transforming growth factor β-1 in a group of hyperuricemic patients with CKD 33. The putative mechanisms by which increased serum uric acid may contribute to CKD onset and progression are illustrated in Figure 2.
Figure 2.
Putative mechanisms by which elevated serum uric acid level may contribute to CKD development and progression.
Uric acid as a predictor of human kidney disease
In the last two decades, a large number of observational studies have examined the potential link between increased serum uric acid levels and CKD 34–51. These studies (summarized in Table 1) have shown conflicted results in some instances. For example, an analysis of the Cardiovascular Health Study (CHS), 45 which involved 5,808 participants with 5 years of follow-up, showed no significant association between serum uric acid levels and incident CKD, and yet there was a significant relationship between increased serum uric acid and CKD progression even after adjustment for age, sex, race, serum creatinine, body mass index, waist circumference, blood pressure, use of anti-hypertensive drugs, use of allopurinol, blood glucose, lipids, ankle-arm index, carotid intima-media thickness, major electrocardiogram abnormalities, hemoglobin, C-reactive protein, and albumin levels. However, the older age of this population may have precluded the identification of serum uric acid as a predictor of incident CKD. Consistent with this, serum uric acid was found to be an independent risk factor for incident CKD in a pooled analysis of the Atherosclerosis Risks in Communities (ARIC) study and the CHS. This analysis involved 13,338 participants with intact kidney function at baseline who were followed for a mean period of 8.5 years 35. Similar findings were reported from the Vienna Health Screening Project, where an analysis of 21,475 healthy participants followed for 7 years indicated that elevated baseline uric acid levels were associated with an increased risk of incident CKD (defined as GFR <60 ml/min/1.73 m2), independently of age, sex, waist circumference, plasma lipids, fasting plasma glucose, estimated GFR, blood pressure, and use of anti-hypertensive drugs 36. Such an independent association between serum uric acid and incident CKD also has been corroborated by many other studies in Asian populations 34, 37–39. In addition, elevated levels of serum uric acid appear to be associated with an increased risk of diabetic nephropathy in both type 1 and type 2 diabetes 41–43.
TABLE 1.
Main prospective studies of the association between elevated serum uric acid level and CKD development or progression.
| Investigators | Study Population (N) |
F/U (y) |
Independent Variable |
Study Outcome | Adjustments Considered | Findings | |
|---|---|---|---|---|---|---|---|
| Iseki et al. 81 |
Okinawa General Health Maintenance Association (6,403) |
2 | Hyperuricemia (≥8 mg/dl) |
Incident CKD (SCr level ≥1.4 [♂] or ≥ 1.2[♀]) |
Age, sex, BMI, SBP, fasting glucose, sAlb, total cholesterol, proteinuria, hematuria, smoking, alcohol intake, physical activity |
Hyperuricemia independently associated with risk of developing higher SCr, aHR: 2.91 (1.8–4.7) in ♂, 10.4 (1.9–56.6) in ♀ |
|
| Domrongk itchaiporn et al.34 |
Employees of the Electric Generation Authority of Thailand |
3, 49 9 |
12 | Hyperuricemia (≥6.3 mg/dl) |
Incident CKD (eGFR <60) |
BMI, SBP, DBP, DM status, proteinuria, serum cholesterol, smoking history |
Hyperuricemia independently associated with increased risk of incident CKD: aOR, 1.82(1.12–2.98) |
| Weiner et al.35 |
ARIC and CHS |
13 ,3 38 |
8.5 | Serum uric acid levels (per 1 mg/dL increase) |
Incident CKD (eGFR decrease of ≥15 with final eGFR <60, or SCr increase of ≥0.4 with final SCr > 1.4 [♂] or ≥1.2[♀]) |
Age, sex, race, DM status, SBP, HTN status, CVD, LVH, smoking, alcohol use, education, total cholesterol, HDL- C, sAlb, Hct, baseline kidney function |
Serum uric acid independently associated with increased risk for incident CKD: aOR, 1.07(1.01–1.14) |
| Obermayr et al.36 |
The Vienna Health Screening Project |
21 ,4 75 |
7 | Hyperuricemia (moderate [7–8.9 mg/dL] and significant [≥9 mg/dL]) |
Incident CKD (eGFR <60) |
Age, sex, waist circumference, HDL-C, blood glucose, triglycerides, eGFR, mean BP, HTN medications |
Moderately elevated serum uric acid independently associated with increased risk of incident CKD: aOR, 1.26 (1.02–1.55); for significantly elevated serum uric acid: aOR, 1.63 (1.18–2.27) |
| Sonoda et al.37 |
Health check- up screening of non-DM healthy people |
7, 07 8 |
4.5 | Uric acid levels (per 1 mg/dL increase) |
Incident CKD (eGFR <60) |
BMI, smoking, SBP, fasting glucose, LDL-C, HDL-C, Hb, eGFR, smoking |
Serum uric acid an independent predictor of incident CKD: aOR, 1.09 (1.01–1.18), P=0.03 |
| Wang et al.38 |
Retrospective cohort study of Taiwanese adults |
94 ,4 22 |
3.5 | Hyperuricemia (≥7.3 mg/dl) |
Incident CKD (eGFR <60) |
Age, sex, education status, alcohol intake, smoking, HTN, DM status, physical activity, BMI, lipid profile, sAlb, Hb, CRP, GGT, SUN, eGFR, proteinuria, hematuria, medication usec |
Hyperuricemia independently associated with increased risk of incident CKD: aHR, 1.15(1.01–1.30), P<0.05 |
| Mok et al. 39 |
The Severance cohort study in Korea |
14 ,9 39 |
10. 2 |
Hyperuricemia ( ≥6.6 mg/dl [♂] or ≥4.6 mg/dl [♀]) |
Incident CKD (eGFR <60) |
Age, smoking, alcohol consumption, physical activity, BMI, total cholesterol, HTN status, DM |
Increased risk of incident CKD with hyperuricemia, aHR: 2.1 (1.6–2.9) in ♂ (P<0.0001), 1.3 (1.0–1.8) in ♀ (P=0.13) |
| Kuo et al., 82 |
Retrospective study of hospital based cohort |
63 ,7 85 |
3 | Hyperuricemia > 7.7 mg/dL [♂] or > 6.6 mg/dL [♀]) |
Annual eGFR decline of ≥3 |
Age, sex, baseline eGFRb, azotemia, hypercholesterolemia, hyperglycemia |
Hyperuricaemia associated with accelerated eGFR decline: HR, 1.28 (1.23–1.33), p< 0.001 |
| Hovind et al.40 |
T1DM | 26 3 |
18. 1 |
Serum uric acid levels (per 1 mg/dL increase) |
Incident Micro- or macro-albuminuria |
Age, sex, BMI, HbA1c, albuminuria, SCr, total cholesterol, mean BP |
Serum uric acid independently associated with subsequent development of persistent macroalbuminuria: aHR, 2.93 (1.25– 6.86) per 100 μmol/l increase in uric acid(P=0.013) |
| Jalal et al. 41 |
Coronary Artery Calcification in T1 DM Study |
32 4 |
6 | Serum uric acid levels (per 1 mg/dL increase) |
Composite outcome: incident micro- or macroalbuminuria |
Age, sex, duration of DM, BMI, waist circumference, SBP, smoking, HbA1c, albuminuria, SCr, SCysC, HDL-C, triglycerides, use of RAAS blockers |
Serum uric acid associated with micro-/macroalbuminuria: aOR, 1.8 (1.2–2.8) per 1-mg/dl increase in uric acid (P=0.005) |
| Ficociello et al.42 |
Second Joslin Kidney Study; T1DM |
35 5 |
6 | Serum uric acid categories (<3.0, 3.0–3.9, 4.0–4.9, 5.0–5.9, ≥6 mg/dl) |
Early eGFR loss, defined as eGFRcys decline of >3.3%/y |
Age, sex, HbA1c, eGFRcys, albuminuria |
Risk of early eGFR loss increased linearly: 9%, 13%, 20%, 29%, and 36% for uric acid categories in increasing order |
| Zoppini et al.43 |
Verona DM Study; T2DM |
1, 44 9 |
5 | Hyperuricemia (>7.0 mg/dl [♂] or >6.5 mg/dl [♀]) or allopurinol use |
Incident CKD (eGFR <60 or overt proteinuria) |
Age, sex, BMI, smoking status, DM duration, SBP, HTN treatment, insulin therapy, HbA1c, eGFR, albuminuria |
Hyperuricemia independently associated with increased risk of incident CKD: aOR, 2.10 (1.16–3.76), P<0.01 |
| Altemtam et al.83 |
Retrospective cohort study of elderly patients with T2DM & CKD3–4 |
27 0 |
8 | Serum uric acid (per each 1-mg increase) |
Progression of CKD (eGFR decline of >2/y) |
Age, race, SBP, eGFR, HbA1c, proteinuria, vascular co-morbidities |
Serum uric acid independently associated with faster kidney disease progression: aOR, 1.16(1.09–1.39), P=0.016 |
| Iseki et al. 46 |
Okinawa General Health Maintenance Association |
48 ,1 77 |
7 | Hyperuricemia (≥7 mg/dl [♂] or >6 mg/dl [♀]) |
ESRD | Age, SBP, DBP, BMI, proteinuria, Hct, total cholesterol, triglycerides, fasting blood glucose, SCr |
Hyperuricemia an independent risk factor for ESRD in ♀(aHR, 5.77 ([2.3– 14.4], P<0.001) but not ♂(aHR, 2.0 [0.90–4.44], P=NS) |
| Yen et al. 44 |
Community- based cohort of elderly Taiwanese |
51 9 |
2.7 | Serum uric acid levels (per 1 mg/dL increase) |
eGFR <60 or kidney disease progression (decrease in eGFR of ≥ 3/y) |
Age, sex, BMI, proteinuria, smoking, SCr, Hb, WBC count, HTN and DM status |
Serum uric acid independently associated with an increased risk of decline in eGFR (aOR, 1.21 [1.05– 1.39]) but not with incident CKD (OR, 0.99 [0.85–1.17]) |
| Sturm et al.50 |
The Mild to Moderate Kidney Disease Study |
22 7a |
7 | Serum uric acid levels (per 1 mg/dL) increase |
Progression of CKD (doubling of baseline SCr or ESRD) |
Age, sex, proteinuria, GFR, allopurinol use |
Serum uric acid not associated with increased risk of CKD progression, aHR: 0.95 (0.80–1.13) in all, 1.03 (0.85–1.26) if exclude those taking allopurinol |
| Chonchol et al.45 |
CHS | 5, 80 8 |
5 | Quintiles of uric acid |
Incident CKD (eGFR<60)or kidney disease progression (decrease in eGFR of ≥ 3/y) |
Age, sex, race, SCr, BMI, waist circumference, BP, HTN medications use, diuretics use, allopurinol use, glucose, HDL-C, triglycerides, ankle- arm index, carotid IMT, major ECG abnormalities, Hb, CRP, sAlb |
Independent association of serum uric acid with progression of kidney disease (aORs of 1.0, 0.88, 1.23, 1.47, and 1.49 for uric acid quintiles 1 through 5, respectively) but no significant association with incident CKD(aOR, 1.0 [0.89–1.14]) |
| Madero et al.51 |
MDRD Study | 84 0 |
10 | Serum uric acid levels (per 1 mg/dL increase) |
ESRD and death | Age, sex, history of CVD, DM, BMI, HDL-C, SBP, eGFR, sAlb, diuretic use, proteinuria, allopurinol use |
In CKD3–4, hyperuricemia not an independent risk factor for ESRD (HR, 1.02 [0.97–1.07]); serum uric acid significantly associated with all-cause and CV mortality |
| Ishani et al.84 |
MRFIT | 12 ,8 66e |
25 | Serum uric acid levels (per 1 mg/dL increase) |
Initiation of treatment for ESRDd |
Age, race, family history of DM, smoking, BMI, SBP, fasting glucose, triglycerides, HDL-C, LDL-C, eGFR, Hct, proteinuria |
Serum uric acid independently associated with increased risk of ESRD: aHR, 1.16(1.04–1.29), P=0.0006 |
| Hsu et al. 47 |
A large integrated health care delivery system |
17 7, 57 0 |
24. 5 |
Uric acid quartiles | ESRD | Age, sex, race, educational level, BMI, HTN status, DM status, history of kidney disease, history of nocturia, LVH, smoking, alcohol intake, occupational exposure to solvents/fumes/ chemicals, SCr, Hb, proteinuria |
Higher serum uric acid an independent risk factor for ESRD: HR, 2.14 (1.65–2.77) for highest vs lowest quartile |
| Bellomo et al.48 |
Healthy normotensive adult blood donors |
90 0 |
5 | Serum uric acid levels (per 1 mg/dL increase) |
eGFR loss (of >10) | Age, sex, BMI, mean BP, fasting glucose, total cholesterol, triglycerides, UACR, smoking |
Serum uric acid an independent risk factor for decreased kidney function: aHR, 1.23(1.09–1.39), P=0.001 |
| Ben-Dov et al.49 |
The Jerusalem Lipid Research Clinic cohort study |
24 49 |
25 | Hyperuricemia (upper quintile >6.5 mg/dl [♂] or > 5.3 mg/dl [♀]) |
ESRD and AKI defined by hospital discharge records |
None | Hyperuricemia conferred increased risk of ESRD, aHR: 1.94(1.20–3.14) in ♂, 5.20 (1.90–14.2) in ♀; also significantly associated with increased risk of AKI and all-cause mortality |
| Syrjanen et al.85 |
IgA nephropathy |
22 3 |
10 | Hyperuricemia (≥7.0 mg/dl [♂] or ≥ 6.5 mg/dl [♀]) |
Progression of CKD (elevation of SCr above normal and/or SCr ≥20% of baseline) |
Age, sex, BMI, proteinuria, HTN status, DM status, dyslipidemia |
Hyperuricemia independently associated with CKD progression only among those with initially normal kidney function: aHR, 4.60 (1.1–19.4) |
| Ohno et al.86 |
IgA nephropathy |
56 | 8 | Hyperuricemia (≥7.0 mg/dl) |
Progression of CKD (change in CCr) |
HTN and kidney pathology | Hyperuricemia associated with increased risk of kidney disease progression (unadjusted change in CCr: −22.3±20.8% vs +2.6±39.4%, P=0.0238); Uric acid associated with decline in CCr in adjusted analysis (P=0.046) |
Note: eGFR values given in mL/min/1.73 m2; SCr in mg/dL. Values in parentheses for HRs and ORs are 95% confidence intervals
number that completed the study = 177
By MDRD Study equation.
allopurinol, lipid-lowering drug, Chinese herbal medicine
ascertained with USRDS registry data
all men
Abbreviations: aHR, adjusted hazard ratio; AKI, acute kidney injury; aOR, adjusted odds ratio; ARIC, Atherosclerosis Risks in Communities; BMI: body mass index; BP, blood pressure; CCr, creatinine clearance; CHS, Cardiovascular Health Study; CKD, chronic kidney disease; CRP: C-reactive protein; CVD, cardiovascular disease; DBP, diastolic blood pressure; DM, diabetes mellitus; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; eGFRcys, estimated glomerular filtration rate based on serum cystatin C level; ESRD, end-stage renal disease; F/U, follow-up; GFR, glomerular filtration rate; GGT: gamma-glutamyl-transpeptidase; Hb, hemoglobin; HbA1c, hemoglobin A1c; Hct, hematocrit; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; HTN, hypertension; IMT, intima-media thickness; LDL-C, low-density lipoprotein cholesterol; LVH, left ventricular hypertrophy; MDRD, Modiication of Diet in Renal Disease; MRFIT, Multiple Risk Factor Intervention Trial; NS, nonsignificant; OR, odds ratio; RAAS: renin angiotensin aldosterone system; sAlb, serum albumin; SBP: systolic blood pressure; SCr, serum creatinine; SCysC, serum cystatin C; SUN, serum urea nitrogen; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; UACR, urine albumin-creatinine ratio; USRDS, United States Renal Data System; WBC, white blood cell
Several epidemiological studies have examined whether higher serum uric acid levels predict an increased risk of CKD progression. Hsu et al. evaluated this in a cohort of 177,570 participants and found that increased serum uric acid was associated with an increased risk of ESRD over a 25 year follow-up period, independently of age, race, sex, body mass index, educational level, blood pressure, diabetes status, serum creatinine, hemoglobin, and proteinuria47. This association between increased serum uric acid and CKD progression has been supported further by some other studies 45–46 but not all 50–51. For example, an analysis of 840 individuals with stage 3–4 CKD participating in the Modification of Diet in Renal Disease (MDRD) Study did not find uric acid levels to be an independent risk factor for progression to chronic kidney failure despite a 10-year follow-up 51. A potential explanation for these conflicted results may lie in the fact that uric acid clearance is impaired in CKD 52 and as such serum uric acid is increased even early on in kidney disease 53. The MDRD Study adjusted for measured GFR, and perhaps that adjustment may account for its negative findings. In other words, it is possible that uric acid is a sensitive indicator of compromised kidney function and that adjustment for accurate measurement of GFR would offset a potential association.
Another issue that is commonly raised when reviewing the results of prospective observational studies on uric acid is that xanthine oxidase, the enzyme that produces uric acid, also produces reactive oxidative species. As such, serum uric acid might be simply a marker of oxidative stress rather than a mediator of disease per se. Unfortunately, such observational studies are naturally incapable of addressing these concerns. To the skeptics, the significant association between uric acid levels and CKD may be explained by such confounders, i.e., the renal clearance of uric acid and the xanthine oxidase system. The believers, however, may contend that if high uric acid decreases kidney function early on in the disease process, thus increasing the risk of CKD progression, then adjusting for baseline GFR would be expected to attenuate any relation between serum uric acid and chronic kidney failure and that does not exclude a contributing role for hyperuricemia in CKD.
Uric acid and xanthine oxidase
As indicated above, a major challenge in understanding the potential role of uric acid in CKD is that it is a product of xanthine oxidase in conjunction with reactive oxidative species. Xanthine oxidoreductase (XOR) exists in two forms, xanthine dehydrogenase (XDH) and xanthine oxidase (XO). When grouped together, the dehydrogenase and oxidase forms catalyze the final step in purine metabolism by converting hypoxanthine to xanthine and xanthine to urate/uric acid. XOR plays an important role in survival and development, as XOR knockout mice die within the first month of their birth secondary to severe renal dysplasia 54. This seems counterintuitive, but these findings can be explained by impaired COX-2 expression 54 and the accumulation of triglyceride-rich substances, xanthine, and hypoxanthine in the renal tubules, which lead to interstitial fibrosis during early development 55. Xanthine, albeit rarely, can crystallize in supersaturated urine leading to stone formation 56. In contrast, in older rats, an increase in xanthine oxidase activity may contribute to tubulointerstitial injury in experimental hyperlipidemia 57. While these detrimental effects of xanthine oxidase may be merely due to increased oxidative stress, preliminary evidence suggests that uric acid is a mediator of xanthine oxidase effects. For example, the renal dysplasia phenotype found in XOR knockout animals is identical to that seen in COX-2 deficiency. Considering that uric acid ingestion has been shown to stimulate COX-2 expression both in vivo and in vitro 54, it is logical to conclude that uric acid may be at least a partial mediator of XOR effects. Unfortunately, little is known about XOR activity in models of kidney disease; we identified only one study in our review where xanthine oxidase activity is reportedly reduced in the 5/6th nephrectomy model 58. Although the findings of this experimental model suggest that the increase in uric acid seen in CKD may be related to reduced renal clearance as opposed to increased XOR activity, virtually no studies have examined XOR activity in human CKD.
Treatment of hyperuricemia in CKD
In general, xanthine oxidase inhibitors such as allopurinol or febuxostat are the preferred agents to lower uric acid due to their effectiveness in both “over-producers” and “under-secretors” of uric acid. Allopurinol is metabolized by xanthine oxidase to oxypurinol, and both substrates act to inhibit xanthine oxidase 59. Patients with CKD may be at increased risk of toxicity with allopurinol (e.g., rash, gastrointestinal intolerance, leukopenia, and severe hypersensitivity reaction), as oxypurinol is cleared by the kidney60. In addition, some investigators have suggested that insufficient dosing of allopurinol in CKD patients with gout leads to undertreatment61. Thus, it is widely recommended to start with low dosages of allopurinol in CKD patients and to slowly titrate it to an effective dose. Febuxostat, a non-purine selective xanthine oxidase inhibitor, has been shown to be safe and effective for lowering serum uric acid levels 62 and represents a pharmacological alternative to allopurinol in hyperuricemic patients who are unable to tolerate allopurinol Other agents that can be used to lower uric acid levels include uricosuric agents such as probenecid and benzbromarone (the latter is unavailable in the US) in addition to losartan and fenofibrates (both drugs exert mild uricosuric effects).
The use of an uricosuric agent such as probenecid is generally lauded as a better approach than xanthine oxidase inhibition to evaluate the potential role of uric acid in disease states, given that such treatment would eliminate the confounding effect of xanthine oxidase inhibition. However, this may differ in CKD. Uricosuric agents would obviously increase urinary uric acid excretion, and this increase of uric acid on the luminal side of the nephron may be associated with the same deleterious effects. In addition, increased urinary uric acid excretion may increase the risk of crystallization, thus leading to further inflammation and a higher risk of kidney stones.
In any event, the potential benefit of lowering uric acid on CKD progression has been evaluated in only a handful of studies. In a small randomized trial by Siu et al. 63, 54 hyperuricemic patients with mild-to-moderate CKD were assigned to allopurinol (100–300 mg/day with the goal of normalizing serum uric acid levels) versus no therapy (control) and followed for 12 months. At the end of follow-up period, a significantly larger number of participants in the control group (16% vs. 46%; P =0.015) achieved the combined endpoint of a serum creatinine increase of 40% or more, dialysis, or death.. More recently, a larger study conducted by Goicoechea et al. included 113 hyperuricemic patients with CKD randomized either to allopurinol (100 mg/day) or to a control group (no therapy). 64 At the end of the 2 year follow-up period, estimated GFR decreased by 3.3 ± 1.2 ml/min / 1.73 m2 in the control group compared to the allopurinol group, where estimated GFR increased by 1.3 ± 1.3 ml/min / 1.73 m2 (P =0.018). The major limitations of both of these interventional studies are the relatively small number of patients and the absence of a placebo arm. One recent open label randomized controlled trial conducted by Shi Y et al evaluated allopurinol treatment in 40 patients with IgA nephropathy 65. After 6 months of treatment, allopurinol did not significantly alter kidney disease progression or proteinuria, although it did significantly improve blood pressure in these patients. In addition to the open label design and the small number of participants, the short duration of follow-up is a major limitation of this study. The only double blinded randomized placebo controlled trial that examined the effect of lowering uric acid on diabetic nephropathy 66 included 40 patients with type 2 diabetes followed the participants for a period of 4 months, and evaluated proteinuria as an outcome. The small number of participants, short duration of follow-up, and lack of assessment of kidney function are notable limitations of this study, although it did show a significant reduction in proteinuria with allopurinol treatment, which appeared to be complementary to RAAS blockade.
The potential nephro-protective effect of lowering uric acid in addition to traditional therapies of CKD is further supported by the findings of a post-hoc analysis of the RENAAL (Reduction of Endpoints in Non-Insulin-Dependent Diabetes Mellitus With the Angiotensin II Antagonist Losartan) Trial 67. In this study, the risk of renal events was decreased by 6% for every 0.5-mg/dL decrement in serum uric acid during the first 6 months of treatment with losartan. The lower uric acid levels in the losartan group are most likely due to the uricosuric effect of this drug. While such studies suggest that lowering uric acid may slow CKD progression, considering their many limitations, a role for uric acid lowering therapies in CKD cannot be advocated based on their results. Rather, the results of such studies imply that properly designed, randomized, placebo-controlled studies need to be conducted to assess objectively whether uric acid lowering therapies would benefit patients with CKD.
In addition to the aforementioned trials, some recent studies suggest that treating hyperuricemia may prevent or delay the onset of CKD. A randomized double blinded study by Feig et al. 68 showed that treating hyperuricemia in adolescents with newly diagnosed hypertension was effective at lowering blood pressure. Similar to the studies mentioned above, allopurinol was used to lower serum uric acid levels and resulted in significant improvement in systolic and diastolic blood pressure when compared to placebo. It remains uncertain if this is an effective anti-hypertensive approach as opposed to the current standard of care, but the results of this small clinical trial raise the possibility that lowering uric acid early on may prevent the onset of kidney disease. In attempt to evaluate if lowering uric acid would prevent kidney disease onset, Kanbay et al. 69 conducted a small case controlled study. Here, 59 hyperuricemic individuals with estimated GFR ≥60 ml/min / 1.73 m2 were treated with 300-mg allopurinol daily over a 3 months period and were noted to have improvements in systolic and diastolic blood pressure as well as a significant increase in estimated GFR (proteinuria was unchanged in this trial). Results of such a study, however, need to be confirmed in larger placebo controlled trials. Whether pharmacological lowering of uric acid is more effective than dietary and life-style modifications prior to CKD onset will also need to be assessed.
Hyperuricemia in Kidney Transplantation
Hyperuricemia is common in patients post kidney transplantation 70. While increased uric acid levels in this setting may represent reduced graft function, hyperuricemia has been reported even in patients with intact graft function 71. Several factors contribute to hyperuricemia post transplantion, such as cyclosporine therapy, use of diuretics, and the high prevalence of metabolic syndrome and diabetes in kidney transplant recipients 72. Although hyperuricemia contributes to cyclosporine- associated nephrotoxicity in animal models 73, we are unaware of any studies that have evaluated the role of uric acid—lowering therapies in kidney transplant patients, and observational studies evaluating uric acid as a predictor of graft dysfunction have shown conflicted results 71, 74–80. These studies are summarized in Table 3.
TABLE 3.
Observational studies evaluating uric acid as a predictor of graft dysfunction in patients with a kidney transplant
| Investigators | N | F/ U (y) |
Independent Variable | Study Outcome | Adjustments Considered | Findings |
|---|---|---|---|---|---|---|
| Armstrong et al.76 |
90 | 7 | Hyperuricemia (> 7 mg/dl for men, and > 6 mg/dL for women) at least 6 mo posttransplantation |
eGFR, and change eGFRa |
Age, sex, race, weight, BMI, time since transplantation, history of CVD, dyslipidemia, DM, smoking status, baseline eGFR and proteinuria, calcium, phosphate, albumin, Hb, homocysteine, CRP, medications |
Hyperuricemia independently predictive of eGFR (β estimate, − 22.2; 95% CI, −41 to −3.2; P = 0.02); hyperuricemia not associated with change in eGFR |
| Akalin et al. 75 | 30 7 |
4.3 | Hyperuricemia 6 mo posttransplantation ( ≥ 7 mg/dl for men, and ≥ 6.5 mg/dL for women) |
Composite of death, graft loss, new CV event, or biopsy proven CAN |
Age, race, sex, eGFR < 50, cyclosporine use, cadaveric kidney |
Hyperuricemia associated with more events (P <0.001 for K-M curve); among group with eGFR < 50, hyperuricemia associated with 45% event rate vs 21% in normouricemia (p=0.038) |
| Meier- Kriesche et al. 74 |
85 2 |
3 | Serum uric acid levels 1 month posttransplantation (per 1 mg/dL) and uric acid tertiles |
eGFRb | Donor type, immunosuppressive treatment arm, ethnicity, baseline eGFR |
Uric acid and eGFR were collinear; Uric acid associated with reduced eGFR at 3 y (p=0.005), but this became not significant after adjusting for baseline eGFR |
| Akgul et al. 77 | 13 3 |
3 | Hyperuricemia 1 month posttransplantation (> 7 mg/dl for men, and > 6 mg/dL for women) |
CAN (biopsy proven) |
Age, donor source, no. of HLA mismatches, duration of dialysis, HTN, acute rejection, and serum cholesterol |
Uric acid not associated with CAN (p> 0.05) |
| Haririan et al. 78 |
21 2 |
6 | Serum uric acid level within the first 6 mo posttransplantation (per 1 mg/dL) and hyperuricemia (> 7 mg/dl for men, and > 6.5 mg/dL for women) |
Graft and patient survival, graft function |
Age, sex, race, re- transplantation, BMI, HLA mismatch, early graft function, SCr, DM, induction agent, acute rejection |
Uric acid associated with graft loss (HR, 1.26 [95% CI 1.03–1.53] per1 mg/dL increase, p=0.026) and hyperuricemia independently predicted graft loss (HR, 1.92 [95% CI 1.1–3.4], p=0.029) |
| Kim et al.80 | 55 6 |
4 | hyperuricemia (> 7 mg/dl for men, and > 6 mg/dL for women) |
Graft dysfunction with >50% loss of kidney function (eGFRa) |
Age, sex, weight, donor type, time since transplantation, HTN, DM, immunosuppressive regimen, serum calcium, serum phosphorus |
Hyperuricemia associated with graft dysfunction: unadjusted HR = 1.31 (p<0.001), aHR=1.45 (p<0.001) |
| Kim et al. 71 | 35 6 |
5 | Mean uric acid levels obtained every 3 mo (starting 6 mo posttransplantation) modeled as a continuous variable (per 1 mg/dL) and categorical hyperuricemia (> 7 mg/dL for men and >6 mg/dL for women) |
eGFRa | Age, sex, weight, donor type, time since transplantation, HTN, DM, immunosuppressive regimen, serum calcium, serum phosphorus |
Uric acid did not predict eGFR |
| Boratynska et al.79 |
98 | 2.5 | Hyperuricemia (> 7 mg/dL for men and >6 mg/dL for women) |
eGFR | NA | eGFR significantly higher in normo- vs hyperuricemic patients at baseline, but SCr and eGFR were similar in both groups at study end |
Note: eGFR values given in mL/min/1.73 m2;
eGFR calculated using the MDRD Study equation.
eGFR calculated using creatinine clearance
Abbreviations: eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; BMI, body mass index; CVD, cardiovascular disease; HR, hazard ratio, CI, confidence interval; CAN, Chronic allograft nephropathy; NA, not applicable; K-M, Kaplan- Meier; DM, diabetes mellitus; CRP, C-reactive protein; HTN, hypertension; CV, cardiovascular; SCr, serum creatinine
Conclusions
Hyperuricemia is common in CKD. Experimental evidence suggests that uric acid itself may harm CKD patients by contributing to increased inflammation and CKD progression. While controversial, these observations are supported by many large prospective, observational studies that show increased levels of serum uric acid that predict the development and progression of CKD in various populations. Interventional studies, while sparse, suggest that lowering uric acid in hyperuricemic patients with CKD is safe and might slow CKD progression. The currently published studies are promising and suggest that there may be a role for uric acid lowering therapy in patients with CKD. It is important to note, however, that these studies are limited by the small number of participants and the lack of a placebo arm. Considering the significant limitations of the current literature, further studies are needed before we can advocate lowering uric acid in patients with CKD. In addition, the best therapeutic strategy to lower uric acid in this patient population needs to be determined.
TABLE 2.
Main interventional studies to lower serum uric acid levels in CKD
| Investigators | Study Design and Population |
Treatment | Study Endpoints |
Main findings |
|---|---|---|---|---|
| Siu et al. 63 | RCT of 54 hyperuricemic patients with mild to moderate CKD |
12 mo of either allopurinol 100–300 mg/d or no treatment |
Decreased kidney function with SCr level ≥40% of baseline, or initiation of dialysis, or death |
Nonsignificant trend toward a lower SCr level in the treatment group (P=0.08); overall, 16% (4/25) of allopurinol group reached the combined end- points, vs 46.1% (12/ 26) in control group (P=0.015) |
| Goicoechea et al. 64 |
RCT of 113 hyperuricemic patients with mild to moderate CKD |
24 mo of either allopurinol 100 mg/d or no treatment |
Progression of CKD (defined as eGFR decrease > 0.2/mo), or CV events, or hospitalizations of any cause, or death |
ΔeGFRs of −3.3 ±1.2 (control) and +1.3 ±1.3 (allopurinol group), P =0.018; compared with controls, allopurinol treatment slowed CKD progression in a Cox regression model (adjusted for age, sex, diabetes, uric acid), and reduced risk of CV events and number of hospitalizations (aHR, 0.29; 95%CI, 0.09–0.86; P=0.026) |
| Kanbay et al.87 | Case-control study of 59 hyperuricemic patients with eGFR >60 and 21 normouricemic controls; only hyperuricemic patients received allopurinola |
3 mo of allopurinol 300 mg/d |
eGFR <60 | eGFR significantly increased (from 79.2±32 to 92.9±37; P=0.008) and BP and plasma CRP decreased in the allopurinol group; no significant change in the control group |
| Talaat et al.33 | Intervention trial of allopurinol withdrawal in 50 hyperuricemic patients with CKD3–4 treated with allopurinol |
12 mo after allopurinol withdrawal |
Changes in eGFR and urinary TGF- β1 |
Significant acceleration of the rate of eGFR loss and significant increases in BP values and urinary TGF- β1 only among those who were not receiving ACEi |
| Miao et al67 | Placebo-conrolled RCT of patients treated with losartan in a post-hoc analysis of the RENAAL Trial, N=1,342 patients with type 2 diabetes and nephropathy |
Post-hoc analysis of the first 6 mo of treatment |
Progression of CKD defined as doubling of SCr or ESRD |
Losartan lowered serum uric acid by 0.16 (95% CI, 0.30–0.01) mg/dL (P=0.031) vs placebo; risk of renal events was decreased by 6% (95% CI, 10%–3%) per 0.5-mg/dL decrement in serum uric acid during the first 6 mo of treatment after adjustment for age, sex, treatment assignment (losartan or placebo), eGFR, SBP, albuminuria, serum albumin, ACEi or ARB use at baseline, changes in albuminuria and eGFR |
Note: eGFR values given in mL/min/1.73 m2;
Ie, not a placebo-controlled RCT
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; OR, odds ratio; HR, hazard ratio, CI, confidence interval, SBP: systolic blood pressure; SCr, serum creatinine; RENAAL, Reduction of Endpoints in Non-Insulin Dependent Diabetes Mellitus With the Angiotensin II Antagonist Losartan; aHR, adjusted hazard ratio; TGF, transforming growth factor; BP, blood pressure, ACEi, angiotensin-converting enzyme inhibitor; ARB, antiotensin receptor blocker; RCT, randomized clinical trial; CV, cardiovascular; CRP, C-reactive protein
Acknowledgements
Support: This review was supported by the following grants: 1K23DK088833, 1R01 DK081473-01A1, and 1R01DK078112-01A2, as well as ISN Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007 Aug;72(3):247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried LF. Higher incidence of ESRD than mortality in the AASK study. J Am Soc Nephrol. 2010 Aug;21(8):1244–1246. doi: 10.1681/ASN.2010060623. [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Stevens LA, Coresh J. Conceptual model of CKD: applications and implications. Am J Kidney Dis. 2009 Mar;53(3 Suppl 3):S4–S16. doi: 10.1053/j.ajkd.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 5.Eijkelkamp WB, Zhang Z, Remuzzi G, et al. Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. J Am Soc Nephrol. 2007 May;18(5):1540–1546. doi: 10.1681/ASN.2006050445. [DOI] [PubMed] [Google Scholar]
- 6.United States Renal Data System-2011 Atlas of CKD and ESRD. 2011
- 7.Rosenfeld L. Four Centuries of Clinical Chemistry. New York: Taylor and Francis; 1999. [Google Scholar]
- 8.McCrudden F. Uric acid: the chemistry, physiology and pathology of uric acid and the physiologically important purine bodies, with a discussion of the metabolism in gout. Paul Hoeber Medical Books; 1905. [Google Scholar]
- 9.Croftan A. Uric Acid Theories. JAMA. 1899;33:59–63. [Google Scholar]
- 10.Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Rheum. 2004 Jun 15;51(3):321–325. doi: 10.1002/art.20405. [DOI] [PubMed] [Google Scholar]
- 11.Wright AF, Rudan I, Hastie ND, Campbell H. A 'complexity' of urate transporters. Kidney Int. 2010 Sep;78(5):446–452. doi: 10.1038/ki.2010.206. [DOI] [PubMed] [Google Scholar]
- 12.Edwards NL. The role of hyperuricemia and gout in kidney and cardiovascular disease. Cleve Clin J Med. 2008 Jul;75(Suppl 5):S13–S16. doi: 10.3949/ccjm.75.suppl_5.s13. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Lario B, Macarron-Vicente J. Uric acid and evolution. Rheumatology (Oxford) 2010 Nov;49(11):2010–2015. doi: 10.1093/rheumatology/keq204. [DOI] [PubMed] [Google Scholar]
- 14.Enomoto A, Kimura H, Chairoungdua A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002 May 23;417(6887):447–452. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 15.Preitner F, Bonny O, Laverriere A, et al. Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc Natl Acad Sci U S A. 2009 Sep 8;106(36):15501–15506. doi: 10.1073/pnas.0904411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waisman J, Mwasi LM, Bluestone R, Klinenberg JR. Acute hyperuricemic nephropathy in rats. An electron microscopic study. Am J Pathol. 1975 Nov;81(2):367–378. [PMC free article] [PubMed] [Google Scholar]
- 17.Spencer HW, Yarger WE, Robinson RR. Alterations of renal function during dietary-induced hyperuricemia in the rat. Kidney Int. 1976 Jun;9(6):489–500. doi: 10.1038/ki.1976.63. [DOI] [PubMed] [Google Scholar]
- 18.Coe FL. Uric acid and calcium oxalate nephrolithiasis. Kidney Int. 1983 Sep;24(3):392–403. doi: 10.1038/ki.1983.172. [DOI] [PubMed] [Google Scholar]
- 19.Koka RM, Huang E, Lieske JC. Adhesion of uric acid crystals to the surface of renal epithelial cells. Am J Physiol Renal Physiol. 2000 Jun;278(6):F989–F998. doi: 10.1152/ajprenal.2000.278.6.F989. [DOI] [PubMed] [Google Scholar]
- 20.Umekawa T, Chegini N, Khan SR. Increased expression of monocyte chemoattractant protein-1 (MCP-1) by renal epithelial cells in culture on exposure to calcium oxalate, phosphate and uric acid crystals. Nephrol Dial Transplant. 2003 Apr;18(4):664–669. doi: 10.1093/ndt/gfg140. [DOI] [PubMed] [Google Scholar]
- 21.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001 Nov;38(5):1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Lozada LG, Tapia E, Lopez-Molina R, et al. Effects of acute and chronic L-arginine treatment in experimental hyperuricemia. American journal of physiology. 2007 Apr;292(4):F1238–F1244. doi: 10.1152/ajprenal.00164.2006. [DOI] [PubMed] [Google Scholar]
- 24.Zharikov S, Krotova K, Hu H, et al. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol. 2008 Nov;295(5):C1183–C1190. doi: 10.1152/ajpcell.00075.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gersch C, Palii SP, Kim KM, Angerhofer A, Johnson RJ, Henderson GN. Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids. 2008 Aug;27(8):967–978. doi: 10.1080/15257770802257952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazzali M, Kanellis J, Han L, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002 Jun;282(6):F991–F997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 27.Han HJ, Lim MJ, Lee YJ, Lee JH, Yang IS, Taub M. Uric acid inhibits renal proximal tubule cell proliferation via at least two signaling pathways involving PKC, MAPK, cPLA2, and NF-kappaB. Am J Physiol Renal Physiol. 2007 Jan;292(1):F373–F381. doi: 10.1152/ajprenal.00104.2006. [DOI] [PubMed] [Google Scholar]
- 28.Netea MG, Kullberg BJ, Blok WL, Netea RT, van der Meer JW. The role of hyperuricemia in the increased cytokine production after lipopolysaccharide challenge in neutropenic mice. Blood. 1997 Jan 15;89(2):577–582. [PubMed] [Google Scholar]
- 29.Roncal CA, Mu W, Croker B, et al. Effect of elevated serum uric acid on cisplatin-induced acute renal failure. American journal of physiology. 2007 Jan;292(1):F116–F122. doi: 10.1152/ajprenal.00160.2006. [DOI] [PubMed] [Google Scholar]
- 30.Kang DH, Nakagawa T, Feng L, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002 Dec;13(12):2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez-Lozada LG, Tapia E, Soto V, et al. Effect of febuxostat on the progression of renal disease in 5/6 nephrectomy rats with and without hyperuricemia. Nephron Physiol. 2008;108(4):p69–p78. doi: 10.1159/000127837. [DOI] [PubMed] [Google Scholar]
- 32.Kosugi T, Nakayama T, Heinig M, et al. The Effect of Lowering Uric Acid on Renal Disease in the Type 2 Diabetic db/db Mice. Am J Physiol Renal Physiol. 2009 Aug;297(2):F481–F488. doi: 10.1152/ajprenal.00092.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talaat KM, el-Sheikh AR. The effect of mild hyperuricemia on urinary transforming growth factor beta and the progression of chronic kidney disease. Am J Nephrol. 2007;27(5):435–440. doi: 10.1159/000105142. [DOI] [PubMed] [Google Scholar]
- 34.Domrongkitchaiporn S, Sritara P, Kitiyakara C, et al. Risk factors for development of decreased kidney function in a southeast Asian population: a 12-year cohort study. J Am Soc Nephrol. 2005 Mar;16(3):791–799. doi: 10.1681/ASN.2004030208. [DOI] [PubMed] [Google Scholar]
- 35.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008 Jun;19(6):1204–1211. doi: 10.1681/ASN.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol. 2008 Dec;19(12):2407–2413. doi: 10.1681/ASN.2008010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonoda H, Takase H, Dohi Y, Kimura G. Uric acid levels predict future development of chronic kidney disease. Am J Nephrol. 2011;33(4):352–357. doi: 10.1159/000326848. [DOI] [PubMed] [Google Scholar]
- 38.Wang S, Shu Z, Tao Q, Yu C, Zhan S, Li L. Uric acid and incident chronic kidney disease in a large health check-up population in Taiwan. Nephrology (Carlton) 2011 Nov;16(8):767–776. doi: 10.1111/j.1440-1797.2011.01513.x. [DOI] [PubMed] [Google Scholar]
- 39.Mok Y, Lee SJ, Kim MS, Cui W, Moon YM, Jee SH. Serum uric acid and chronic kidney disease: the Severance cohort study. Nephrol Dial Transplant. 2012 May;27(5):1831–1835. doi: 10.1093/ndt/gfr530. [DOI] [PubMed] [Google Scholar]
- 40.Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving HH. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes - an inception cohort study. Diabetes. 2009 Jul;58(7):1668–1671. doi: 10.2337/db09-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jalal DI, Rivard CJ, Johnson RJ, et al. Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: findings from the Coronary Artery Calcification in Type 1 Diabetes study. Nephrol Dial Transplant. 2010 Jun;25(6):1865–1869. doi: 10.1093/ndt/gfp740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ficociello LH, Rosolowsky ET, Niewczas MA, et al. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes Care. 2010 Jun;33(6):1337–1343. doi: 10.2337/dc10-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zoppini G, Targher G, Chonchol M, et al. Serum uric Acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabetes Care. 2012 Jan;35(1):99–104. doi: 10.2337/dc11-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yen CJ, Chiang CK, Ho LC, et al. Hyperuricemia associated with rapid renal function decline in elderly Taiwanese subjects. J Formos Med Assoc. 2009 Dec;108(12):921–928. doi: 10.1016/S0929-6646(10)60004-6. [DOI] [PubMed] [Google Scholar]
- 45.Chonchol M, Shlipak MG, Katz R, et al. Relationship of uric acid with progression of kidney disease. Am J Kidney Dis. 2007 Aug;50(2):239–247. doi: 10.1053/j.ajkd.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 46.Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004 Oct;44(4):642–650. [PubMed] [Google Scholar]
- 47.Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009 Feb 23;169(4):342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bellomo G, Venanzi S, Verdura C, Saronio P, Esposito A, Timio M. Association of uric acid with change in kidney function in healthy normotensive individuals. Am J Kidney Dis. 2010 Aug;56(2):264–272. doi: 10.1053/j.ajkd.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 49.Ben-Dov IZ, Kark JD. Serum uric acid is a GFR-independent long-term predictor of acute and chronic renal insufficiency: the Jerusalem Lipid Research Clinic cohort study. Nephrol Dial Transplant. 2011 Aug;26(8):2558–2566. doi: 10.1093/ndt/gfq740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sturm G, Kollerits B, Neyer U, Ritz E, Kronenberg F. Uric acid as a risk factor for progression of non-diabetic chronic kidney disease? The Mild to Moderate Kidney Disease (MMKD) Study. Exp Gerontol. 2008 Apr;43(4):347–352. doi: 10.1016/j.exger.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Madero M, Sarnak MJ, Wang X, et al. Uric acid and long-term outcomes in CKD. Am J Kidney Dis. 2009 May;53(5):796–803. doi: 10.1053/j.ajkd.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson RJ, Kang DH, Feig D, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003 Jun;41(6):1183–1190. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 53.Rosolowsky ET, Ficociello LH, Maselli NJ, et al. High-normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2008 May;3(3):706–713. doi: 10.2215/CJN.04271007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohtsubo T, Rovira II, Starost MF, Liu C, Finkel T. Xanthine oxidoreductase is an endogenous regulator of cyclooxygenase-2. Circ Res. 2004 Nov 26;95(11):1118–1124. doi: 10.1161/01.RES.0000149571.96304.36. [DOI] [PubMed] [Google Scholar]
- 55.Ohtsubo T, Matsumura K, Sakagami K, et al. Xanthine oxidoreductase depletion induces renal interstitial fibrosis through aberrant lipid and purine accumulation in renal tubules. Hypertension. 2009 Oct;54(4):868–876. doi: 10.1161/HYPERTENSIONAHA.109.135152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pais VM, Jr, Lowe G, Lallas CD, Preminger GM, Assimos DG. Xanthine urolithiasis. Urology. 2006 May;67(5):1084e9–1084e11. doi: 10.1016/j.urology.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 57.Gwinner W, Scheuer H, Haller H, Brandes RP, Groene HJ. Pivotal role of xanthine oxidase in the initiation of tubulointerstitial renal injury in rats with hyperlipidemia. Kidney Int. 2006 Feb;69(3):481–487. doi: 10.1038/sj.ki.5000121. [DOI] [PubMed] [Google Scholar]
- 58.Vaziri ND, Freel RW, Hatch M. Effect of chronic experimental renal insufficiency on urate metabolism. J Am Soc Nephrol. 1995 Oct;6(4):1313–1317. doi: 10.1681/ASN.V641313. [DOI] [PubMed] [Google Scholar]
- 59.Gaffo AL, Saag KG. Management of hyperuricemia and gout in CKD. Am J Kidney Dis. 2008 Nov;52(5):994–1009. doi: 10.1053/j.ajkd.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 60.Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984 Jan;76(1):47–56. doi: 10.1016/0002-9343(84)90743-5. [DOI] [PubMed] [Google Scholar]
- 61.Dalbeth N, Stamp L. Allopurinol dosing in renal impairment: walking the tightrope between adequate urate lowering and adverse events. Semin Dial. 2007 Sep-Oct;20(5):391–395. doi: 10.1111/j.1525-139X.2007.00270.x. [DOI] [PubMed] [Google Scholar]
- 62.Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12(2):R63. doi: 10.1186/ar2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006 Jan;47(1):51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 64.Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010 Aug;5(8):1388–1393. doi: 10.2215/CJN.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi Y, Chen W, Jalal D, et al. Clinical Outcome of Hyperuricemia in IgA Nephropathy: A Retrospective Cohort Study and Randomized Controlled Trial. Kidney Blood Press Res. 2011 Nov 23;35(3):153–160. doi: 10.1159/000331453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Momeni A, Shahidi S, Seirafian S, Taheri S, Kheiri S. Effect of allopurinol in decreasing proteinuria in type 2 diabetic patients. Iran J Kidney Dis. 2010 Apr;4(2):128–132. [PubMed] [Google Scholar]
- 67.Miao Y, Ottenbros SA, Laverman GD, et al. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension. 2011 Jul;58(1):2–7. doi: 10.1161/HYPERTENSIONAHA.111.171488. [DOI] [PubMed] [Google Scholar]
- 68.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. Jama. 2008 Aug 27;300(8):924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kanbay M, Huddam B, Azak A, et al. A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011 Aug;6(8):1887–1894. doi: 10.2215/CJN.11451210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baroletti S, Bencivenga GA, Gabardi S. Treating gout in kidney transplant recipients. Prog Transplant. 2004 Jun;14(2):143–147. doi: 10.1177/152692480401400208. [DOI] [PubMed] [Google Scholar]
- 71.Kim KM, Kim SS, Han DJ, Yang WS, Park JS, Park SK. Hyperuricemia in kidney transplant recipients with intact graft function. Transplant Proc. 2010 Nov;42(9):3562–3567. doi: 10.1016/j.transproceed.2010.07.104. [DOI] [PubMed] [Google Scholar]
- 72.Mazali FC, Mazzali M. Uric acid and transplantation. Semin Nephrol. 2011 Sep;31(5):466–471. doi: 10.1016/j.semnephrol.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 73.Mazali FC, Johnson RJ, Mazzali M. Use of uric acid-lowering agents limits experimental cyclosporine nephropathy. Nephron Exp Nephrol. 2012;120(1):e12–e19. doi: 10.1159/000330274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meier-Kriesche HU, Schold JD, Vanrenterghem Y, Halloran PF, Ekberg H. Uric acid levels have no significant effect on renal function in adult renal transplant recipients: evidence from the symphony study. Clin J Am Soc Nephrol. 2009 Oct;4(10):1655–1660. doi: 10.2215/CJN.02700409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akalin E, Ganeshan SV, Winston J, Muntner P. Hyperuricemia is associated with the development of the composite outcomes of new cardiovascular events and chronic allograft nephropathy. Transplantation. 2008 Sep 15;86(5):652–658. doi: 10.1097/TP.0b013e3181814f5b. [DOI] [PubMed] [Google Scholar]
- 76.Armstrong KA, Johnson DW, Campbell SB, Isbel NM, Hawley CM. Does uric acid have a pathogenetic role in graft dysfunction and hypertension in renal transplant recipients? Transplantation. 2005 Dec 15;80(11):1565–1571. doi: 10.1097/01.tp.0000183895.88572.13. [DOI] [PubMed] [Google Scholar]
- 77.Akgul A, Bilgic A, Ibis A, Ozdemir FN, Arat Z, Haberal M. Is uric acid a predictive factor for graft dysfunction in renal transplant recipients? Transplant Proc. 2007 May;39(4):1023–1026. doi: 10.1016/j.transproceed.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 78.Haririan A, Nogueira JM, Zandi-Nejad K, et al. The independent association between serum uric acid and graft outcomes after kidney transplantation. Transplantation. 2010 Mar 15;89(5):573–579. doi: 10.1097/TP.0b013e3181c73c18. [DOI] [PubMed] [Google Scholar]
- 79.Boratynska M, Karbowska A, Klinger M. The effect of hyperuricemia on endothelial biomarkers and renal function in kidney allograft recipients. Transplant Proc. 2010 Dec;42(10):4074–4077. doi: 10.1016/j.transproceed.2010.09.069. [DOI] [PubMed] [Google Scholar]
- 80.Kim KM, Kim SS, Yun S, et al. Uric acid contributes to glomerular filtration rate deterioration in renal transplantation. Nephron Clin Pract. 2011;118(2):c136–c142. doi: 10.1159/000320616. [DOI] [PubMed] [Google Scholar]
- 81.Iseki K, Oshiro S, Tozawa M, Iseki C, Ikemiya Y, Takishita S. Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens Res. 2001 Nov;24(6):691–697. doi: 10.1291/hypres.24.691. [DOI] [PubMed] [Google Scholar]
- 82.Kuo CF, Luo SF, See LC, et al. Hyperuricaemia and accelerated reduction in renal function. Scand J Rheumatol. 2011 Mar;40(2):116–121. doi: 10.3109/03009742.2010.507218. [DOI] [PubMed] [Google Scholar]
- 83.Altemtam N, Russell J, El Nahas M. A study of the natural history of diabetic kidney disease (DKD) Nephrol Dial Transplant. 2012 May;27(5):1847–1854. doi: 10.1093/ndt/gfr561. [DOI] [PubMed] [Google Scholar]
- 84.Ishani A, Grandits GA, Grimm RH, et al. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol. 2006 May;17(5):1444–1452. doi: 10.1681/ASN.2005091012. [DOI] [PubMed] [Google Scholar]
- 85.Syrjanen J, Mustonen J, Pasternack A. Hypertriglyceridaemia and hyperuricaemia are risk factors for progression of IgA nephropathy. Nephrol Dial Transplant. 2000 Jan;15(1):34–42. doi: 10.1093/ndt/15.1.34. [DOI] [PubMed] [Google Scholar]
- 86.Ohno I, Hosoya T, Gomi H, Ichida K, Okabe H, Hikita M. Serum uric acid and renal prognosis in patients with IgA nephropathy. Nephron. 2001 Apr;87(4):333–339. doi: 10.1159/000045939. [DOI] [PubMed] [Google Scholar]
- 87.Kanbay M, Ozkara A, Selcoki Y, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39(4):1227–1233. doi: 10.1007/s11255-007-9253-3. [DOI] [PubMed] [Google Scholar]