SUMMARY
Cisplatin, a platinum-derived chemotherapeutic agent, produces mechanical and cold allodynia reminiscent of chemotherapy-induced neuropathy in humans. The endocannabinoid system represents a novel target for analgesic drug development. The endocannabinoid consists of endocannabinoids (e.g. anandamide (AEA) and 2-arachidonoylglycerol (2-AG)), cannabinoid receptors (e.g. CB1 and CB2) and the enzymes controlling endocannabinoid synthesis and degradation. AEA is hydrolyzed by fatty-acid amide hydrolase (FAAH) whereas 2-AG is hydrolyzed primarily by monoacylglycerol lipase (MGL). We compared effects of brain permeant (URB597) and impermeant (URB937) inhibitors of FAAH with an irreversible inhibitor of MGL (JZL184) on cisplatin-evoked behavioral hypersensitivities. Endocannabinoid modulators were compared with agents used clinically to treat neuropathy (i.e. the opioid analgesic morphine, the anticonvulsant gabapentin and the tricyclic antidepressant amitriptyline). Cisplatin produced robust mechanical and cold allodynia but did not alter responsiveness to heat. After neuropathy was fully established, groups received acute intraperitoneal (i.p.) injections of vehicle, amitriptyline (30 mg/kg), gabapentin (100 mg/kg), morphine (6 mg/kg), URB597 (0.1 or 1 mg/kg), URB937 (0.1 or 1 mg/kg) or JZL184 (1, 3 or 8 mg/kg). Pharmacological specificity was assessed by coadministering each endocannabinoid modulator with either a CB1 (AM251 3 mg/kg), CB2 (AM630 3 mg/kg), TRPV1 (AMG9810 3 mg/kg) or TRPA1 (HC030031 8 mg/kg) antagonist. Effects of cisplatin on endocannabinoid levels and transcription of receptors (CB1, CB2, TRPV1, TRPA1) and enzymes (FAAH, MGL) linked to the endocannabinoid system were also assessed. URB597, URB937, JZL184 and morphine reversed cisplatin-evoked mechanical and cold allodynia to pre-cisplatin levels. By contrast, gabapentin only partially reversed the neuropathy while amitriptyline, administered acutely, was ineffective. CB1 or CB2 antagonist completely blocked the anti-allodynic effects of both FAAH (URB597, URB937) and MGL (JZL184) inhibitors to mechanical and cold stimulation, while TRPV1 antagonist AMG9810 blocked only the anti-allodynic efficacy of both FAAH inhibitors, but not the MGL inhibitor,. By contrast, the TRPA1 antagonist HC30031 did not attenuate anti-allodynic efficacy of any endocannabinoid modulator. When the levels of endocannabinoids were examined, cisplatin increased both anandamide (AEA) and 2-arachidonoylglycerol (2-AG) levels in the lumbar spinal cord and decreased 2-AG levels (but not AEA) in dorsal hind paw skin. RT-PCR showed that mRNA for FAAH, but not other markers, was upregulated by cisplatin treatment in dorsal root ganglia. The present studies demonstrate that cisplatin alters endocannabinoid tone and that inhibition of endocannabinoid hydrolysis alleviates chemotherapy-induced mechanical and cold allodynia. The anti-allodynic effects of FAAH and MGL inhibitors are mediated by CB1 and CB2 cannabinoid receptors, whereas TRPV1, but not TRPA1, -dependent mechanisms contribute to the anti-allodynic efficacy of FAAH (but not MGL) inhibitors. Strikingly, endocannabinoid modulators potently suppressed cisplatin-evoked allodynia with a rapid onset and showed efficacy that equaled or exceeded that of major classes of anti-neuropathic pain medications used clinically. Thus, inhibition of endocannabinoid hydrolysis, via FAAH or MGL inhibitors, represents an efficacious pharmacological approach for suppressing chemotherapy-induced neuropathic pain.
Keywords: anandamide, 2-arachidonoyl glycerol, endocannabinoid, neuropathic pain, cold allodynia and hyperalgesia
1. Introduction
Chemotherapeutic agents used in the treatment of cancer are associated with major toxicities including painful neuropathy, renal toxicity and bone marrow suppression [1,2]. Of these toxicities, chemotherapy-evoked neuropathic pain is dose limiting and represents a leading cause of discontinuation of chemotherapy, resulting in suboptimal effects on cancer cell destruction [3–5]. Cisplatin, a platinum-derived chemotherapeutic agent, is widely used to treat breast, ovarian, lung, kidney, liver, thyroid, lymphoma and other cancers [6,7]. Cisplatin produces painful neuropathy through mechanisms that remain poorly understood [8–11].
The development of animal models of chemotherapy-induced toxicities has advanced our understanding about the mechanisms underlying peripheral neuropathy [12]. In preclinical studies, morphine, gabapentin and antidepressants alleviate signs attributed to the development of the neuropathy [13–18]. These observations are consistent with the results of clinical studies suggesting that chemotherapy-evoked neuropathies can be alleviated by the use of morphine, gabapentin and tricyclic antidepressants [4,19–22]. Pharmacodynamic and pharmacokinetic properties of these compounds vary and may affect their time course of action and therapeutic efficacy in vivo [23–25].
In the last decade, the endocannabinoid system has emerged as a target for novel pharmacotherapies aimed at ameliorating neuropathic pain [26,27]. Endocannabinoids are endogenous lipid-signaling molecules that mimic the pharmacological actions of the principal psychoactive component of marijuana, Δ9-tetrahydrocannabinol (Δ9-THC) [28]. Anandamide (AEA) [29] and 2-arachidonoyl glycerol (2-AG) [30,31] are the two best-studied endocannabinoids identified to date. Endocannabinoids possess cannabimimetic properties because they bind and activate cannabinoid CB1 [32,33] and/or CB2 [34] receptor subtypes. AEA is mainly hydrolyzed by the enzyme fatty-acid amide hydrolase (FAAH) [35] whereas 2-AG is mainly, although not exclusively, hydrolyzed by the enzyme monoacylglycerol lipase (MGL) [36–39].
A small number of preclinical studies have recently demonstrated that cannabinoids attenuate chemotherapy-induced neuropathic pain. Indeed, direct agonists such as WIN55,212-2, a mixed CB1 and CB2 agonist, attenuates mechanical allodynia in models of paclitaxel [40], vincristine [41] and cisplatin [42]-induced neuropathy. Moreover, CB2 agonists ((R,S)-AM1241, (R)-AM1241, AM1714, MDA7 and MDA19) also alleviate mechanical allodynia in paclitaxel [43–45] and vincristine-induced neuropathy [41].
An alternative approach to the use of direct cannabinoid agonists is to increase endocannabinoid accumulation indirectly by inhibiting endocannabinoid hydrolysis. This approach aims to harness the therapeutic potential of the endocannabinoid signaling system [46–50], while producing a more limited spectrum of unwanted side-effects compared to direct cannabinoid agonists (for review see [26]). This strategy also offers the potential to improve our current knowledge of the functional roles of endogenous AEA and 2-AG in modulating pain under neuropathological conditions. The beneficial impact of modulating the endocannabinoid system in different neuropathic pain models has recently been reviewed [26]. Indeed, FAAH (URB937, URB597, OL-135, N-Arachidonoyl 5-HT (AA-5-HT)) and MGL (JZL184, URB602) inhibitors alleviate surgically-induced neuropathic pain in animal models [46–48,51–53]. However, efficacy of blocking endocannabinoid hydrolysis through inhibition of FAAH and MGL in models of chemotherapy-induced neuropathy remains relatively uncharacterized [see 54].
The present study was designed to evaluate the impact of inhibition of FAAH and MGL on chemotherapy-induced neuropathy in cisplatin-treated rats. First, we characterized the development of behavioural sensitization to mechanical, cold and heat stimulation following once weekly treatments with cisplatin for three weeks. Second, we used pharmacological inhibitors of FAAH (URB597 (0.1 and 1 mg/kg), URB937 (0.1 and 1 mg/kg)) and MGL (JZL184 (1, 3 and 8 mg/kg)) to evaluate their efficacy in suppressing distinct modalities of cisplatin-induced neuropathic pain in rats. We compared the efficacy of brain permeant (URB597) and impermeant (URB937) inhibitors of the anandamide hydrolyzing enzyme FAAH in suppressing cisplatin-induced mechanical and cold allodynia under identical conditions. Third, we examined the receptor mechanism by which FAAH (URB597, URB937) and MGL (JZL184) inhibitors suppress mechanical and cold allodynia using selective antagonists for CB1 (AM251), CB2 (AM630), TRPV1 (AMG9810) and TRPA1 (HC030031) receptors. Fourth, we evaluated the impact of cisplatin (versus saline) treatment on endocannabinoid levels in both the lumbar spinal cord and hind paw skin. Fifth, we evaluated the impact of cisplatin treatment on transcription of enzymes catalyzing endocannabinoid hydrolysis (FAAH, MGL) and receptors (CB1, CB2, TRPV1, TRPA1). These studies further validate FAAH (URB597, URB937) and MGL (JZL184) inhibitors as important pharmacological tools with high therapeutic potential as anti-allodynic agents. Our studies suggest that pharmacological inhibition of AEA and 2-AG hydrolysis suppresses established mechanical and cold allodynia in cisplatin-treated rats following acute administration with efficacy equaling or exceeding that of anti-neuropathic pain medications employed clinically. Moreover, cisplatin treatment directly modulates the endocannabinoid system. These studies further validate the therapeutic potential of modulating the endocannabinoid system to suppress chemotherapy-induced neuropathy. Our studies also suggest that endocannabinoid modulators exhibit high potency, rapid onset and strong efficacy in suppressing chemotherapy-induced neuropathy in comparison to reference analgesics employed clinically including an opioid (morphine), anti-convulsant (gabapentin) and tricyclic anti-depressant (amitriptyline).
2. Methods
2.1. Subjects
Two hundred and forty-eight adult male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) weighing 260–325 g, at the beginning of the testing, were used in these experiments. Animals were single housed in standard plastic cages with sawdust bedding in a climate-controlled room, under a 12h light/dark cycle. The rats received free access to standard rodent chow and water. All experimental research was carried out in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and protocols approved by the Bloomington Indiana University Institutional Animal Care and Use Committee and all procedures conformed to the guidelines for the treatment of animals established by the International Association for the Study of Pain [55].
2.2. Drugs
URB597 [(3′-(aminocarbonyl)[1,1′-biphenyl]-3-yl)-cyclohexylcarbamate] and JZL184 [4-nitrophenyl-4-(dibenzo[d][1,3]dioxol-5-yl(hydroxyl)methyl)piperidine-1-carboxylate] were kindly provided by the National Institute on Drug Abuse Chemical Synthesis and Drug Supply Program (Bethesda, MD, USA). URB937 [3′-carbamoyl-6-hydroxy-[1,1′-biphenyl]-3-yl cyclohexylcarbamate], AM251 [1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3carboxamide] and AM630 [[6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl)-methanone] were purchased from Cayman Chemical (Ann Arbor, MI, USA). Amitriptyline hydrochloride [3-(10,11-dihydro-5H-dibenzo[a,d]cycloheptene-5-ylidene)-N,N-dimethylpropan-1-amine], gabapentin [1-(aminomethyl)-cyclohexaneacetic acid], morphine sulfate [(5α,6α)-7,8-didehydro-4,5-epoxy-17-methylmorphinan-3,6-diol], AMG9810 (TRPV1 antagonist) [1,2,3,6-Tetrahydro-1,3-dimethyl-N-[4-(1-methylethyl)phenyl]-2,6-dioxo-7H-purine-7-Acetamide] and HC030031 (TRPA1 antagonist) [2E-N-(2,3-Dihydro-1,4-benzodioxin-6-yl)-3-[4-(1,1-dimethylethyl)phenyl]-2-Propenamide] were purchased from Sigma-Aldrich (St-Louis, MO, USA). Doses of URB937, URB597, JZL184, AM251, AM630, AMG9810 and HC030031 were selected based upon efficacy demonstrated in previous studies [48,49,56–59], respectively. Doses of amitriptyline, gabapentin and morphine were those shown previously to be efficacious in paclitaxel/oxaliplatin neuropathy models [15,17,41]. URB597, URB937, JZL184, AM251, AM630, AMG9810 and HC030031 were dissolved in dimethylsulfoxide (DMSO). Gabapentin, amitriptyline hydrochloride and morphine were dissolved in normal saline (0.9 % NaCl in water) [15,17,41].
To evaluate the receptor mechanism underlying anti-allodynic efficacy of FAAH and MGL inhibitors, URB597, URB937 and JZL184 were co-administered in cocktails with either CB1 (AM251, 3 mg/kg i.p.), CB2 (AM630, 3 mg/kg i.p.), TRPV1 (AMG9810, 3 mg/kg i.p.) or TRPA1 (HC030031, 8 mg/kg i.p.) antagonists. URB597, URB937, JZL184, AM251 and AM630 were stored at −20°C. Amitriptyline and AMG9810 were stored at 4°C. Morphine and HC030031 were stored at room temperature. The drugs or vehicle were prepared fresh on the day of the experiment and administered intraperitoneally (i.p.) either alone or co-administered with antagonists in single volume of 1 ml/kg body weight in all studies.
2.3. Development of neuropathy
Cisplatin (Tocris, Ellisville, MO, USA) was administered intraperitoneally once a week at a dose of 3 mg/kg for 3 weeks (cumulative dose: 9 mg/kg i.p.) [10]. Cisplatin was diluted in normal saline (0.9 % NaCl) and delivered in a volume of 10 ml/kg body weight, ensuring that volumes less than 4 ml were injected into the peritoneal cavity. Control groups were injected with an equivalent volume of saline (i.p.) in lieu of cisplatin. All animals received 4% sodium bicarbonate (NaHCO3 dissolved in 0.9% NaCl) (Tocris, Ellisville, MO, USA) subcutaneously (2 ml s.c.) before each i.p. injection of cisplatin or saline to help buffer effects of cisplatin.
2.3.1. Behavioral Testing
The same animals were used to evaluate mechanical and cold allodynia. Responsiveness to heat stimulation was examined in separate groups of rats to prevent development of behavioral sensitization to the stimuli.
2.3.2. Assessment of Mechanical Allodynia
Mechanical withdrawal thresholds were assessed using a digital Electrovonfrey Anesthesiometer (IITC Life Sciences, Woodland Hills, CA) equipped with a rigid tip as described previously [41,44]. Rats were placed in individual plastic cages on an elevated wire mesh platform, and were allowed to habituate to the testing apparatus for at least 15 minutes until exploratory behavior was no longer observed. Force was applied to the midplantar region of the left and right hind paws. Stable baselines were obtained prior to experimental testing. Mechanical stimulation was terminated upon paw withdrawal; consequently, there was no upper threshold limit set for termination of a trial. Two thresholds were taken for each paw. Testing order of the paws was: right, left, right, left. Approximately 3 minutes interstimulation intervals were allowed between tests. Mechanical withdrawal thresholds were measured on days 0, 4, 8, 12 and 16 (Fig. 1). On day 16, mechanical withdrawal thresholds were obtained at −60, 30, 90, and 150 minutes post-injections of the different drug treatments.
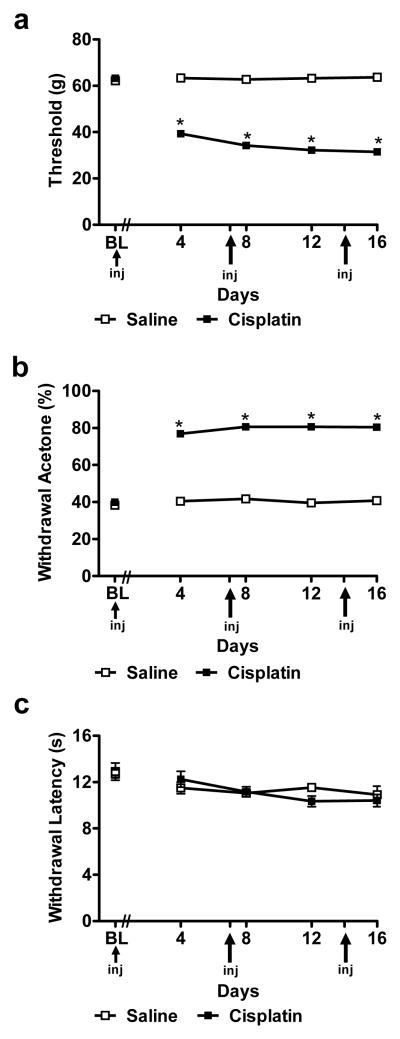
Fig. 1.
Cisplatin produces time-dependent sensitization to mechanical and cold stimulation without altering sensitivity to heat. Time course of development of (a) mechanical and (b) cold allodynia in cisplatin-treated animals (n = 168) relative to saline-treated animals (n = 24). (c) The same cisplatin dosing paradigm did not produce either hypersensitivity or hyposensitivity to heat (n = 6 per group). Data are expressed as mean ± s.e.m. * P < 0.0001 vs. saline (NaCl 0.9 %) group (one way ANOVA).
2.3.3. Assessment of Cold Allodynia
Cold allodynia was assessed by applying drops of room temperature acetone to the plantar surface of the hind paw as previously described [60]. Rats were placed in individual plastic cages on an elevated wire mesh platform, and were allowed to habituate for at least 15 minutes until exploratory behavior was no longer observed. Acetone was loaded into a one ml syringe barrel with no needle tip. Air bubbles were cleared from the syringe prior to acetone application. One drop of acetone (approximately 20 μl) was applied through the mesh platform onto the plantar surface of the hind paw. Care was taken to gently apply the bubble of acetone to the skin on the paw without inducing mechanical stimulation through contact of the syringe barrel with the paw. Paw withdrawal (presence or absence of paw withdrawal) responses were recorded. Paw withdrawal was sometimes associated with a secondary response from the animal (i.e. rapid flicking of the paw, chattering, biting, and/or licking of the paw). Testing order alternated between paws (i.e. right and left) until five measurements were taken for each paw. An interstimulation interval of approximately 3 minutes was allowed between testing of right and left paws. Cold allodynia testing took place on days 0, 4, 8, 12 and 16 for all animals. On day 16, mechanical withdrawal thresholds were obtained at −60, 30, 90, and 150 minutes post-injections of the different drug treatments.
2.3.4. Assessment of Behavioral Responsivity to Heat
Paw withdrawal latencies to radiant heat were measured in duplicate for each paw using the Hargreaves test [61] and a commercially available plantar stimulation unit (IITC model 336; Woodland Hills, CA). Rats were placed on an elevated glass platform in individual plastic cages and radiant heat was applied through the glass to the midplantar surface of the right and left hindpaws in alternating duplicates. Rats were allowed to habituate to the apparatus for at least 15 minutes until exploratory behavior was no longer observed. Stable baseline latencies (about 12 seconds) were obtained prior to experimental testing. A ceiling latency of 20 seconds was implemented to prevent development of behavioral sensitization. Two paw withdrawal latencies were measured for each paw. Testing order of the paws was: right, left, right, left. Approximately 4 minutes interstimulation intervals were allowed between tests. Thermal withdrawal latencies were evaluated before (day 0) and on days 4, 8, 12 and 16.
2.4. Protocol
First, we evaluated the effects of saline (n = 24) or cisplatin (n = 168) treatment on mechanical paw withdrawal threshold (to electro von Frey stimulation) and frequency of paw withdrawal to acetone. Paw withdrawal latency to radiant heat was evaluated in a separate group of rats (n = 6 per group for saline or cisplatin-treated rats). Second, we assessed the anti-allodynic effects of FAAH (URB597 and URB937; 0.1 or 1 mg/kg i.p. for each inhibitor) and MGL (JZL184; 1, 3 or 8 mg/kg i.p.) inhibitors on mechanical and cold allodynia. Then, we compared the efficacy of an MGL inhibitor (JZL184; 8 mg/kg i.p.) with a brain permeant (URB597; 1 mg/kg i.p.) and brain impermeant (URB937; 1 mg/kg i.p.) FAAH inhibitor in suppressing cisplatin-induced mechanical and cold allodynia. Endocannabinoid modulators were compared with reference compounds (morphine, gabapentin or amitriptyline) used clinically to treat neuropathic pain. Finally, we evaluated pharmacological specificity of the observed anti-allodynic effects of FAAH and MGL inhibitors using cannabinoid CB1 (AM251; 3 mg/kg i.p.) and the CB2 (AM630; 3 mg/kg i.p.) antagonists as well as TRPV1 (AMG9810; 3 mg/kg i.p.) and TRPA1 (HC030031; 8 mg/kg i.p.) antagonists. URB597 (0.3 mg/kg i.p.) was previously validated to increase accumulation of anandamide but not 2-AG in rat brain [62]. URB937 (1 mg/kg i.p.) was previously shown to elevate AEA (and other fatty-acid amides) outside the CNS in rats without altering endocannabinoid levels in the brain [48]. JZL184 (8 mg/kg i.p.) was previously shown to produce CB1-dependent anxiolytic effects in rats and enhance antinociceptive effects of exogenous cannabinoids without altering basal nociceptive thresholds [49]. We also previously showed that JZL184, administered locally in the paw, enhanced antinociceptive effects of exogenous 2-AG [57] and produced modality-specific antinociceptive effects in rats that did not overlap with effects of URB597 [58]. The doses employed for AM251 (3 mg/kg i.p.) and AM630 (3 mg/kg i.p.) were those used previously in a surgically-induced neuropathic pain model [56]. The doses for AMG9810 (3 mg/kg i.p.) and HC030031 (8 mg/kg i.p.) have been demonstrated to be efficacious in an osteoarthritis pain model [59]. All drugs were injected intraperitoneally (i.p.) and administered, either alone or in combination, in the same total volume (1 ml/kg). Preliminary experiments (n = 6 per group; data not shown) verified that mechanical (P = 0.076) and cold (P = 0.567) allodynia data did not differ in cisplatin-treated rats receiving either NaCl (0.9 %) or DMSO vehicle. Animals used in the first study receiving either saline (n = 24) or cisplatin (n = 168) were randomly assigned to the different drug conditions. All subsequent studies employed n = 6 animals per group.
2.5. Sample preparation for LC/MS/MS and RT-PCR analysis
Animals receiving cisplatin (n = 8) or saline (n = 8) vehicle (see methods) were killed by rapid decapitation without anesthesia 16 days following initiation of cisplatin dosing to generate samples used in LC/MS/MS analysis of endocannabinoids and other lipid mediators. Lumbar spinal cord and dorsal hind paw skin was rapidly dissected and fast frozen in isopentane precooled on dry ice (−30 °C) and stored at −80°C until use as described previously [57]. Lumbar spinal cords and dorsal root ganglia (DRG) were similarly obtained from separate groups of cisplatin (n = 8) and saline (n = 8)-treated rats. These latter samples were placed directly in RNAlater and stored at −20°C until use for subsequent RNA quantitation via real time reverse transcriptase polymerase chain reaction (RT-PCR) analysis.
2. 6. Lipid Extraction
Lipids were extracted from spinal cord tissue in the same method as brain tissue as described previously [63]. In brief, frozen tissue was weighed and placed in centrifuge tubes on ice. 40:1 volumes of methanol were added to each tube followed by 10 μL of 100 pM deuterium-labeled N-arachidonoyl glycine (d8NAGly; Cayman Chemical, Ann Arbor, MI, USA) to act as an internal standard. Samples were then covered with parafilm and left on ice and in darkness for approximately 2 hours. Remaining on ice, samples were homogenized using a polytron for approximately 1 minute. Samples were centrifuged at 19,000xG at 24°C for 20 minutes. Supernatants were collected and placed in polypropylene tubes and HPLC-grade water was added making the final supernatant/water solution 25% organic. To isolate the compound of interest partial purification of the 25% solution was performed on a Preppy apparatus assembled with 500 mg C18 solid-phase extraction columns. The columns were conditioned with 5 mL of HPLC-grade methanol immediately followed by 2.5 mL of HPLC-grade water. The supernatant/water solution was then loaded onto the C18 column, and then washed with 2.5 mL of HPLC grade water followed by 1.5 mL of 40% methanol. Elutions of 1.5 mL of 70%, 85%, and 100% methanol were collected in individual autosampler vials and then stored in a −20°C freezer until mass spectrometer analysis.
2.7. LC/MS/MS Quantification
Samples were removed from the −20°C freezer, allowed to warm to room temperature for 10 minutes, and vortexed for approximately 1 minute before being placed into the autosampler, where they were held at 24°C (Agilent 1100 series autosampler, Palo Alto, CA) for LC/MS/MS analysis. 10–20μL of eluants were injected separately for each sample to be rapidly separated using a C18 Zorbax reversed-phase analytical column to scan for each individual lipid. Gradient elution (200 μL/min) then occurred, under the pressure created by two Shimadzu 10AdVP pumps (Columbia, MD). Next, electrospray ionization was accomplished using an Applied Biosystems/MDS Sciex (Foster City, CA) API3000 triple quadrupole mass spectrometer. A multiple reaction monitoring (MRM) setting on the LC/MS/MS was then used to analyze levels of each lipid present in the sample injection. Synthetic standards were used to generate optimized MRM methods and standard curves for analysis.
2.8. LC/MS/MS Analysis
The amount of analyte in each sample was calculated by using a combination of calibration curves of the synthetic standards obtained from the Analyst software and recovery adjusted by the deuterium-labeled internal standard. The standards provided a reference for the retention times by which the analytes could be compared. They also helped to identify the specific precursor ion and fragment ion for each analyte which enabled their isolation. These processes provide confidence in the claim that the compounds measured were, in fact, the compound of interest. The amount of each compound in each tissue was then converted to moles per gram tissue.
2.9. mRNA Quantification
Real time RT-PCR was used to measure mRNA levels (n = 3–8 per group). Total RNA from lumbar spinal cord and DRG from saline or cisplatin-treated rats were extracted using a TRIzol (Invitrogen)/RNeasy (Qiagen) hybrid protocol according to manufacturer’s instructions. All RNA samples had A260/280 ratios of 1.8 to 2.0. Purified RNA from each sample was treated with DNase 1. One step RT-PCR was performed in a Matercycler ep realplex RT-PCR machine (Eppendorf Norh America Inc., Hauppauge, NY) using PowerSYBR green PCR kit (Applied Biosystems, Carlsbad, CA). Positive results from one step RT-PCR were further confirmed using two step RT-PCR. Each reaction was run in duplicate and contained 30–100 ng of RNA in a final reaction volume of 10 μl. Reverse transcription was first performed for 30 min at 48°C. Cycling parameters for RT-PCR were 95°C for 15 sec, then 40 cycles of 95°C for 30 sec, 60°C for 1 min, and 72°C for 1 min. Melting curves analysis were performed to ensure only a single product was amplified. Single annealed products for each primer set were also confirmed on agarose gels. mRNA levels were normalized using GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as internal standard. The primer set for rat MGL and FAAH [64], and for rat TRPV1 and TRPA1 from [65] were those published previously. Primers for rat MGL (sense: 5′ CATGGAGCTGGGGAACACTG-3′, anti-sense: 5′-GGAGATGGCACCGCCCATGGAG-3′); rat FAAH (sense: 5′-GTTACAGAGTGGAGAGCTGTC-3′, anti-sense: 5′-GAGGGTTACTGCAGTCAAAGC-3′); rat TRPV1 (sense: 5′-GGTGTGCCTGCACCTAGC-3′ and anti-sense: 5′-CTCTTGGGGTGGGGACTC-3′); rat TRPA1 (sense: 5′-ATTTGCGGCCTGAGTTTTT-3′ and anti-sense: 5′-TCCATCATTGTCCTCATCCA-3′); rat CB1 (sense: 5′-CTACTGGTGCTGTGTGTCATC-3′ and anti-sense: 5′-GCTGTCTTTACGGTGGAATAC-3′); rat CB2 (sense: 5′-GCAGCCTGCTGCTGACCGCTG-3′, anti-sense: 5′-TGCTTTCCAGAGGACATACCC-3′); rat GAPDH (sense: 5′-ATGACTCTACCCACGGCAAG-3′, anti-sense: 5′-CATACTCTGCACCAGCATCTC-3′).
2.10. Statistical analysis
All experiments were conducted in a blinded manner. Animals were randomly assigned to experimental conditions. Paw withdrawal thresholds (mechanical), frequencies (cold) and latencies (heat) were calculated for each paw and averaged. Data were analyzed using analysis of variance (ANOVA) for repeated measures or one-way ANOVA as appropriate. The Greenhouse-Geisser correction was applied to all repeated factors; degrees of freedom reported for significant interactions are the uncorrected values. The source of significant interactions was further evaluated by performing one way ANOVAs at each individual time point, followed by Bonferroni post hoc tests. The different components of the total variation were settled a priori using multiple regression analysis [66]. Paired t-tests were used to compare post-injection responses of the analgesics (endocannabinoid modulators and reference compounds) with corresponding baseline for each group at the time point of maximal effect (i.e. 30 min post drug). Effects of cisplatin on the endocannabinoid/lipid content and mRNA levels were analyzed using unpaired t-tests (two-tailed). Analyses were performed using SPSS statistical software (version 19.0; SPSS Incorporated, Chicago, IL, USA). P < 0.05 was considered significant.
3. Results
3.1. Assessment of mechanical/cold allodynia and heat hyperalgesia/hypoalgesia in cisplatin-treated rats
3.1.1 Mechanical allodynia
Cisplatin decreased mechanical withdrawal thresholds relative to saline treatment (F1,190 = 1736.98, P < 0.0001), consistent with the development of mechanical allodynia (Fig. 1a). Mechanical allodynia was present from day 4 to day 16 (P < 0.0001) (Fig. 1a).
3.1.2. Cold allodynia
Cisplatin increased the frequency of paw withdrawal to acetone relative to saline treatment (F1,190 = 1456.46, P < 0.0001), consistent with the development of cold allodynia (Fig. 1b). Cold allodynia was similarly present from day 4 to day 16 (P < 0.0001) (Fig. 1b).
3.1.3. Heat hyperalgesia/hypoalgesia
Cisplatin did not alter paw withdrawal latencies to radiant heat relative to saline treatment (F1,10 = 0.83, P = 0.779) throughout the observation interval (P > 0.076). These observations document the absence of cisplatin-evoked heat hyperalgesia or hypoalgesia in our study (Fig. 1c).
3.2. Anti-allodynic effects of FAAH (URB597, URB937) and MGL (JZL184) inhibitors on cisplatin-induced mechanical allodynia
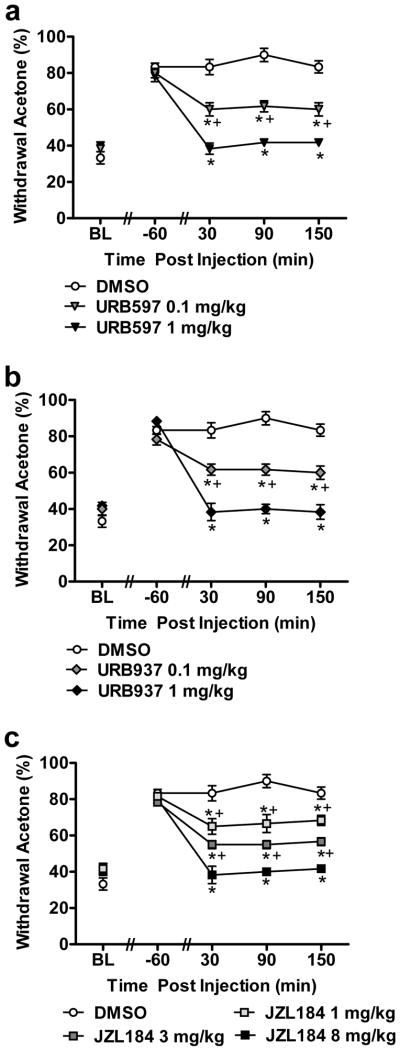
URB597 (0.1 and 1 mg/kg) suppressed cisplatin-evoked mechanical allodynia relative to vehicle treatment (F2,15 = 118.89, P < 0.0001; P < 0.0001 for each comparison). Both doses produced time-dependent attenuations of mechanical allodynia relative to pre-injection baseline thresholds (F8,60 = 62.34, P < 0.0001) (Fig. 2a). The antiallodynic effects of URB597 were observed relative to vehicle throughout the 150 (P < 0.0001) min post-injection observation interval. The high dose of URB597 (1 mg/kg i.p.) increased mechanical paw withdrawal thresholds to a greater extent than the low (0.1 mg/kg i.p.) dose at all post-injection intervals (P < 0.0001) (Fig. 2a).
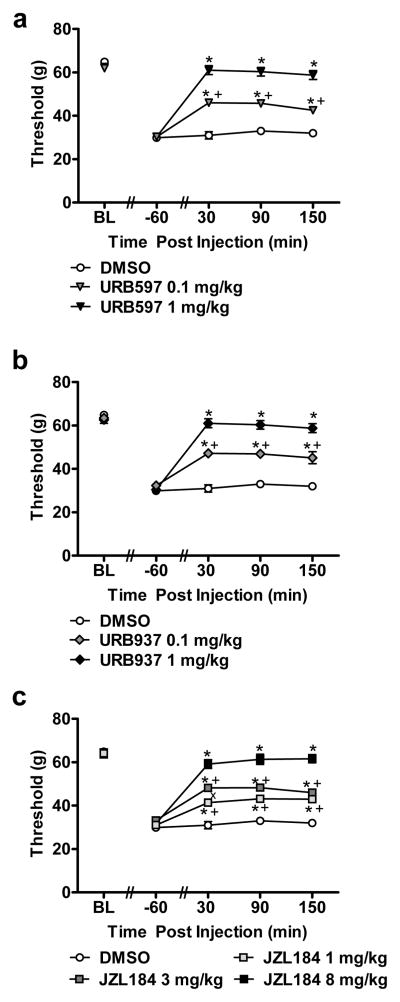
Fig. 2.
FAAH (URB597 and URB937) and MGL (JZL184) inhibitors produce dose-related suppressions of cisplatin-induced mechanical allodynia. (a) Acute treatment with URB597 (0.1 and 1 mg/kg i.p.), (b) URB937 (0.1 and 1 mg/kg i.p.) and (c) JZL184 (1, 3 and 8 mg/kg i.p.) suppressed mechanical allodynia in cisplatin-treated animals. Data are expressed as mean ± s.e.m. (n = 6 per group). * P < 0.001 vs. vehicle group (ANOVA, Bonferroni post hoc); + P < 0.001 vs. high dose of endocannabinoid modulator (i.e. URB597 (1 mg/kg i.p.), URB937 (1 mg/kg) or JZL184 (8 mg/kg i.p.); x P < 0.04 vs. JZL184 (1 mg/kg) (ANOVA, Bonferroni post hoc).
URB937 (0.1 and 1 mg/kg i.p.) attenuated cisplatin-evoked mechanical allodynia relative to vehicle treatment (F2,15 = 66.58, P < 0.0001; P < 0.0001 for each comparison). Both doses produce time-dependent attenuations of mechanical allodynia relative to pre-injection baselines (F8,60 =39.89, P < 0.0001) (Fig. 2b). Anti-allodynic efficacy of URB937 was observed relative to vehicle throughout the 150 min observation interval (P < 0.001). The high dose of URB937 (1 mg/kg i.p.) increased mechanical paw withdrawal thresholds to a greater extent than the low (0.1 mg/kg i.p.) dose at 30 (P < 0.0001), 90 (P < 0.0001) and 150 (P < 0.001) minutes post-injection (Fig. 2b).
JZL184 (1, 3 and 8 mg/kg i.p.) suppressed cisplatin-evoked mechanical allodynia relative to vehicle treatment (F3,20 = 82.68, P < 0.0001; P < 0.0001 for each comparison). JZL184 produced time-dependent attenuations of mechanical allodynia relative to pre-injection baseline thresholds (F12,80 = 31.23, P < 0.0001) (Fig. 2c). Anti-allodynic efficacy of JZL184 was observed relative to vehicle throughout the 150 min observation interval (P < 0.001 at each time point). The high dose of JZL184 (8 mg/kg i.p.) increased mechanical paw withdrawal thresholds to a greater extent than either of the lower (1 and 3 mg/kg) doses at 30 (P < 0.001), 90 (P < 0.0001) and 150 (P < 0.0001) minutes post-injection. The middle dose (3 mg/kg i.p.) of JZL184 produced a greater antinociceptive effect than the low (1 mg/kg i.p.) dose only at 30 min (P < 0.04) post-injection. Anti-allodynic efficacy of these two doses did not differ at 90 (P =0.124 ) or 150 (P =0.764) minutes post-injection (Fig. 2c).
3.3. Comparison of anti-allodynic efficacy of morphine, gabapentin, amitriptyline, FAAH (URB597, URB937) and MGL (JZL184) inhibitors on cisplatin-evoked mechanical allodynia
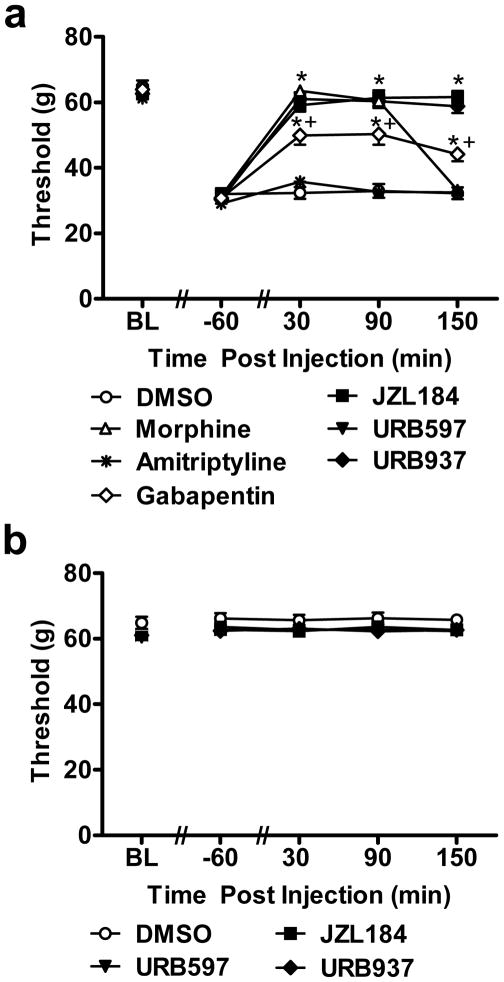
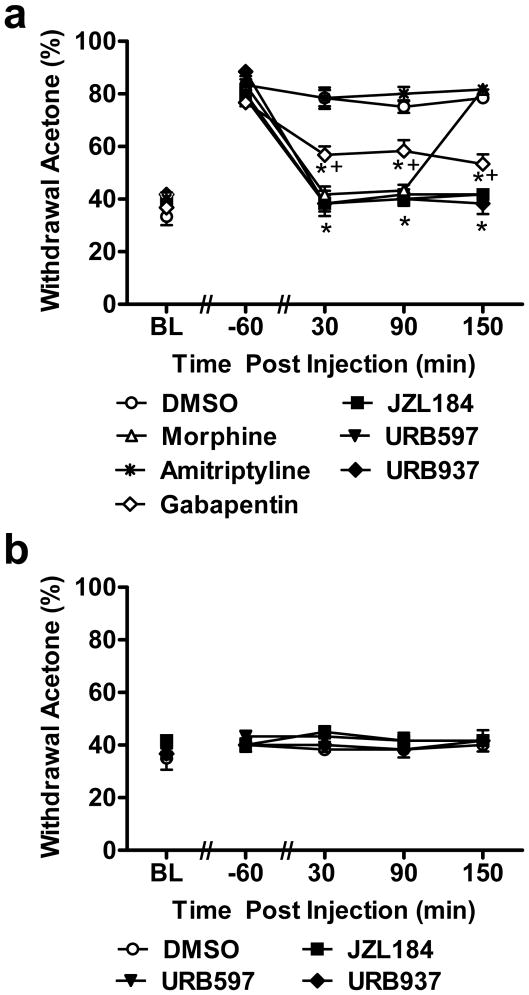
We compared anti-allodynic efficacy of reference analgesics used clinically to treat cisplatin-induced neuropathy with the maximally efficacious doses of FAAH and MGL inhibitors identified in the dose response studies. Pharmacological manipulations attenuated cisplatin-evoked mechanical allodynia (F6,35 = 78.72, P < 0.001; Fig. 3a). Morphine, gabapentin, FAAH (URB597, URB937) and MGL (JZL184) inhibitors (P < 0.001 for each comparison) all suppressed cisplatin-induced mechanical allodynia relative to vehicle whereas amitriptyline (P = 0.998) failed to suppress mechanical hypersensitivity. Morphine, gabapentin, FAAH (URB597, URB937) and MGL (JZL184) inhibitors also produced time-dependent attenuations of mechanical allodynia relative to pre-injection baselines (F24,140 = 27.44, P < 0.001). This attenuation was observed relative to either vehicle or amitriptyline-treated groups (Fig. 3a) from 30 – 150 (P < 0.001) minutes post-injection. Acute amitriptyline treatment failed to suppress cisplatin-induced mechanical allodynia relative to vehicle (P = 1.000). Morphine, FAAH and MGL inhibitors all increased mechanical paw withdrawal thresholds to a greater extent than gabapentin-treated groups at both 30 (P < 0.05) and 90 (P < 0.022) minutes post-injection. At 150 (P < 0.001) minutes post-injection, paw thresholds were lower in gabapentin-treated groups in comparison to FAAH and MGL inhibitors, and morphine no longer suppressed mechanical allodynia. Thus, acute gabapentin treatment was less effective in suppressing mechanical allodynia compared to the endocannabinoid modulators or morphine. At 30 min post-injection, both FAAH (P = 0.07 for URB597; P = 0.39, for URB937) and MGL (P = 0.11 for JZL184) inhibitors normalized paw withdrawal thresholds to preinjection levels, as observed with morphine (P = 0.62), whereas gabapentin (P = 0.02) and amitriptyline (P = 0.0001) failed to do so. Anti-allodynic effects of endocannabinoid modulators also outlasted that of morphine (P < 0.001 for each comparison); by 150 minutes post-injection, paw withdrawal thresholds observed following morphine treatment did not differ from those observed in vehicle- or amitriptyline-treated groups (P= 1.000) (Fig. 3a).
Fig. 3.
Comparison of suppressions of cisplatin-evoked mechanical allodynia produced by acute treatment with FAAH (URB597, URB937) and MGL (JZL184) inhibitors and reference compounds morphine, gabapentin and amitriptyline. (a) URB597 (1 mg/kg i.p.), URB937 (1 mg/kg i.p.) or JZL184 (8 mg/kg i.p.) suppressed cisplatin-induced mechanical allodynia throughout the 150 min post-injection observation interval. The anti-allodynic effects of endocannabinoid modulators outlasted that of morphine (6 mg/kg i.p.). Amitriptyline (30 mg/kg i.p.) failed to attenuate cisplatin-evoked mechanical allodynia, whereas gabapentin (100 mg/kg i.p.) was only partially effective in elevating mechanical withdrawal thresholds. (b) FAAH (URB597, URB937) and MGL (JZL184) inhibitors did not alter mechanical withdrawal thresholds in saline-treated rats. Data are expressed as mean ± s.e.m. (n = 6 per group). * P < 0.001 vs. vehicle (DMSO) group (ANOVA, Bonferroni post hoc); + P < 0.05 vs. endocannabinoid modulators and morphine(ANOVA, Bonferroni post hoc).
In saline-treated rats, FAAH (URB597, URB937) and MGL (JZL184) inhibitors failed to alter (F3,20 = 1.327, P = 0.294) mechanical withdrawal thresholds relative to vehicle treatment (Fig. 3b) at any post-injection time point (F12,80 = 0.561, P = 0.867) (P > 0.157). In previous work, we showed that lower doses of morphine (2 mg/kg s.c.) increased mechanical paw withdrawal thresholds (from 65.7 to 79 ± 4 g) using the same digital Electrovonfrey anesthesiometer [67], suggesting that the higher morphine dose employed here was analgesic rather than anti-allodynic.
3.4. Pharmacological specificity of anti-allodynic effects of FAAH (URB597, URB937) and MGL (JZL184) inhibitors on cisplatin-evoked mechanical allodynia
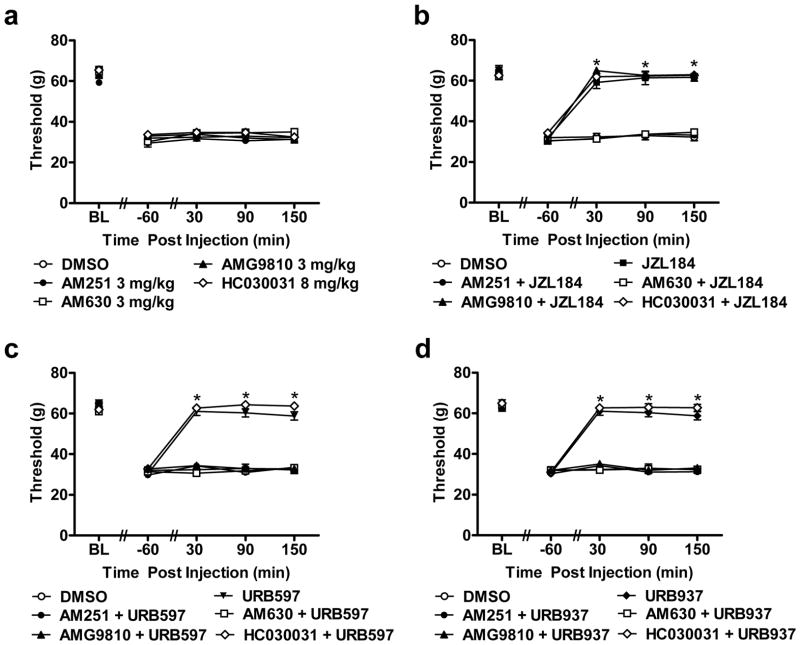
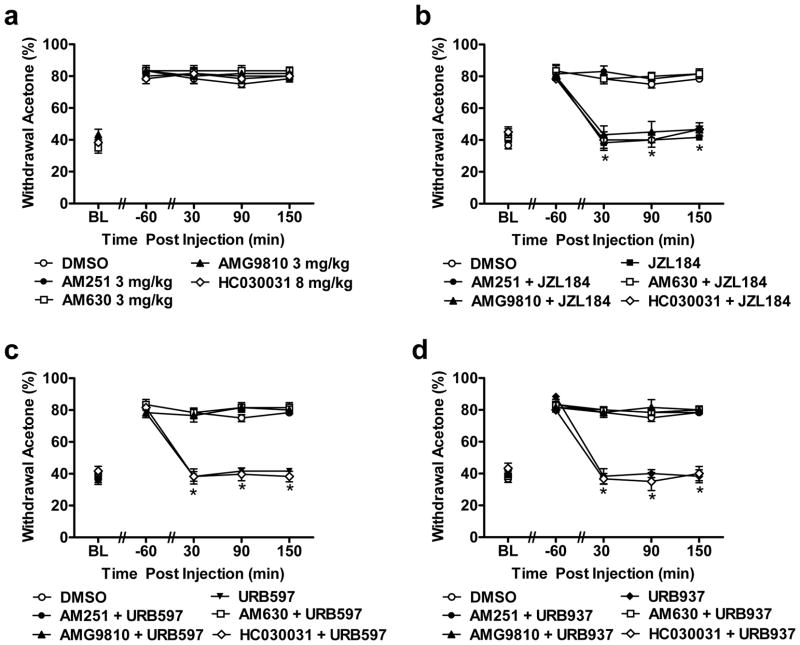
The CB1 (AM251; 3 mg/kg i.p.), CB2 (AM630; 3 mg/kg i.p.), TRPV1 (AMG9810; 3 mg/kg i.p.) and TRPA1 (HC030031; 8 mg/kg i.p.) antagonists failed to alter (F4,25 = 2.48, P = 0.07) cisplatin-evoked mechanical allodynia relative to vehicle treatment at any time point (F16,100 = 0.99, P = 0.46) (Fig. 4a). However, AM251, AM630 and AMG9810, administered alone, completely blocked the ability of URB597 (1 mg/kg; F5,30 = 162.80, P < 0.001) or URB937 (1 mg/kg i.p.; F5,30 = 194.10, P < 0.001) to attenuate cisplatin-induced mechanical allodynia (P < 0.001 for all post-injection time points for each study; Fig. 4c–d). Only AM251 or AM630, in the absence of the other antagonist, completely blocked the anti-allodynic effects of JZL184 (8 mg/kg i.p.; F5,30 = 219.57, P < 0.001) in response to mechanical stimulation (P < 0.001 for all post-injection time points; Fig. 4b). By contrast, AMG9810 (P = 0.843) failed to block the JZL184-induced suppression of mechanical allodynia (P > 0.067 for all post-injection time points; Fig. 4b). The TRPA1 antagonist HC030031 (8 mg/kg i.p.) failed to block the ability of URB597 (P = 0.292), URB937 (P = 0.296) or JZL184 (P = 1.000) to attenuate cisplatin-induced mechanical allodynia (P > 0.116 for all post-injection time points for each study; Fig. 4b–d). Mechanical withdrawal thresholds were similar in groups receiving vehicle or AM251, AM630 or AMG9810 combined with FAAH (URB597 or URB937) modulators at all post-injections time points (P = 1.000). Mechanical paw withdrawal thresholds were similar in vehicle-treated groups and group that received AM251 or AM630 combined with JZL184 at all post-injections time points (P = 1.000).
Fig. 4.
FAAH (URB597, URB937) and MGL (JZL184) inhibitors suppress cisplatin-induced mechanical allodynia through CB1 and CB2 but not TRPA1 receptor mechanisms whereas FAAH inhibitors additionally engage TRPV1 dependent mechanisms. (a) The CB1 (AM251; 3 mg/kg i.p.), CB2 (AM630; 3 mg/kg i.p.), TRPV1 (AMG9810; 3 mg/kg) and TRPA1 (HC030031; 8 mg/kg) antagonists did not alter cisplatin-induced mechanical allodynia relative to vehicle treatment (i.p.). (b) JZL184 (8 mg/kg i.p.), (c) URB597 (1 mg/kg i.p.) and (d) URB937 (1 mg/kg i.p.) suppressed cisplatin-induced mechanical allodynia through mechanisms blocked by either the CB1 (AM251; 3 mg/kg i.p.) or the CB2 (AM630; 3 mg/kg i.p.) antagonists, but not the TRPA1 (HC030031; 8 mg/kg i.p) antagonist. Anti-allodynic effects of FAAH (URB597, URB937), but not MGL (JZL184), inhibitors were also blocked by the TRPV1 (AMG9810; 3 mg/kg i.p.) antagonist. Data are expressed as mean ± s.e.m. (n = 6 per group). * P < 0.001 vs. DMSO, AM251 + MGL/FAAH inhibitors, AM630 + MGL/FAAH inhibitors and AMG9810 + FAAH inhibitors (ANOVA, Bonferroni post hoc).
3.5 Anti-allodynic effects of FAAH (URB597, URB937) and MGL inhibitors on cisplatin-induced cold allodynia
URB597 (0.1 and 1 mg/kg i.p.) suppressed cisplatin-evoked cold allodynia in a dose-related fashion relative to vehicle (F2,15 = 67.64, P < 0.0001; P < 0.0001 for each comparison). Both doses produced time-dependent attenuations of cold allodynia relative to pre-injection baselines (F8,60 = 22.41, P < 0.0001) (Fig. 5a). Anti-allodynic efficacy of URB597 was observed relative to vehicle from 30–150 (P < 0.001) minutes post-injection. The high (1 mg/kg i.p.) dose of URB597 suppressed the frequency of withdrawal to acetone to a greater extent than the low (0.1 mg/kg i.p.) dose at 30 (P < 0.002), 90 (P < 0.001) and 150 (P < 0.002) minutes post-injection (Fig. 5a).
Fig. 5.
FAAH (URB597 and URB937) and MGL (JZL184) inhibitors produce dose-related suppressions of cisplatin-induced cold allodynia. (a) Acute treatment with URB597 (0.1 and 1 mg/kg), (b) URB937 (0.1 and 1 mg/kg) and (c) JZL184 (1, 3 and 8 mg/kg) suppressed cold allodynia in cisplatin-treated animals. Data are expressed as mean ± s.e.m. (n = 6 per group). * P < 0.024 vs. vehicle group (ANOVA, Bonferroni post hoc); + P < 0.048 vs. URB597 (1 mg/kg) or URB937 (1 mg/kg) or JZL184 (1 or 3 mg/kg) (ANOVA, Bonferroni post hoc).
URB937 (0.1 and 1 mg/kg i.p.) also suppressed cisplatin-evoked cold allodynia in a dose-related manner relative to vehicle (F2,15 = 60.09, P < 0.0001; P < 0.0001 for each comparison). Both doses produced time-dependent attenuations of cold allodynia (F8,60 = 18.98, P < 0.0001) (Fig. 5b). Anti-allodynic efficacy of URB937 was observed relative to vehicle from 30–150 (P < 0.006) minutes post-injection. The high dose of URB937 (1 mg/kg i.p.) suppressed the frequency of withdrawal to acetone to a greater extent than the low (0.1 mg/kg i.p.) dose at 30 (P < 0.003), 90 (P < 0.001) and 150 (P < 0.002) minutes post-injection (Fig. 5b).
JZL184 (1, 3 and 8 mg/kg i.p.) exhibited dose-dependent anti-allodynic effects relative to vehicle in suppressing cisplatin-evoked cold allodynia (F3,20 = 41.58, P < 0.0001; P < 0.004 for 1 mg/kg i.p. dose, P < 0.0001 for 3 and 8 mg/kg i.p. doses). All doses of JZL184 produced time-dependent attenuations of cold allodynia relative to pre-injection baselines (F12,80 = 15.64, P < 0.0001) (Fig. 5c). This attenuation by JZL184 was observed relative to vehicle from 30–150 (P < 0.024) min post-injection. The high dose of JZL184 (8 mg/kg i.p.) suppressed the frequency of withdrawal to acetone to a greater extent than either the low (1 mg/kg i.p.; P< 0.001) or the middle (3 mg/kg i.p.; P < 0.007) dose at 30 (P < 0.048), 90 (P < 0.024) and 150 (P < 0.021) minutes post-injection. However, the anti-allodynic efficacy of the low and middle doses of JZL184 did not differ from each other at any time point: 30 (P = 0.553), 90 (P = 0.121) and 150 (P = 0.109) minutes post-injection (Fig. 5c).
3.6. Comparison of anti-allodynic efficacy of morphine, gabapentin, amitriptyline, FAAH (URB597, URB937) and MGL (JZL184) inhibitors on cisplatin-evoked cold allodynia
Pharmacological manipulations differentially suppressed cisplatin-evoked cold allodynia (F6,35 = 46.01, P < 0.001; Fig. 6a). Morphine, gabapentin, FAAH (URB597, URB937) and MGL (JZL184) inhibitors (P < 0.001 for each comparison) all suppressed cisplatin-evoked cold allodynia whereas amitriptyline (P = 1.000) failed to attenuate withdrawal frequency to acetone stimulation. Moreover, morphine, FAAH (URB597, URB937) and MGL (JZL184) inhibitors all produced time-dependent attenuations of cold allodynia relative to pre-injection baselines (F24,140 = 18.02, P < 0.001) (Fig. 6a). This attenuation was observed throughout the entire observation interval (i.e. 30–150 (P < 0.005) min post-injection) relative to vehicle or amitriptyline treatment. Acute amitriptyline treatment failed to suppress cisplatin-induced cold allodynia relative to vehicle (P = 0.772). Moreover, morphine, FAAH and MGL inhibitors suppressed the frequency of withdrawal to acetone to a greater extent than gabapentin at 30 (P < 0.025) and 90 (P < 0.002) minutes post-injection. At 150 (P < 0.037) minutes post-injection, frequency of paw withdrawals were higher in gabapentin-treated groups in comparison to FAAH and MGL inhibitors. At 30 min post-injection, both FAAH (P = 0.61 for URB597; P = 0.53, for URB937) and MGL (P = 0.74 for JZL184) inhibitors normalized paw withdrawal responses to preinjection levels, as observed with morphine (P = 0.36), whereas gabapentin (P = 0.003) and amitriptyline (P = 0.0005) failed to do so. Effects of the FAAH and MGL inhibitors outlasted that of morphine (P < 0.001 for each comparison); by 150 minutes post-injection, morphine failed to produce antinociceptive effects in comparison to vehicle- or amitriptyline-treated groups (P = 0.990; Fig. 6a).
Fig. 6.
Comparison of suppressions of cisplatin-evoked cold allodynia produced by acute treatment with FAAH (URB597, URB937) and MGL (JZL184) inhibitors and reference compounds morphine, gabapentin and amitriptyline. (a) URB597 (1 mg/kg i.p.), URB937 (1 mg/kg i.p.) and JZL184 (8 mg/kg i.p.) suppressed cisplatin-induced cold allodynia throughout the 150 min observation interval. The anti-allodynic effects of endocannabinoid modulators outlasted that of morphine (6 mg/kg i.p.). Amitriptyline (30 mg/kg i.p.) failed to attenuate cisplatin-evoked cold allodynia whereas gabapentin (100 mg/kg i.p.) was only partially effective in reducing frequency of withdrawal to acetone stimulation. (b) FAAH (URB597, URB937) and MGL (JZL184) inhibitors did not alter frequency of withdrawal to cold in saline-treated rats. Data are expressed as mean ± s.e.m. (n = 6 per group). * P < 0.005 vs. vehicle (DMSO) group (ANOVA, Bonferroni post hoc); + P < 0.037 vs. endocannabinoid modulators or morphine (ANOVA, Bonferroni post hoc).
In saline-treated rats, FAAH (URB597, URB937) and MGL (JZL184) inhibitors failed to alter ((F3,20 = 2.74, P = 0.071) the frequency of withdrawal to acetone relative to vehicle treatment at any timepoint (F12,80 = 0.348, P = 0.977) (Fig. 6b).
3.7. Pharmacological specificity of anti-allodynic effects of FAAH (URB597, URB937) and MGL (JZL184) inhibitors on cisplatin-evoked cold allodynia
The CB1 (AM251; 3 mg/kg i.p.), CB2 (AM630; 3 mg/kg i.p.), TRPV1 (AMG9810; 3 mg/kg i.p.) and TRPA1 (HC030031; 8 mg/kg i.p.) antagonists failed to alter (F4,25 = 0.805, P = 0.534) cisplatin-evoked cold allodynia relative to vehicle treatment at any time point (F16,100 = 0.829, P = 0.651) (Fig. 7a). However, the CB1 antagonist AM251, the CB2 antagonist AM630 and the TRPV1 antagonist AMG9810, in the absence of the other antagonists, each blocked the suppressions of cold allodynia produced by either URB597 (1 mg/kg; F5,30 = 59.30, P < 0.001) or URB937 (1 mg/kg i.p.; F5,30 = 51.46, P < 0.001) (P < 0.001 for all post-injection time points; Fig. 7c–d). Only AM251 and AM630, in the absence of the other antagonist completely blocked the ability of JZL184 (8 mg/kg i.p.; F5,30 = 38.19, P < 0.001) to attenuate cisplatin-induced cold allodynia and normalize paw withdrawal thresholds (P < 0.001 for all post-injection time points; Fig. 7b). The TRPV1 antagonist AMG9810 (P = 1.000) was ineffective in blocking the ability of JZL184 to suppress cisplatin-induced cold allodynia (P = 1.000 at all post-injection time points; Fig. 7b). Moreover, the TRPA1 antagonist HC030031 failed to block the anti-allodynic efficacy of URB597 (P = 1.000), URB937 (P = 1.000) or JZL184 (P = 1.000) in the acetone test (P = 1.000 for all post-injection time points for each study; Fig. 7b–d). Paw withdrawal responses to cold stimulation were similar in groups receiving vehicle, AM251, AM630 or AMG9810 combined with FAAH (URB597 or URB937) modulators at all post-injections time point (P = 1.000 for all studies; Fig. 7b–d). Paw withdrawal responses to acetone were only similar to vehicle in the CB1 and CB2 antagonist groups that received coadministration of JZL184; similar effects were observed at all post-injections time points (P = 1.000).
Fig. 7.
FAAH (URB597, URB937) and MGL (JZL184) inhibitors suppress cisplatin-induced cold allodynia through CB1 and CB2 but not TRPA1 receptor mechanisms whereas FAAH inhibitors additionally engage TRPV1 dependent mechanisms. (a) The CB1 (AM251; 3 mg/kg i.p.), CB2 (AM630; 3 mg/kg i.p.), TRPV1 (AMG9810; 3 mg/kg i.p.) and TRPA1 (HC030031; 8 mg/kg i.p.) antagonists did not alter cisplatin-induced cold allodynia relative to vehicle treatment (i.p.). (b) JZL184 (8 mg/kg i.p.), (c) URB597 (1 mg/kg i.p.) and (d) URB937 (1 mg/kg i.p.) suppressed cisplatin-induced cold allodynia in a manner blocked by either the CB1 (AM251; 3 mg/kg i.p.) or the CB2 (AM630; 3 mg/kg i.p.) antagonists, but not the TRPA1 (HC030031; 8 mg/kg i.p.) antagonist. Anti-allodynic effects of FAAH (URB597, URB937), but not MGL (JZL184), inhibitors were also blocked by the TRPV1 (AMG9810; 3 mg/kg i.p.) antagonist. Data are expressed as mean ± s.e.m. (n = 6 per group). * P < 0.001 vs. DMSO, AM251 + MGL/FAAH inhibitors, AM630 + MGL/FAAH inhibitors and AMG9810 + FAAH inhibitors (ANOVA, Bonferroni post hoc).
3.8 Lipid levels in the spinal cord
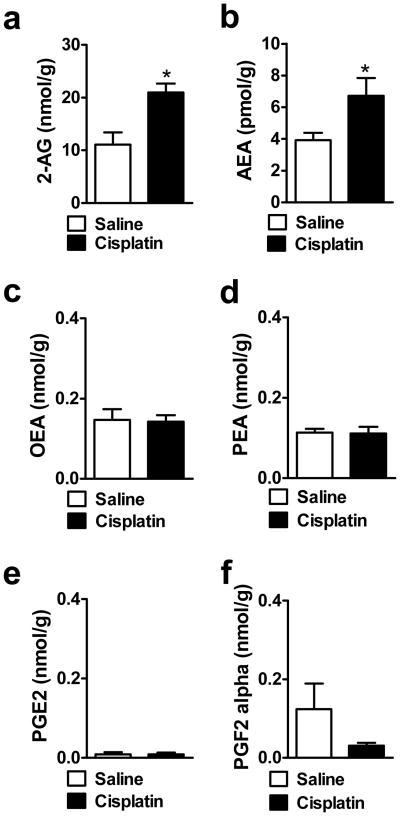
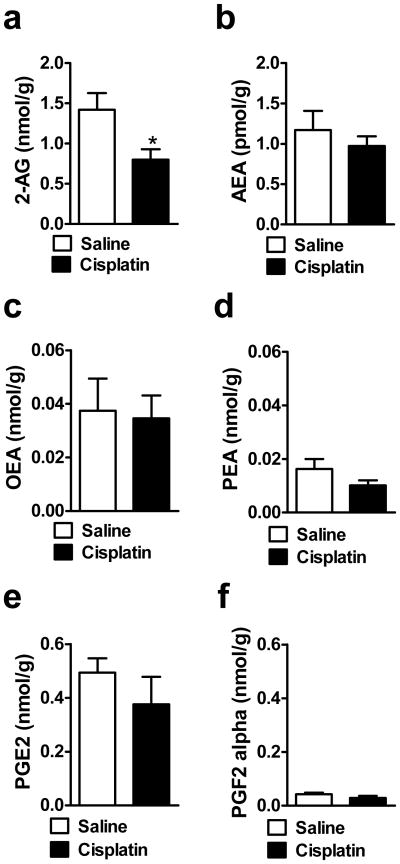
Cisplatin increased both 2-AG (t14 = 3.47, P < 0.004) and AEA (t13 = 2.16, P < 0.049) levels in the lumbar spinal cord relative to saline-treated groups (Fig. 8a–b). Cisplatin did not alter OEA (P = 0.916), PEA (P = 0.895), PGE2 (P = 0.997) or PGF2α (P = 0.113) levels in the same lumbar spinal cord samples (Fig. 8c–f).
Fig. 8.
Cisplatin increases levels of (a, b) endocannabinoids (2-AG, AEA) but not (c, d) fatty-acid amides (OEA, PEA) or (e,f) other inflammatory mediators (PGE2, PGF2α) levels in the lumbar spinal cord relative to saline treatment. Data are expressed as mean ± s.e.m. (n = 5–8 per group). * P < 0.049 vs. Saline (t-test, two-tailed).
3.9 Lipid levels in hind paw skin
Cisplatin decreased 2-AG levels in the dorsal hind paw skin relative to saline treatment (t13 = 2.46, P < 0.029) (Fig. 9a)) without altering the levels of AEA (P = 0.472), OEA (P = 0.844), PEA (P = 0.173), PGE2 (P = 0.325) and PGF2α (P = 0.190) (Fig. 9b–f).
Fig. 9.
Cisplatin decreases levels of (a) the endocannabinoid 2-AG in rat hind paw skin without altering levels of (b) AEA relative to saline treatment. Levels of (c, d) fatty-acid amides (OEA, PEA) and (e,f) other inflammatory mediators (PGE2,PGF2α) were not altered in rat hind paw skin relative to saline treatment. Data are expressed as mean ± s.e.m. (n = 6–8 per group). * P < 0.029 vs. Saline (t-test, two-tailed).
3.10 mRNA Quantitation of FAAH, MGL, CB1, CB2, TRPV1 and TRPA1 in lumbar spinal cord and DRG
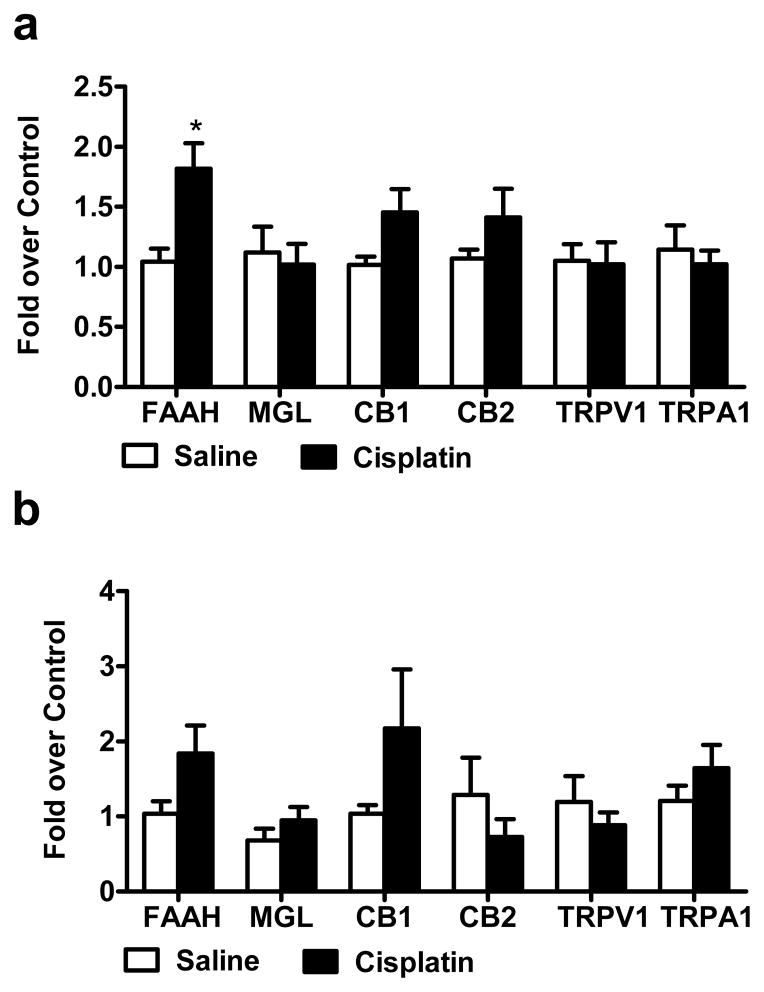
In rats treated with cisplatin for 16 days, levels of FAAH mRNA were reliably increased in the lumbar spinal cord (t12 = 3.242, P < 0.0071) (Fig. 10a), but not in the DRG (P = 0.1217) (Fig. 10b). Real time RT-PCR analysis revealed that mRNA levels of MGL (P = 0.7155 and 0.3182, respectively), CB1 (P = 0.0541 and 0.1509 and, respectively), CB2 (P = 0.1998 and 0.3702, respectively), TRPV1 (P = 0.9034 and 0.4427, respectively) and TRPA1 (P = 0.5879 and 0.3040, respectively) were largely similar between cisplatin and saline-treated rats in both lumbar spinal cord and DRG, respectively (Fig. 10a–b). The increase in FAAH mRNA was confirmed using two step RT-PCR which also failed to show upregulation of TRPV1 and TRPA1 mRNAs (data not shown). Levels of CB2 mRNA were near the threshold for detection in both cisplatin- and saline-treated rats.
Fig. 10.
(a) Cisplatin increases levels of FAAH, but not MGL, mRNA in the lumbar spinal cord without altering levels of CB1, CB2, TRPV1 or TRPA1 mRNAs. (b) Cisplatin did not reliably alter mRNAs for either endocannabinoid hydrolyzing enzymes (MGL, FAAH) or receptors (CB1, CB2, TRPV1, TRPA1) in DRG. Data are expressed as mean ± s.e.m. (n = 7–8 for spinal cord and n = 3–7 per group for DRG). * P < 0.0071 vs. Saline (t-test, two-tailed).
4. Discussion
Our study demonstrates that pharmacological inhibitors of endocannabinoid degradation, targeting either FAAH (URB597, URB937) or MGL (JZL184), suppress chemotherapy-induced neuropathic pain. We found that cisplatin-induced mechanical and cold allodynia was exquisitely sensitive to endocannabinoid modulators; all endocannabinoid modulators reversed cisplatin-evoked mechanical and cold allodynia to pre-cisplatin levels following acute administration. In each case, the anti-allodynic effects of FAAH inhibitors were mediated by CB1, CB2 and TRPV1 receptors whereas those of the MGL inhibitor were mediated by CB1 and CB2 receptors only. Moreover, brain permeant (URB597) and impermeant (URB937) inhibitors of FAAH are equally efficacious in suppressing cisplatin-induced neuropathy and exhibit identical patterns of pharmacological specificity. We also found that cisplatin treatment itself was sufficient to alter endocannabinoid tone in both the spinal cord and peripheral paw skin. We observed a selective decrease in 2-AG in the periphery in dorsal hind paw skin (with no changes in AEA levels) and an increase in accumulation of both AEA and 2-AG centrally in the lumbar spinal cord. It is possible that neurotoxicity of chemotherapy induces a selective loss in basal levels of 2-AG in peripheral paw skin, and that, compensatory, adaptive changes in endocannabinoid levels are then induced centrally in response to peripheral nerve injury. Consistent with this hypothesis, cisplatin has recently been reported to decrease AEA levels in plantar paw skin in mice with a different dosing paradigm (1 mg/kg i.p. for 7 days), although spinal cord levels of endocannabinoids were not evaluated [54]. Moreover, spinal nerve ligation has been shown to increase accumulation of endocannabinoids in rat DRG [68]. In our study long term transcriptional changes in expression of FAAH (anandamide hydrolyzing enzyme), but not MGL (2-AG hydrolyzing enzyme) mRNAs were observed in the spinal cord, with no significant changes observed in the DRG. Levels of MGL, CB1, CB2, TRPV1 and TRPA1 mRNAs remained relatively unaltered in both the lumbar spinal cord and DRG. Thus, it is unlikely that downregulation of MGL (or FAAH) can explain the increases in spinal 2-AG (or anandamide) content observed here following cisplatin treatment. By contrast, upregulation of FAAH mRNA was, in fact, observed, in the lumbar spinal cord. More work is necessary to demonstrate that increases in 2-AG and AEA observed here in the lumbar spinal cord reflect changes in endocannabinoid synthesis rather than in the hydrolysis. Effects of cisplatin on activity of enzymes catalyzing endocannabinoid synthesis and hydrolysis remains to be characterized at both central and peripheral levels. Our studies, nonetheless, demonstrate that endocannabinoid tone is reduced in peripheral paw skin, but elevated in the lumbar spinal cord following cisplatin treatment, an effect which may contribute to the effectiveness of FAAH and MGL inhibitors in reversing established cisplatin-induced neuropathy. Our results further suggest that modulation of the endocannabinoid system through inhibition of either FAAH or MGL represents an effective strategy for suppressing cisplatin-induced neuropathic pain.
A noteworthy observation from our studies was that endocannabinoid modulators, administered acutely, were as effective as morphine in suppressing cisplatin-evoked mechanical and cold allodynia. However, endocannabinoid modulators exhibited a longer duration of anti-allodynic action and did not alter basal nociceptive thresholds in the absence of cisplatin-induced neuropathy, in contrast to the analgesic dose of morphine evaluated here. Dose response studies with URB597 (0.1 and 1 mg/kg), URB937 (0.1 and 1 mg/kg) and JZL184 (1, 3 and 8 mg/kg) have further documented that endocannabinoid modulators are highly potent in suppressing cisplatin-induced neuropathy; doses of FAAH inhibitors as low as 0.1 mg/kg i.p. attenuated cisplatin-induced allodynia. Inhibitors of endocannabinoid hydrolysis also produced a greater suppression of mechanical and cold allodynia compared to acute treatment with gabapentin, which showed only partial attenuation of allodynia at an acute dose of 100 mg/kg i.p. By contrast, acute treatment with the anti-depressant amitriptyline was ineffective in altering cisplatin-evoked mechanical or cold allodynia, consistent with previous observations that repeated amitriptyline treatment was required to attenuate paclitaxel-evoked allodynia [17]. Pharmacodynamic and pharmacokinetic properties are likely to contribute to the partial attenuation of allodynia observed with gabapentin and ineffectiveness of amitriptyline to suppress mechanical/cold allodynia [23,24]. For example, amitriptyline possesses a low bioavailability since it is characterized by a significant intestinal first-pass effect in rats [23]. Different pharmacodynamic/kinetic properties could also impact the bioavailability of compounds used in this study [25,69–71].
In our study, cisplatin produced mechanical and cold allodynia that was manifest by day 4 post initial cisplatin dosing and was maintained until the end of the experiment (day 16). Hyperalgesia or hypoalgesia to heat was notably absent. Cisplatin-evoked mechanical and cold allodynia was observed relative to saline-treated rats tested at the same times under the same experimental conditions. These observations are consistent with other published reports [9–11,42,72,73]. Using different methodologies and species, cisplatin [10,74] and oxaliplatin [73,75] have been shown to produce cold allodynia to tail immersion (10°C water). Cisplatin-treated mice also exhibit hypersensitivity to cold in the cold plate (− 4.2°C) test [76]. These latter effects are likely to be TRPA1-dependent because TRPA1 receptors are required for the development of oxaliplatin-induced mechanical and cold allodynia in mice and also contribute to cisplatin-evoked mechanical allodynia [74]. However, in our study, performed in rats, the TRPA1 antagonist HC030031 (8 mg/kg i.p.) did not reverse cisplatin-induced mechanical and cold allodynia when neuropathy was already established and did not alter anti-allodynic efficacy of endocannabinoid modulators.
In our study, cisplatin-treated rats failed to exhibit heat hyperalgesia or hypoalgesia (i.e. increase or decrease in paw withdrawal latencies in response to radiant heat) using the Hargreaves test [61]. Our finding is corroborated by another study which has demonstrated that, after the 6th cisplatin injection around day 15 (cisplatin 1 and 2 mg/kg (i.p.) alternatively every 3 days; cumulative dose: 15 mg/kg), slight, but non-significant, changes in sensitivity to heat applied to the plantar surface of the hindpaw were observed [9]. Other studies similarly failed to demonstrate hypoalgesia to heat following cisplatin administration [10,76]. These findings are largely consistent with the observation that TRPV1−/− mice develop cisplatin-evoked mechanical allodynia, but fail to develop hypersensitivity to heat using a radiant heat assay [65]. Both native mouse trigeminal ganglia (cisplatin 5 days treatment- 5 days of rest for 2 cycles: cumulative dose 23 mg/kg) and cultured rat DRG neurons (upregulation from cisplatin treatment (6.7 μM) after 6, 24 and 48 h) treated with cisplatin showed increases in both TRPV1 and TRPA1 mRNA expression [65]. In our study, we administered a lower cumulative dose of cisplatin (9 mg/kg i.p. over 3 weeks in our study vs. 23 mg/kg over 20 days [65]), which also failed to produce reliable changes in TRPV1 or TRPA1 expression in lumbar DRG. In other studies, a single injection of cisplatin or oxaliplatin (2 mg/kg i.v. by tail vein) produced a reduction in paw-withdrawal latency (i.e. hyperalgesia) to radiant heat stimulation [73] using other dosing paradigms. Cisplatin (1 mg/kg ip for 3 consecutive days) transiently increases paw withdrawal latencies (i.e produced hypoalgesia) compared to saline treated rats from day 3 to day 7 post initial cisplatin dosing [11]; decreased withdrawal latencies to heat were not observed after the third injection of cisplatin in other studies employing the dosing paradigm used by Cata and colleagues [10, 11]. However, cisplatin (1 mg/kg i.p. for 7 days) has also been shown to induce heat hyperalgesia in mice [54]. Thus, the dose and timing of cisplatin doses and species employed (mouse vs. rat) may contribute to the presence (or absence) of observed changes in heat thresholds and changes in TRPV1/TRPA1 expression. In our study, we attempted to closely mimic the clinical situation in which humans receive cycles (i.e. discontinuous) of cisplatin at regular intervals [77,78].
Few studies have evaluated effects of cannabinoids in chemotherapy-induced neuropathic pain models. The mixed CB1 and CB2 receptor agonist WIN55,212-2 attenuates mechanical allodynia induced by paclitaxel [40], vincristine [41] and cisplatin [42] treatment. More recently, CB2 agonists (AM1710, AM1714, (R,S)-AM1241, (R)-AM1241, MDA7 and MDA19) have been shown to alleviate mechanical allodynia in paclitaxel [43–45,93], vincristine [41] and cisplatin [93]-induced neuropathies. Surprisingly, no prior study has compared effects of FAAH and MGL inhibition on chemotherapy-induced peripheral neuropathy. More work is necessary to characterize the impact of FAAH and MGL inhibitors on nociceptor excitability in the cisplatin model and determine whether these agents also suppress neurotoxic effects of chemotherapy that result in aberrant changes in neuronal excitability. Thus, it is noteworthy that URB597 have recently been shown to normalized cisplatin-induced decrease in conduction velocity of Aα/Aβ fibers and reduced increases in ATF-3 and TRPV1 immunoreactivity in DRG neurons in a different cisplatin-dosing paradigm [54].
Although FAAH and MGL inhibition alleviate neuropathic pain resulting from surgically-induced traumatic nerve injury (for review see [26,27]), the impact of inhibiting endocannabinoid hydrolysis on chemotherapy-induced neuropathy has remained relatively unexplored (but see [54]). Our study is the first to demonstrate that MGL (JZL184) inhibitors as well as brain permeant and impermeant inhibitors of FAAH (URB937, URB597) suppress chemotherapy-induced neuropathic pain. Quite interestingly, the peripherally-restricted FAAH inhibitor, URB937, was as efficacious in suppressing cisplatin-evoked mechanical and cold allodynia as URB597. Both of these FAAH inhibitors produce their antinociceptive effects through a CB1/CB2 mechanism of action consistent with the pattern of pharmacological specificity observed following URB597 treatment in surgically-induced neuropathic pain models [47,52,53]. It is thus important to emphasize that anandamide, a product of FAAH inhibition, in addition to acting as an endocannabinoid at CB1 receptors also acts as an endovanilloid at TRPV1 receptors [79,94]. Thus, it is noteworthy that the anti-allodynic efficacy of FAAH (URB597 and URB937) inhibitors, but not MGL inhibitors, is additionally dependent upon TRPV1 receptors. Meanwhile, few studies have evaluated the implication of TRPV1 receptors in the antinociceptive effects of URB597 following acute [95,96], inflammatory [96] and neuropathic [54,94,97] pain models. In our study, anti-allodynic efficacy of FAAH and MGL inhibitors did not require TRPA1 receptors because the TRPA1 antagonist HC030031 (8 mg/kg i.p.) did not suppress anti-allodynic efficacy of either agent. Furthermore, in our study, mRNA levels of TRPV1 and TRPA1 were not reliably altered in either lumbar spinal cord or DRG following cisplatin treatment (rats killed at 16 days post initiation of cisplatin dosing). These observations suggest the absence of long term transcriptional changes in TRPV1 and TRPA1 mRNAs, at the level of the lumbar spinal cord and DRG, in the present cisplatin (3 mg/kg i.p./weekly for 3 weeks: cumulative dose 9 mg/kg) dosing paradigm. Our findings further suggest that transcriptional regulation of TRPV1 and TRPA1 mRNAs is not a universal effect of cisplatin treatment but, rather, depends on the dose and frequency of cisplatin injections, and time points and species evaluated. It is possible that increased expression of TRPV1 would, in fact, be observed with a different cisplatin dosing paradigm that altered sensitivity to heat. More work is necessary to characterize the time course (i.e early vs. late) of changes in transcriptional regulation of these markers during the development of cisplatin-evoked neuropathy.
Whereas FAAH inhibitors attenuated cisplatin-evoked neuropathy through cannabinoid (CB1 and CB2) and TRPV1-dependent mechanisms, the MGL inhibitor suppressed established mechanical and cold allodynia through cannabinoid CB1 and CB2 dependent mechanisms only. In our study, the MGL inhibitor JZL184 also completely reversed cisplatin-evoked mechanical and cold allodynia to pre-cisplatin levels. The antiallodynic effects of JZL184 were also mediated selectively by CB1 and CB2 receptor mechanisms as neither TRPV1 (AMG9810) nor TRPA1 (HC030031) antagonists blocked the anti-allodynic effects of JZL184 after the neuropathy was fully established. More work is necessary to characterize the impact of these treatments on the development of cispalin-evoked neuropathy. Our study is the first to demonstrate a role for TRPV1 receptors in the anti-allodynic effects of brain permeant and impermeant inhibitors of FAAH (i.e. URB597 and URB937, respectively) on cisplatin-evoked allodynia. In contrast to the cisplatin model, JZL184 produced a CB1-mediated suppression of behavioral hypersensitivities (mechanical and cold) following chronic constriction injury of the sciatic nerve [46,47]. Moreover, in inflammatory pain models, peripheral antinociceptive effects of JZL184 are mediated by both CB1 and CB2 cannabinoid receptors [57,58]. Thus, it appears that endocannabinoid modulators recruit CB1, CB2 as well as TRPV1 mechanisms to attenuate chemotherapy-induced peripheral neuropathy. Peripheral nerve injury and chemotherapy-induced neuropathy have both been linked to an inflammatory component [80,81]. However, in our study, inflammatory mediators such as PGE2 and PGF2α levels were not reliably increased in either the lumbar spinal cord or dorsal hind paw skin by cisplatin treatment. Recent findings suggest that cisplatin may decrease the expression of cyclooxygenase-2 (COX-2), thereby reducing PGE2 levels [82,83]. Such effects may reduce oxidative metabolism of endocannabinoids by COX-2 [84], further enhancing the anti-allodynic efficacy of FAAH and MGL inhibitors through actions at CB1 and CB2 (and in the case of FAAH inhibitors TRPV1) receptors.
It is important to emphasize that inhibitors of FAAH and MGL are not specific for the endocannabinoid system, but also inhibit hydrolysis of other fatty-acid amides and monoacylglycerols that do not bind to cannabinoid receptors. Endocannabinoids (e.g. AEA and 2-AG) serve as substrates for metabolism by a complex array of enzymes (cyclooxygenase, lipoxygenase, P450 hydrolase, P450 epoxygenase and others) [85–88]. Although oxidative metabolites of endocannabinoids produce physiological products independent of the cannabinoid system, effects of FAAH and MGL inhibitors in our study were blocked completely by either CB1 or CB2 antagonists, and in the case of FAAH (URB597, URB937) inhibitors also by TRPV1 antagonists. TRPA1 antagonists did not alter anti-allodynic efficacy of either FAAH or MGL inhibitors. Our results raise the possibility that a better understanding of the pathways controlling in vivo metabolism of endocannabinoids may be exploited to further optimize the therapeutic potential of the endocannabinoid signalling system through drug discovery efforts focused on directed multi-targeting [85,86,88].
Morphine, gabapentin and tricyclic antidepressants (TCA) are known to relieve chemotherapy-evoked pain in animal models [14,15,17,89,90] and in humans [4,19,22]. Interestingly, in our study, morphine fully reversed mechanical and cold allodynia with efficacy comparable to either FAAH (URB937, URB597) or MGL (JZL184) inhibitors, whereas gabapentin only partially reversed cisplatin-evoked mechanical and cold allodynia. Amitriptyline, administered acutely, failed to produce antinociceptive effects at any time point. The failure of anti-allodynic effects of amitriptyline following acute administration has also been observed in models of neuropathy produced by traumatic nerve injury [91,92] and paclitaxel [17] treatment. In our studies, endocannabinoid modulators were effective in normalizing responses to mechanical and cold stimulation to pre-cisplatin levels following acute administration and did not alter responses to the same stimuli in the absence of chemotherapy treatment. These observations suggest that inhibitors of FAAH and MGL could represent first line treatments for chemotherapy-induced neuropathy.
5. Conclusions
The present study provides evidence that inhibition of FAAH (URB937, URB597) and MGL (JZL184) suppresses cisplatin-evoked mechanical and cold allodynia. A brain impermeant inhibitor of FAAH was as efficacious as a brain permeant inhibitor of either FAAH or MGL. Moreover, endocannabinoid modulators were effective following acute administration, exhibiting efficacy comparable to morphine and superior to that of gabapentin, administered via the same route. In all cases, the anti-allodynic effects of FAAH and MGL inhibitors were mediated by cannabinoid CB1 and CB2 receptors, but not TRPA1 receptors. In the case of FAAH inhibitors, anti-allodynic efficacy of both brain permeable and impermeable inhibitors was additionally mediated by TRPV1 receptors. Our studies also suggest that cisplatin treatment selectively decreases 2-AG content in the dorsal hind paw, but increases levels of both 2-AG and AEA in the lumbar spinal cord, possibly as an adaptive central response to peripheral injury induced by chemotherapy. These observations support the effectiveness of FAAH and MGL inhibitors in reversing established cisplatin-induced peripheral neuropathic pain by elevating and/or reinstating endocannabinoid levels. Modulation of the endocannabinoid system through inhibition of FAAH and MGL, thus, represents a promising approach to suppress chemotherapy-induced neuropathic pain. Our findings are the first to provide evidence that pharmacological inhibition of MGL and FAAH modulates the endocannabinoid system to block the maintenance of chemotherapy-induced neuropathic pain. More work is necessary to determine whether inhibition of endocannabinoid degradation represents an effective strategy for suppressing chemotherapy-induced neuropathy in cancer patients.
Acknowledgments
Supported by DA021644 and DA028200 (to AGH).
Abbreviations
- 2-AG
2-arachidonoyl glycerol
- AEA
anandamide
- ANOVA
analysis of variance
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
- CNS
central nervous system
- COX-2
cyclooxygenase-2
- Δ9-THC
delta 9-tetrahydrocannabinol
- DMSO
dimethylsulfoxide
- DRG
dorsal root ganglion
- FAAH
fatty-acid amide hydrolase
- HPLC
high performance liquid chromatography
- i.p
intraperitoneal
- LC/MS
liquid chromatography-mass spectrometry
- MGL or MAGL
monoacylglycerol lipase
- OEA
oleoylethanolamide
- PEA
palmitoylethanolamide
- PGE2
prostaglandin E2
- PGF2α
prostaglandin F2 alpha
- s.c
subcutaneous
- RT-PCR
reverse transcription polymerase chain reaction
- TRPA1
Transient Receptor Potential A1 channel
- TRPV1
Transient Receptor Potential V1 channel
Footnotes
Conflict of interest
The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alberts DS, Noel JK. Cisplatin-associated neurotoxicity: can it be prevented? Anticancer Drugs. 1995;6:369–83. doi: 10.1097/00001813-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 3.Polomano RP, Bennett GJ. Chemotherapy-evoked painful peripheral neuropathy. Pain Med. 2001;2:8–14. doi: 10.1046/j.1526-4637.2001.002001008.x. [DOI] [PubMed] [Google Scholar]
- 4.Verstappen CC, Heimans JJ, Hoekman K, Postma TJ. Neurotoxic complications of chemotherapy inpatients with cancer: Clinical signs and optimal management. Drugs. 2003;63:1549–63. doi: 10.2165/00003495-200363150-00003. [DOI] [PubMed] [Google Scholar]
- 5.Dougherty PM, Cata JP, Cordella JV, Burton A, Weng HR. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:32–42. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 6.Perry MC. Companion Handbook to the Chemotherapy Sourcebook. Baltimore: Williams & Wilkins; 1999. [Google Scholar]
- 7.Boyiadzis MM, Lebowitz PF, Frame JN, Fojo T. Hematology-Oncology Therapy. New York: McGraw Hill, Medical Publishing Division; 2007. [Google Scholar]
- 8.Apfel SC, Arezzo JC, Lipson L, Kessler JA. Nerve growth factor prevents experimental cisplatin neuropathy. Ann Neurol. 1992;31:76–80. doi: 10.1002/ana.410310114. [DOI] [PubMed] [Google Scholar]
- 9.Authier N, Fialip J, Eschalier A, Coudoré F. Assessment of allodynia and hyperalgesia after cisplatin administration to rats. Neurosci Lett. 2000;291:73–6. doi: 10.1016/s0304-3940(00)01373-2. [DOI] [PubMed] [Google Scholar]
- 10.Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. An animal model of nociceptive peripheral neuropathy following repeated cisplatin injections. Exp Neurol. 2003;182:12–20. doi: 10.1016/s0014-4886(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 11.Cata JP, Weng HR, Dougherty PM. Behavioral and electrophysiological studies in rats with cisplatin-induced chemoneuropathy. Brain Res. 2008;1230:91–8. doi: 10.1016/j.brainres.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Authier N, Balayssac D, Marchand F, Ling B, Zangarelli A, Descoeur J, et al. Animal models of chemotherapy-evoked painful peripheral neuropathies. Neurotherapeutics. 2009;6:620–9. doi: 10.1016/j.nurt.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchand F, Alloui A, Pelissier T, Hernández A, Authier N, Alvarez P, et al. Evidence for an antihyperalgesic effect of venlafaxine in vincristine-induced neuropathy in rat. Brain Res. 2003;980:117–20. doi: 10.1016/s0006-8993(03)02946-9. [DOI] [PubMed] [Google Scholar]
- 14.Lynch JJ, 3rd, Wade CL, Zhong CM, Mikusa JP, Honore P. 2004 Attenuation of mechanical allodynia by clinically utilized drugs in a rat chemotherapy-induced neuropathic pain model. Pain. 2004;110:56–63. doi: 10.1016/j.pain.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Ling B, Authier N, Balayssac D, Eschalier A, Coudore F. Behavioral and pharmacological description of oxaliplatin-induced painful neuropathy in rat. Pain. 2007;128:225–34. doi: 10.1016/j.pain.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 16.Xiao W, Boroujerdi A, Bennett GJ, Luo ZD. Chemotherapy-evoked painful peripheral neuropathy: analgesic effects of gabapentin and effects on expression of the alpha-2-delta type-1 calcium channel subunit. Neuroscience. 2007;144:714–20. doi: 10.1016/j.neuroscience.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao W, Naso L, Bennett GJ. Experimental studies of potential analgesics for the treatment of chemotherapy-evoked painful peripheral neuropathies. Pain Med. 2008;9:505–17. doi: 10.1111/j.1526-4637.2007.00301.x. [DOI] [PubMed] [Google Scholar]
- 18.Gauchan P, Andoh T, Ikeda K, Fujita M, Sasaki A, Kato A, et al. Mechanical allodynia induced by paclitaxel, oxaliplatin and vincristine: different effectiveness of gabapentin and different expression of voltage-dependent calcium channel alpha(2)delta-1 subunit. Biol Pharm Bull. 2009;32:732–4. doi: 10.1248/bpb.32.732. [DOI] [PubMed] [Google Scholar]
- 19.Uhm JH, Yung WK. Neurologic Complications of Cancer Therapy. Curr Treat Options Neurol. 1999;1:428–37. doi: 10.1007/s11940-996-0006-x. [DOI] [PubMed] [Google Scholar]
- 20.Bosnjak S, Jelic S, Susnjar S, Luki V. Gabapentin for relief of neuropathic pain related to anticancer treatment: a preliminary study. J Chemother. 2002;14:214–9. doi: 10.1179/joc.2002.14.2.214. [DOI] [PubMed] [Google Scholar]
- 21.Caraceni A, Zecca E, Bonezzi C, Arcuri E, Yaya Tur R, Maltoni M, et al. Gabapentin for neuropathic cancer pain: a randomized controlled trial from the Gabapentin Cancer Pain Study Group. J Clin Oncol. 2004;22:2909–17. doi: 10.1200/JCO.2004.08.141. [DOI] [PubMed] [Google Scholar]
- 22.Pachman DR, Barton DL, Watson JC, Loprinzi CL. Chemotherapy-induced peripheral neuropathy: prevention and treatment. Clin Pharmacol Ther. 2011;90:377–87. doi: 10.1038/clpt.2011.115. [DOI] [PubMed] [Google Scholar]
- 23.Bae SK, Yang KH, Aryal DK, Kim YG, Lee MG. Pharmacokinetics of amitriptyline and one of its metabolites, nortriptyline, in rats: little contribution of considerable hepatic first-pass effect to low bioavailability of amitriptyline due to great intestinal first-pass effect. J Pharm Sci. 2009;98:1587–601. doi: 10.1002/jps.21511. [DOI] [PubMed] [Google Scholar]
- 24.Aryal B, Tae-Hyun K, Yoon-Gyoon K, Hyung-Gun K. A comparative study of the pharmacokinetics of traditional and automated dosing/blood sampling systems using gabapentin. Indian J Pharmacol. 2011;43:262–9. doi: 10.4103/0253-7613.81512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Gregori S, De Gregori M, Ranzani GN, Allegri M, Minella C, Regazzi M. Morphine metabolism, transport and brain disposition. Metab Brain Dis. 2012;27:1–5. doi: 10.1007/s11011-011-9274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS Neurol Disord Drug Targets. 2009;8:403–21. doi: 10.2174/187152709789824660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics. 2009;6:713–37. doi: 10.1016/j.nurt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–7. [Google Scholar]
- 29.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–9. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 30.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 31.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 32.Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–13. [PubMed] [Google Scholar]
- 33.Matsuda L, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–4. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 34.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–5. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 35.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–7. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 36.Goparaju SK, Ueda N, Taniguchi K, Yamamoto S. Enzymes of porcine brain hydrolyzing 2-arachidonoylglycerol, an endogenous ligand of cannabinoid receptors. Biochem Pharmacol. 1999;57:417–23. doi: 10.1016/s0006-2952(98)00314-1. [DOI] [PubMed] [Google Scholar]
- 37.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002;99:10819–24. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–56. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Ueda N. Biology of endocannabinoid synthesis system. Prostaglandins Other Lipid Mediat. 2009;89:112–9. doi: 10.1016/j.prostaglandins.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Pascual D, Goicoechea C, Suardíaz M, Martín MI. A cannabinoid agonist, WIN 55,212-2, reduces neuropathic nociception induced by paclitaxel in rats. Pain. 2005;118:23–34. doi: 10.1016/j.pain.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Rahn EJ, Makriyannis A, Hohmann AG. Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. Br J Pharmacol. 2007;152:765–77. doi: 10.1038/sj.bjp.0707333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vera G, Chiarlone A, Cabezos PA, Pascual D, Martín MI, Abalo R. WIN 55,212-2 prevents mechanical allodynia but not alterations in feeding behaviour induced by chronic cisplatin in the rat. Life Sci. 2007;81:468–79. doi: 10.1016/j.lfs.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 43.Naguib M, Diaz P, Xu JJ, Astruc-Diaz F, Craig S, Vivas-Mejia P, et al. MDA7: a novel selective agonist for CB2 receptors that prevents allodynia in rat neuropathic pain models. Br J Pharmacol. 2008;155:1104–16. doi: 10.1038/bjp.2008.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rahn EJ, Zvonok AM, Thakur GA, Khanolkar AD, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB2 receptors suppresses neuropathic nociception induced by treatment with the chemotherapeutic agent paclitaxel in rats. J Pharmacol Exp Ther. 2008;327:584–91. doi: 10.1124/jpet.108.141994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu JJ, Diaz P, Astruc-Diaz F, Craig S, Munoz E, Naguib M. Pharmacological characterization of a novel cannabinoid ligand, MDA19, for treatment of neuropathic pain. Anesth Analg. 2010;111:99–109. doi: 10.1213/ANE.0b013e3181e0cdaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinsey SG, Long JZ, O’Neal ST, Abdullah RA, Poklis JL, Boger DL, et al. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther. 2009;330:902–10. doi: 10.1124/jpet.109.155465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kinsey SG, Long JZ, Cravatt BF, Lichtman AH. Fatty acid amide hydrolase and monoacylglycerol lipase inhibitors produce anti-allodynic effects in mice through distinct cannabinoid receptor mechanisms. J Pain. 2010;11:1420–8. doi: 10.1016/j.jpain.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, et al. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci. 2010;13:1265–70. doi: 10.1038/nn.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sciolino NR, Zhou W, Hohmann AG. Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol Res. 2011;64:226–34. doi: 10.1016/j.phrs.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yapa U, Prusakiewicz JJ, Wrightstone AD, Christine LJ, Palandra J, Groeber E, et al. High-performance liquid chromatography-tandem mass spectrometry assay of fatty acid amide hydrolase (FAAH) in blood: FAAH inhibition as clinical biomarker. Anal Biochem. 2012;421:556–65. doi: 10.1016/j.ab.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 51.Maione S, De Petrocellis L, de Novellis V, Moriello AS, Petrosino S, Palazzo E, et al. Analgesic actions of N-arachidonoyl-serotonin, a fatty acid amide hydrolase inhibitor with antagonistic activity at vanilloid TRPV1 receptors. Br J Pharmacol. 2007;150:766–81. doi: 10.1038/sj.bjp.0707145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russo R, Loverme J, La Rana G, Compton TR, Parrott J, Duranti A, et al. The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3′-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J Pharmacol Exp Ther. 2007;322:236–42. doi: 10.1124/jpet.107.119941. [DOI] [PubMed] [Google Scholar]
- 53.Desroches J, Guindon J, Lambert C, Beaulieu P. Modulation of the anti-nociceptive effects of 2-arachidonoyl glycerol by peripherally administered FAAH and MGL inhibitors in a neuropathic pain model. Br J Pharmacol. 2008;155:913–24. doi: 10.1038/bjp.2008.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khasabova IA, Khasabov S, Paz J, Harding-Rose C, Simone DA, Seybold VS. Cannabinoid type-1 receptor reduces pain and neurotoxicity produced by chemotherapy. J Neurosci. 2012;32:7091–101. doi: 10.1523/JNEUROSCI.0403-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 56.Guindon J, Desroches J, Dani M, Beaulieu P. Pre-emptive antinociceptive effects of a synthetic cannabinoid in a model of neuropathic pain. Eur J Pharmacol. 2007;568:173–6. doi: 10.1016/j.ejphar.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 57.Guindon J, Guijarro A, Piomelli D, Hohmann AG. 2011 Peripheral antinociceptive effects of inhibitors of monoacylglycerol lipase in a rat model of inflammatory pain. Br J Pharmacol. 2011;163:1464–78. doi: 10.1111/j.1476-5381.2010.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spradley JM, Guindon J, Hohmann AG. Inhibitors of monoacylglycerol lipase, fatty-acid amide hydrolase and endocannabinoid transport differentially suppress capsaicin-induced behavioral sensitization through peripheral endocannabinoid mechanisms. Pharmacol Res. 2010;62:249–58. doi: 10.1016/j.phrs.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okun A, Liu P, Davis P, Ren J, Remeniuk B, Brion T, et al. Afferent drive elicits ongoing pain in a model of advanced osteoarthritis. Pain. 2012;153:924–33. doi: 10.1016/j.pain.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–76. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 61.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 62.Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 63.Bradshaw HB, Rimmerman N, Krey JF, Walker JM. Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am J Physiol Regul Integr Comp Physiol. 2006;291:R349–58. doi: 10.1152/ajpregu.00933.2005. [DOI] [PubMed] [Google Scholar]
- 64.Serrano A, Rivera P, Pavon FJ, Decara J, Suárez J, Rodriguez de Fonseca F, et al. Differential effects of single versus repeated alcohol withdrawal on the expression of endocannabinoid system-related genes in the rat amygdala. Alcohol Clin Exp Res. 2012;36:984–94. doi: 10.1111/j.1530-0277.2011.01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ta LE, Bieber AJ, Carlton SM, Loprinzi CL, Low PA, Windebank AJ. Transient Receptor Potential Vanilloid 1 is essential for cisplatin-induced heat hyperalgesia in mice. Mol Pain. 2010;6:1–15. doi: 10.1186/1744-8069-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Draper NR, Smith H. Applied regression analysis. New York: Wiley; 1998. [Google Scholar]
- 67.Rahn EJ, Zvonok AM, Makriyannis A, Hohmann AG. Antinociceptive effects of racemic AM1241 and its chirally synthesized enantiomers: lack of dependence upon opioid receptor activation. AAPS J. 2010;12:147–57. doi: 10.1208/s12248-009-9170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitrirattanakul S, Ramakul N, Guerrero AV, Matsuka Y, Ono T, Iwase H, et al. Site-specific increases in peripheral cannabinoid receptors and their endogenous ligands in a model of neuropathic pain. Pain. 2006;126:102–14. doi: 10.1016/j.pain.2006.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clapper JR, Vacondio F, King AR, Duranti A, Tontini A, Silva C, et al. A second generation of carbamate-based fatty acid amide hydrolase inhibitors with improved activity in vivo. Chem Med Chem. 2009;4:1505–13. doi: 10.1002/cmdc.200900210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Long JZ, Nomura DK, Cravatt BF. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol. 2009;16:744–53. doi: 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vera G, Chiarlone A, Martín MI, Abalo R. Altered feeding behaviour induced by long-term cisplatin in rats. Auton Neurosci. 2006;126–127:81–92. doi: 10.1016/j.autneu.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 73.Joseph EK, Levine JD. Comparison of oxaliplatin- and cisplatin-induced painful peripheral neuropathy in the rat. J Pain. 2009;10:534–41. doi: 10.1016/j.jpain.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nassini R, Gees M, Harrison S, De Siena G, Materazzi S, Moretto N, et al. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain. 2011;152:1621–31. doi: 10.1016/j.pain.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 75.Joseph EK, Chen X, Bogen O, Levine JD. Oxaliplatin acts on IB4-positive nociceptors to induce an oxidative stress-dependent acute painful peripheral neuropathy. J Pain. 2008;9:463–72. doi: 10.1016/j.jpain.2008.01.335. [DOI] [PubMed] [Google Scholar]
- 76.Ta LE, Low PA, Windebank AJ. Mice with cisplatin and oxaliplatin-induced painful neuropathy develop distinct early responses to thermal stimuli. Mol Pain. 2009;5:1–9. doi: 10.1186/1744-8069-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perry MC. Companion Handbook to the Chemotherapy Sourcebook. Baltimore: Williams & Wilkins; 1999. [Google Scholar]
- 78.Bruijnincx PC, Sadler PJ. New trends for metal complexes with anticancer activity. Curr Opin Chem Biol. 2008;12:197–206. doi: 10.1016/j.cbpa.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Starowicz K, Nigam S, Di Marzo V. Biochemistry and pharmacology of endovanilloids. Pharmacol Ther. 2007;114:13–33. doi: 10.1016/j.pharmthera.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 80.Bennett GJ. Pathophysiology and animal models of cancer-related painful peripheral neuropathy. Oncologist. 2010;15 (Suppl 2):9–12. doi: 10.1634/theoncologist.2009-S503. [DOI] [PubMed] [Google Scholar]
- 81.Calvo M, Bennett DL. The mechanisms of microgliosis and pain following peripheral nerve injury. Exp Neurol. 2012;234:271–82. doi: 10.1016/j.expneurol.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 82.Tusgaard B, Norregaard R, Jensen AM, Wang G, Topcu SO, Wang Y, et al. Cisplatin decreases renal cyclooxygenase-2 expression and activity in rats. Acta Physiol (Oxf) 2011;202:79–90. doi: 10.1111/j.1748-1716.2011.02257.x. [DOI] [PubMed] [Google Scholar]
- 83.Yokoyama Y, Xin B, Shigeto T, Mizunuma H. Combination of ciglitazone, a peroxisome proliferator-activated receptor gamma ligand, and cisplatin enhances the inhibition of growth of human ovarian cancers. J Cancer Res Clin Oncol. 2011;137:1219–28. doi: 10.1007/s00432-011-0993-1. [DOI] [PubMed] [Google Scholar]
- 84.Kozak KR, Prusakiewicz JJ, Marnett LJ. Oxidative metabolism of endocannabinoids by COX-2. Curr Pharm Des. 2004;10:659–67. doi: 10.2174/1381612043453081. [DOI] [PubMed] [Google Scholar]
- 85.Ueda N, Yamamoto K, Yamamoto S, Tokunaga T, Shirakawa E, Shinkai H, et al. Lipoxygenase-catalyzed oxygenation of arachidonylethanolamide, a cannabinoid receptor agonist. Biochim Biophys Acta. 1995;1254:127–34. doi: 10.1016/0005-2760(94)00170-4. [DOI] [PubMed] [Google Scholar]
- 86.Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem. 1997;272:21181–6. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]
- 87.Kozak KR, Marnett LJ. Oxidative metabolism of endocannabinoids. Prostaglandins Leukot Essent Fatty Acids. 2002;66:211–20. doi: 10.1054/plef.2001.0359. [DOI] [PubMed] [Google Scholar]
- 88.Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334:809–13. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nozaki-Taguchi N, Chaplan SR, Higuera ES, Ajakwe RC, Yaksh TL. Vincristine-induced allodynia in the rat. Pain. 2001;93:69–76. doi: 10.1016/S0304-3959(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 90.Flatters SJ, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain. 2004;109:150–61. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 91.Esser MJ, Sawynok J. Acute amitriptyline in a rat model of neuropathic pain: differential symptom and route effects. Pain. 1999;80:643–53. doi: 10.1016/S0304-3959(98)00261-9. [DOI] [PubMed] [Google Scholar]
- 92.Walczak JS, Pichette V, Leblond F, Desbiens K, Beaulieu P. Behavioral, pharmacological and molecular characterization of the saphenous nerve partial ligation: a new model of neuropathic pain. Neuroscience. 2005;132:1093–102. doi: 10.1016/j.neuroscience.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 93.Deng L, Guindon J, Vemuri VK, Thakur GA, White FA, Makriyannis A, et al. The maintenance of cisplatin- and paclitaxel-induced mechanical and cold allodynia is suppressed by cannabinoid CB2 receptor activation and independent of CXCR4 signaling in models of chemotherapy-induced peripheral neuropathy. Mol Pain. 2012;8:71. doi: 10.1186/1744-8069-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Starowicz K, Makuch W, Osikowicz M, Piscitelli F, Petrosino S, Di Marzo V, et al. Spinal anandamide produces analgesia in neuropathic rats: possible CB(1)- and TRPV1-mediated mechanisms. Neuropharmacology. 2012;62:1746–55. doi: 10.1016/j.neuropharm.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 95.Maione S, Bisogno T, de Novellis V, Palazzo E, Cristino L, Valenti M, et al. Elevation of endocannabinoid levels in the ventrolateral periaqueductal grey through inhibition of fatty acid amide hydrolase affects descending nociceptive pathways via both cannabinoid receptor type 1 and transient receptor potential vanilloid type-1 receptors. J Pharmacol Exp Ther. 2006;316:969–82. doi: 10.1124/jpet.105.093286. [DOI] [PubMed] [Google Scholar]
- 96.de Novellis V, Palazzo E, Rossi F, De Petrocellis L, Petrosino S, Guida F, et al. The analgesic effect of N-arachidonoyl-serotonin, a FAAH inhibitor and TRPV1 receptor antagonist, associated with changes in rostral ventromedial medulla and locus coeruleus cell activity in rats. Neuropharmacology. 2008;55:1105–13. doi: 10.1016/j.neuropharm.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 97.de Novellis V, Vita D, Gatta L, Luongo L, Bellini G, De Chiaro M, et al. The blockade of the transient receptor potential vanilloid type 1 and fatty acid amide hydrolase decreases symptoms and central sequelae in the medial prefrontal cortex of neuropathic rats. Mol Pain. 2011;7:7. doi: 10.1186/1744-8069-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]