Abstract
Objective
High-fat diets result in increased body weight. However, this is not uniform and determining the factors that make some animals or individual more susceptible to this diet-induced weight gain is a critical research question. The expansion of white adipose tissue (WAT) associated with weight gain requires high rates of angiogenesis to support the expanding tissue mass. We hypothesized that diet-induced obese (DIO) mice have a greater capacity for WAT angiogenesis and remodeling than diet-resistant (DR) mice at a young age, prior to age or diet-induced obesity.
Design
We measured body weight and body composition by NMR. We compared the expression of genes related to lipid metabolism, angiogenesis and inflammation by RT-qPCR and PCR arrays. WAT morphology and distribution of adipocyte size were analyzed. The level of hypoxia and vascular density was assessed by immunohistochemistry in WAT of young mice.
Results
C57Bl/6 mice were DIO and FVB/N mice DR after 8 weeks on a low fat diet or high fat diet (HFD). However, C57Bl/6 mice had lower body weight, lower adiposity, smaller adipocytes and decreased leptin and lipogenic genes expression in AT than FVB/N mice at 9 weeks of age on a chow diet. Despite having smaller adipocytes, the level of hypoxia and the expression of pro-angiogenesis genes were higher in WAT of young C57Bl/6 mice than young FVB/N mice. In addition, expression of genes related to macrophages and their recruitment, and to proinflammatory cytokines, was significantly higher in WAT of young C57Bl/6 mice than young FVB/N mice.
Conclusion
These data suggest that the potential for WAT remodeling in early period of growth is higher in C57Bl/6 mice as compared to FVB/N mice and we hypothesize that it may contribute to the increased susceptibility to DIO of C57Bl/6 mice.
Keywords: adipose tissue, angiogenesis, inflammation, hypoxia, diet-induced obesity, diet-resistant, body weight, high-fat diet
Introduction
Obesity is a complex and multifactorial disease. Early identification of obesity-prone humans may provide a strategy for preventing or reducing the incidence and severity of the disorder. While it is clear that environmental factors such as consumption of calorically-dense, high-fat diets (HFDs) can lead to increased adiposity, this response is not uniform. Rodent experiments make it clear that even when exposed to the same obesogenic diet and housed under the same conditions, there are important genetic differences that contribute to the susceptibility to gain weight on a HFD.
Modulation of genes related to thermogenesis, such as UCP-1, affects the development of diet-induced obesity (DIO) in mice,1 and hypothalamic neural circuits involved with regulation of energy balance are altered in offspring of DIO rats.2 Many hypotheses have been offered to explain the differences between DIO and diet-resistant (DR) animals. One common hypothesis is that differences in the key CNS circuits that regulate energy balance differ between DIO and DR animals. Consistent with this, there is an inborn genetic disposition to develop leptin or insulin resistance, altered glucose sensing, and disturbed metabolism of norepinephrine, serotonin, and dopamine in the hypothalamus of DIO rats.2
An alternative hypothesis is that a key difference between DIO and DR animals relates to the ability of white adipose tissue (WAT) to accommodate the storage of increased calorie intake. Analogous to tumors, WAT has the capability to quickly expand and proliferate throughout adulthood. Also similar to tumors, such expansion is highly dependent on the ability to generate new blood vessels3, 4 and as a result, WAT is highly vascularized and has angiogenic properties5 that are critical to its ability to expand.3, 4 Interestingly, treatments that inhibit WAT angiogenesis potently (but reversibly) reduce body weight and fat mass in rodents and non-human primates, indicating that WAT integrity and sustained weight gain depends on maintaining WAT vasculature.4, 6, 7
WAT angiogenesis is highly correlated with adipogenesis, inflammation and the need for ongoing tissue remodeling. Many different cell types in WAT, including mature adipocytes, preadipocytes, pericytes, endothelial cells and inflammatory cells, are involved in the capacity for WAT to remodel itself under different circumstances.3, 8 Obese rodents and humans have elevated levels of angiogenic factors and macrophages in their WAT.9, 10 Such low-grade inflammation is progressively increased in WAT of mice fed a HFD and these changes have been hypothesized to contribute to obesity and insulin resistance.11 The surface area of adipocytes reflects their volume and is positively correlated with the number of macrophages.11,12 As obesity develops, there is a switch to a more inflammatory phenotype in macrophages in WAT.13
In the present work, we hypothesized that at a young age and before exposure to a HFD, the WAT of mice that are susceptible to weight gain on a HFD have increased capacity for tissue expansion, including the capability of rapid angiogenesis, and that this capacity may contribute to the enhanced susceptibility to develop obesity. To this end, we compared the ability of two mouse strains to gain weight and undergo WAT remodeling when fed a HFD. While C57Bl/6 (C57) mice gained substantially more weight and fat on the HFD than FVB/N (FVB) mice, the latter being almost completely resistant to weight gain on the HFD. Consistent with this, the capacity for WAT tissue remodeling was far greater in C57 compared to FVB mice, and this was the case prior to their exposure to the HFD.
Materials and methods
Animals
Male C57 mice and FVB mice obtained from Jackson Labs at 8 weeks of age were housed individually in standard mouse cages with a 12-h light/12-h dark schedule. Sixteen of each genotype had ad lib access to a high-fat diet providing 40% calories as fat (HFD, D03082706, Research diet, Inc.) or a low-fat diet with 4% fat (LFD, D03082705) for 8 weeks. In two separate experiments, a total of 16 mice of each genotype were fed a chow diet and sacrificed at 9 weeks of age after adaptation to the cage for 1 week for gene expression or histologic studies. All animal protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Body composition
Body composition was analyzed by an NMR (Echo Medical Systems) on Weeks 0, 8, and 12 in the first experiment, and at 9 weeks of age in the second study.
Adipocyte tissue morphometry
To determine adipocyte size, the cross-sectional area of adipocytes was determined on paraffin-embedded hematoxylin eosin-stained sections of epididymal fat at a magnification of 200× by image processing with custom-designed software. The method of determining adipocyte size was modified from previous papers using a computer image analysis.14 The distribution of adipocyte size was determined by relative frequencies of adipocytes having a size within a regular interval. The number of adipocytes was determined by dividing the total fat mass by the mean adipocyte size. A total 500 to 1200 adipocytes were used for the analysis of the mean adipocyte area and the distribution of adipocyte size for each mouse.14
Immunohistochemistry
To assess the degree of hypoxia in WAT, a Hypoxyprobe™-1 Plus Kit (Hypoxyprobe, Inc.) was used. Five mice in each group were intraperitoneally injected with 60 mg/kg of a Hypoxyprobe™-1 (pimonidazole HCl) 30 min before being sacrificed. Epididymal fat was fixed in alcoholic zinc formalin (Anatech LTD.) overnight. The AT was embedded in a paraffin in pairs containing one C57 and one FVB mouse each per block to minimize any differences that might arise during the process of immunohistochemistry and sectioned at the thickness of 5 μm. The immunohistochemistry was performed and a total of 200 images (5 images per section) were captured at a magnification of 100× (AxioVision 4.6 microscope; Carl Zeiss). To correlate hypoxia with adipocyte size, the brown color signifying hypoxia was selected by using HSL color system (Hue 0-25 and 253-255; Saturation 25-255; Luminescence 0-225) and the number and the cross-sectional area of adipocytes were concurrently measured in the same field with the software. The degree of hypoxia was determined by dividing the area of brown color by the number or the averaged cross-sectional area of adipocytes in the same field.
For the detection of PECAM1, sections were incubated overnight at 4 °C with a rat anti-mouse CD31 antibody (1:500; BD Pharmingen). After incubation with biotinylated anti-rat IgG (1:200; Jackson ImmunoResearch Laboratories), sections were revealed with avidin-biotin complex (1:500; Vector Laboratories). Sections were counterstained with hematoxylin and eosin. The numbers of adipocytes and vessels were analyzed by three independent individuals blinded to the experimental conditions.
PCR arrays
Mice (8 per condition) were sacrificed after a 1-h fast. The epididymal fat was quickly frozen in 2-methyl butane on dry ice. An RT2 First Strand Kit and RT2 Profiler™ PCR Array System (SABiosciences Corporation) was used to perform PCR arrays related to angiogenesis. Total RNA was isolated using RNAeasy columns (Qiagen) after processing with Tri-reagent (Molecular research center, Inc.). The first strand cDNA and the cocktail solutions of cDNA including RT2 qPCR Master Mix were prepared for each mouse. PCR arrays (eight in each group) were performed using an iCycler (Bio-rad Laboratories). The quality control tests for genomic DNA control, reverse transcription control, and positive PCR control of each array plates were all passed below the criteria. The array data were analyzed using RT2 Profiler PCR Array Data Analysis. The names of all genes are presented using official symbol of NCBI in this study (Figures and Supplemental table 2). Three housekeeping genes not influenced by the experimental condition (Hsp90ab1, Gapdh and Actb) were selected as an endogenous control. The normalization and analysis of data were performed according to the instructions (SABioscience Corporation).
Real-time quantitative PCR
Total RNA was isolated from epididymal fat of each mouse in the same way as above, and cDNA was synthesized using iScript (Bio-rad). PCR was performed in triplicate using an iCycler and the iQ SYBR Green Supermix (Bio-rad Laboratories) or 7900HT Fast Real-Time PCR system (Applied Biosystems) (Supplemental table 1).14 The expressions of each gene were normalized to constitutively expressed L32 and relative expression was quantified.14 The names of all genes were presented using official symbol of NCBI in this study (Figures and Supplemental table 2).
Statistics
All data are expressed as mean ± SEM. Data were analyzed by Student’s T-test or two-way repeated measures ANOVA followed by Bonferoni’s multiple comparisons using Prism 5.0.
Results
C57 mice are prone to diet-induced obesity whereas FVB mice are resistant
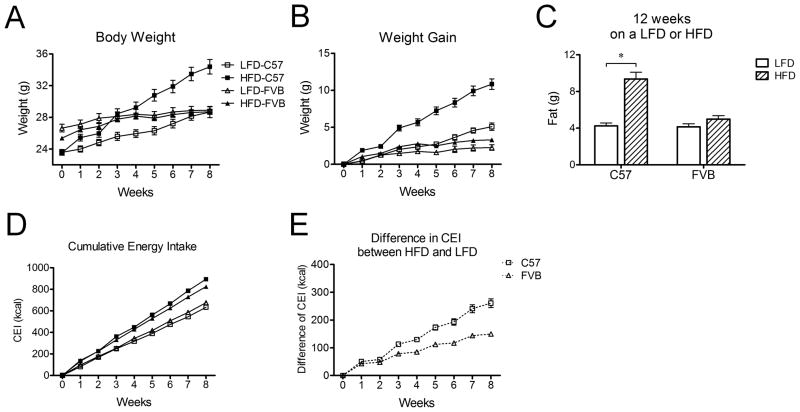
To test whether FVB mice are resistant to diet-induced obesity relative to C57 mice, we compared the change in body weight and energy intake by feeding both genotypes a low-fat diet (LFD) or high-fat diet (HFD) for 8 weeks. The body weight of C57 mice was lighter relative to FVB mice at 9 weeks of age at the start of the experiment (Figure 1A). C57 mice increased their weight by 20% after 8 weeks on the HFD compared to those on the LFD. In contrast, the FVB mice fed the HFD gained comparable weight as those on the LFD (Figure 1B). Likewise, the fat mass of C57 mice on a HFD was greater than that on a LFD while that of FVB mice on a HFD was not significantly different from that of LFD mice after both 5 and 12 weeks (Figure 1C). The difference in cumulative energy intake between LFD and HFD was significantly greater in C57 mice compared to FVB mice (Figure 1D and 1E). Given the lower energy expenditure in C57 mice reported in previous studies,15, 16 these data support a greater energy surplus in C57 mice relative to FVB mice. These data confirm the differential susceptibility to a HFD between DIO C57 and FVB mice.
Figure 1. Changes in body weight, body composition, and energy intake of C57 mice (n=16) and FVB mice (n=16) in response to a LFD or a HFD.
A) Change in body weight of C57 and FVB mice after 8 weeks on a LFD or a HFD
B) Change in weight gain of C57 and FVB mice after 8 weeks on a LFD or a HFD
C) Body composition of C57 and FVB mice after 12 weeks on a LFD or HFD
D) Change in cumulative energy intake of C57 and FVB mice after 8 weeks on a LFD or a HFD
E) Difference in cumulative energy intake between HFD and LFD in each group
* P<0.05. CEI indicates cumulative energy intake.
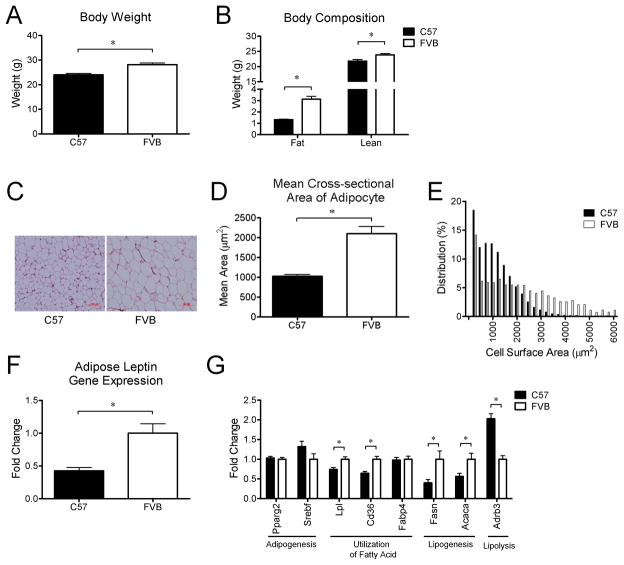
Young C57 mice fed chow have smaller adipocytes and lower adiposity than young FVB mice
The body weight of C57 mice was actually lower due to both lower adiposity and less lean mass relative to FVB mice at 9 weeks of age on a chow diet (Figure 2A and 2B). The cross-sectional area of individual adipocytes was smaller in C57 mice than FVB mice and there were more small adipocytes and fewer large adipocytes in C57 mice (Figure 2C and 2D). There was also a trend toward a decrease in the number of adipocytes in C57 mice relative to FVB mice (P=0.06). Consistent with the lower adiposity and smaller adipocytes, the level of gene expression of Lep was lower in WAT of C57 mice relative to FVB mice (Figure 2E).
Figure 2.
Differences in body weight (A), body composition (B), mean cross-sectional area (D) and relative distribution of adipocyte size (E), adipose leptin gene expression (F), and differential expression of genes related to lipid metabolism in white adipose tissue (G) between C57 mice (n=8) and FVB mice (n=8) at 9 weeks of age on a chow diet. Representative figures of adipocytes (C) in white adipose tissue of C57 mice and FVB mice at 9 weeks of age on a chow diet. * P<0.05. Acaca, acetyl-CoA carboxylase 1; Adrb3, beta 3-adrenergic receptor; Cd36, fatty acid translocase; Fasn, fatty acid synthase; Lpl, lipoprotein lipase; Ppar2, peroxisome proliferator activated receptor, gamma 2; Srebf, sterol regulatory element binding transcription factor.
To determine whether the difference in adipocytes between young C57 mice and FVB mice is associated with differential adipose lipid metabolism, we compared the expression of genes involved in adipogenesis, utilization of fatty acid, lipogenesis and lipolysis in WAT of both groups. There was no significant difference between genotypes in expression of genes for transcriptional factors involved in adipogenesis such as Pparg2 and Srebf. The expression of genes involved in lipogenesis, including Fasn and Acaca were significantly lower in C57 mice relative to FVB mice. Lpl and Cd36 are associated with release of fatty acids from circulating lipoproteins and uptake by WAT.17, 18 These genes involved in fatty acid utilization were significantly lower in WAT of C57 mice relative to FVB mice (Figure 2G). The expression level of Adrb3 (beta 3-adrenergic receptor) is associated with lipolysis and sympathetic activity in WAT,19–21 and is lower in WAT of obese rodents.22 Consistent with the lower adiposity of C57 mice, Adrb3 gene expression was higher in WAT of C57 mice relative to FVB mice (Figure 2G).
Young C57 mice on a chow diet have higher angiogenic activity in adipose tissue than young FVB mice
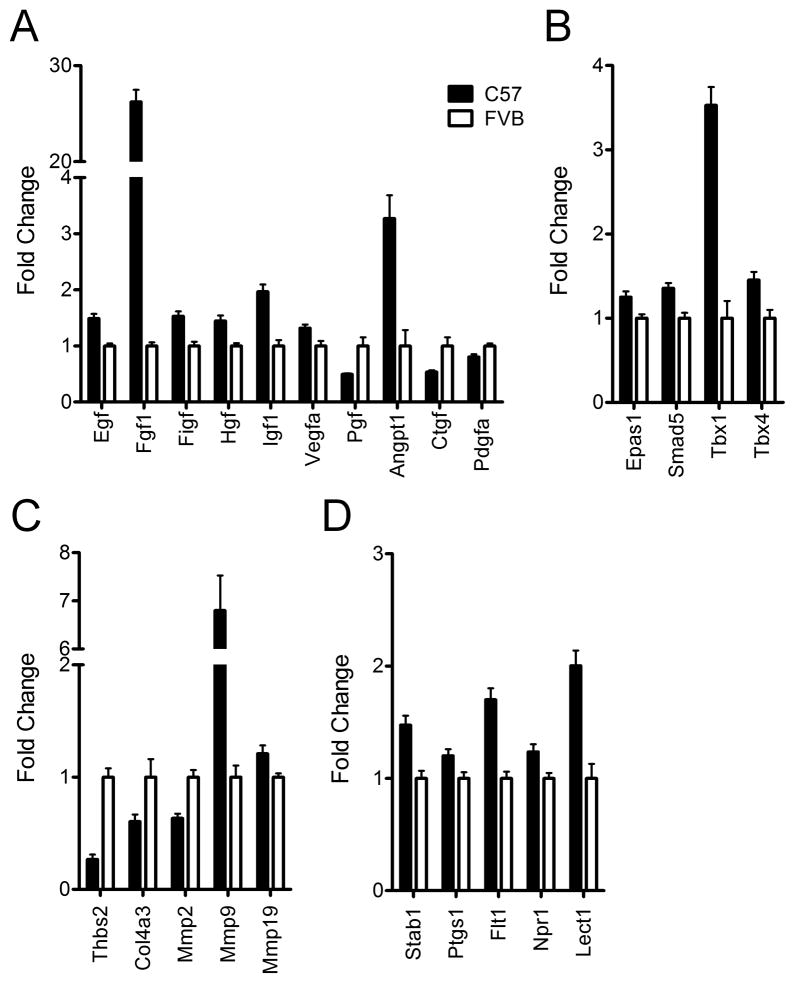
The growth or regression of vasculature depends on the net balance between angiogenic stimulators and angiogenic inhibitors, and the process of angiogenesis comprises modulation of migration and proliferation of endothelial cells (ECs), conversion of ECs to pericytes, recruitment of pericytes and smooth muscle cells, and stabilization of vessels.23–25 Because the expandability of WAT highly depends on its vasculature,4 we hypothesized that C57 mice, with their tendency to develop DIO when fed a HFD, have a higher angiogenic potential in WAT at a young age, prior to exposure to a HFD. To test this, we determined the expression of 84 genes involved in the process of angiogenesis by RT-PCR array. A total of 32% of angiogenic genes were differentially expressed in WAT between C57 mice and FVB mice, with 78% of the significantly changed genes being more highly expressed in C57 relative to FVB mice.
Genes for growth factors that increase migration and proliferation of ECs or stimulation of VEGF such as Vegfa, Egf, Fgf1, Figf, Hgf, and Igf1 were all expressed at higher levels in WAT of C57 mice relative to FVB mice, while Pgf, which stimulates angiogenesis only in pathologic conditions (Figure 3A),25, 26 was lower. Angpt1, which maintains a normalized state of vasculature, was highly expressed in C57 mice while Tek, a receptor for Angpt1 and Angpt2, and Angpt2, an antagonist ligand for Tie-2, were not different, indicating that stabilization of WAT vasculature and pro-angiogenesis was increased in WAT of C57 mice relative to FVB mice.26, 27 However, Ctgf, which is associated with fibrosis, proliferation of mesenchymal cells including pericytes and vascular smooth muscle cells, and inhibition of VEGF-induced angiogenesis,28, 29 and Pdgfa which is related to proliferation of mesenchymal cells and an autocrine regulator of FGF-2 and VEGF, were lower in WAT of C57 mice (Figure 3A).30 This may imply that the process of supporting actively sprouting vessels is less more active in WAT of C57 mice than FVB mice.
Figure 3.
Comparison of genes related to angiogenesis in white adipose tissue of C57 mice (n=8) and FVB mice (n=8) at 9 weeks of age on a chow diet. All genes displayed are differentially expressed between C57 mice and FVB mice (P<0.05). Significantly different growth factors (A), transcriptional factors (B), anti-angiogenic factors and matrix metalloproteinases (C), and two pro-angiogenic genes and three anti-angiogenic genes (D). Angpt1, angiopoietin 1; Col4a3, collagen type IV alpha 3 (tumstatin); Ctgf, connective tissue growth factor; Egf, epidermal growth factor; Epas1, endothelial PAS domain protein 1 (Hif-2alpha); Fgf1, fibroblast growth factor 1; Figf, c-fos induced growth factor (VEGF-D); Hgf, hepatocyte growth factor; Flt-1, FMS-like tyrosine kinase 1 (VEGFR-1); Igf1, insulin-like growth factor 1; Lect1, leukocyte cell derived chemotaxin 1; Mmp2, matrix metallopeptidase 2; Mmp9, matrix metallopeptidase 9; Mmp19, matrix metallopeptidase 19; Npr1, natriuretic peptide receptor 1; Pdgfa, platelet derived growth factor alpha; Pgf, placental growth factor; Ptgs1, prostaglandin-endoperoxide synthase 1 (Cox-1); Smad5, MAD homolog 5; Stab1, Stabilin-1; Tbx1, T-box 1; Tbx4, T-box 4; Thbs2, thrombospondin 2; Vegfa, vascular endothelial growth factor A.
Transcriptional factors such as Epas1(Hif-2alpha), Smad5, Tbx1, and Tbx4 were highly expressed in WAT of C57 mice whereas Hif-1alpha was not different between genotyes (Figure 3B). Epas1 promotes angiogenesis in response to hypoxia and Smad5 is associated with ECs proliferation.31, 32 Tbx1 and 4 are associated with FGF signaling, angiogenesis or morphogenesis during development.33, 34 The difference in expression of these transcription factors is consistent with a more pro-angiogenic environment in WAT of C57 mice (Figure 3B).
The expression of Thbs1 and Thbs2, endogenous anti-angiogenic factors, was lower in WAT of C57 mice (P=0.07 and P<0.05, respectively; Figure 3C). Other anti-angiogenic factors including Col18a1 and Col4a3, a major constituent of basement membrane cleaved by MMP-9, tended to be lower (P=0.07) or were significantly lower in WAT of C57 mice relative FVB mice (Figure 3C).25, 35 These lower anti-angiogenic factors are also consistent with the greater pro-angiogenesis in WAT of young C57 mice. In addition, genes regarding degradation of the extracellular matrix such as Mmp9 and Mmp19 were higher in C57 mice, favoring angiogenesis, but Mmp2 was lower (Figure 3C).
Stab1 is expressed by sinusoidal endothelium or alternatively by activated macrophages and acts as a scavenger receptor for endocytosis of SPARC, a potent inhibitor of angiogenesis.36 Expression of Stab1 was higher in WAT of C57 mice. Ptgs1, which is related to enhanced tube formation or vasodilation, was also higher in WAT of C57 mice (Figure 3D).37
Although most of the significantly changed genes were pro-angiogenic, some of the genes were either anti-angiogenic or have mixed function. Flt-1 is increased by hypoxia, but it is not clear whether it mediates VEGF signaling in its intact form, or plays a role as a vascular permeability receptor, or sequesters VEGF and subsequently antagonizes VEGF action in a soluble form under some circumstances.24, 38, 39 In the present study, WAT of C57 mice had higher Flt-1 gene expression relative to FVB mice. Npr1 is related to vasodilatation via increased guanylyl cyclase activity and inhibits synthesis and function of VEGF activities.40 Lect1 contributes to growth of chondrocytes and inhibits angiogenesis (Figure 3D).41, 42 Npr1 and Lect1 were higher in WAT of C57 mice.
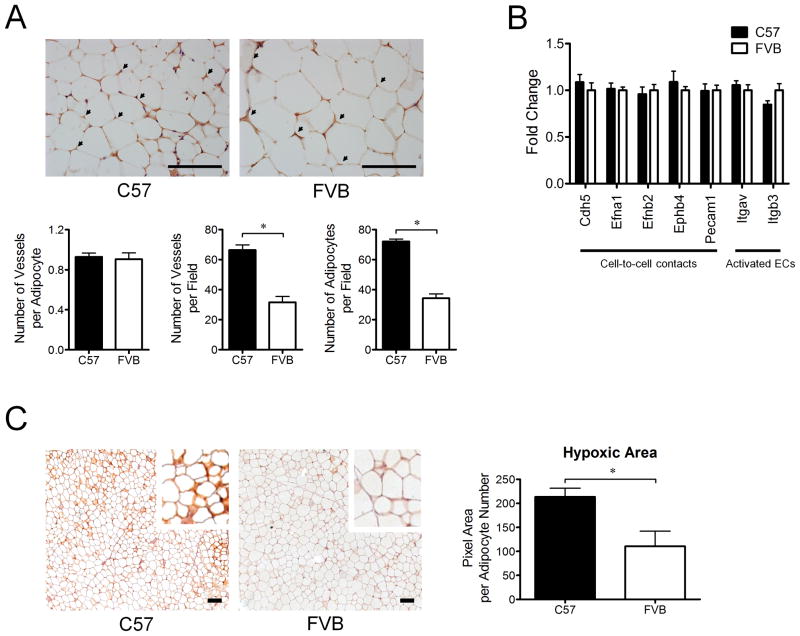
Genes involved in modulation of cell-to-cell contacts, such as Cdh5, Efna1, Efnb2, Ephb4, and Pecam1 were not different between C57 mice and FVB mice. In addition, the expression of Itgav and Itgab3 genes that are highly expressed in activated ECs25 was not different. Consistent with no difference in expression of these genes, the number of vessels per adipocyte was not different although the numbers of adipocytes or vessels per field were higher in WAT of young C57 mice relative to FVB mice (Figure 4A and B). These data are consistent with the hypothesis that the status of ECs does not differ between C57 mice and FVB mice maintained on a chow diet.
Figure 4. Comparison of vessel density and adipose tissue hypoxia in white adipose tissue between C57 mice and FVB mice at 9 weeks of age on a chow diet.
A) Difference in vessel density in white adipose tissue of C57 mice (n=5) and FVB mice (n=4) at 9 weeks of age on a chow diet. Vessel density was calculated by dividing total number of vessels per field by total number of adipocytes per field (×40). Arrows indicate CD31 (PECAM1) staining. Scale bar, 100 μm.
B) Comparison of genes related to the status of endothelial cells (ECs) in white adipose tissue of C57 mice (n=8) and FVB mice (n=8) at 9 weeks of age on a chow diet. No significant differences in the gene expression were observed between C57 mice (n=8) and FVB mice (n=8).
C) Representative figures of adipose tissue hypoxia. Dark brown color indicates the staining area of hypoxia. All samples from C57 mice (n=5) and FVB (n=5) mice were processed in pairs during all the procedures of immunohistochemistry to minimize the occurrence of errors. Hypoxic area was adjusted for either adipocyte number or size in the same field. Scale bar, 100 μm.
* P<0.05. Cdh5, cadherin 5; Efna1, ephrin A1; Efnb2, ephrin B2; Ephb4, Eph receptor B4; Pecam1, platelet/endothelial cell adhesion molecule 1; Itgav, integrin alpha V; Itgb3, integrin beta 3.
In sum, the analysis of these gene expression differences indicates little difference in the state of WAT ECs interacting with other ECs or pericytes between C57 mice and FVB mice. However, WAT of C57 mice had considerably greater expression of angiogenic factors compared to FVB mice. In particular, growth factors involved in proliferation and migration of ECs were mostly higher in WAT of C57 mice, and anti-angiogenic factors such as Thbs1 and Thbs2 were lower.
White adipose tissue of young C57 mice is more hypoxic than that of FVB mice
Within a 100 μm distance from capillaries, the exchange of oxygen or nutrients is the result of diffusion.5 Hypoxia is a key stimulator of angiogenesis. We therefore hypothesized that WAT hypoxia contributes to more pro-angiogenesis in WAT of C57 mice relative to FVB mice. To test this hypothesis, we compared the hypoxic area in WAT between C57 and FVB mice using immunohistochemistry. Because the staining area in WAT is highly dependent on the number and size of adipocytes, we also compared the hypoxic area after adjusting for number and size of adipocytes in the same field. The absolute area of hypoxia was significantly increased in WAT of C57 mice relative to FVB mice. Importantly, the area of hypoxia adjusted for either adipocyte number or size was still significantly increased in C57 mice relative to FVB mice (Figure 4C). These data suggest that WAT hypoxia may contribute to the increased expression of pro-angiogenic genes in WAT of C57 mice relative to FVB mice.
Young C57 mice on a chow diet have more inflammatory adipose tissue relative to young FVB mice
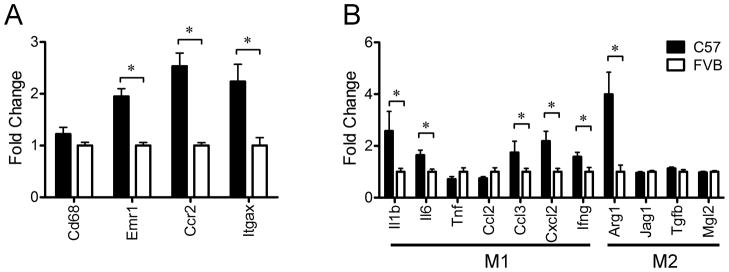
Because angiogenesis contributes to tissue remodeling, we hypothesized that low-grade inflammation may be associated with the greater weight gain on the HFD in C57 mice. To test this hypothesis, we compared the expression of genes related to inflammatory cytokines and macrophages in WAT between young C57 and FVB mice. A macrophage marker, Emr1, as well as genes related to recruitment of macrophages such as Ccr2 and Itgax, were significantly increased in WAT of C57 mice relative to FVB mice. Proinflammatory cytokines including Il1b, Il6, Ccl3 and Cxcl2 were significantly increased in WAT of C57 mice relative to FVB mice while genes related to alternative activation of macrophages such as Jag1, Tgfb1 and Mgl2 were not significantly different. However, Arg1 was significantly increased in WAT of C57 mice relative to FVB mice (Figure 5).
Figure 5. Differential expression of genes related to inflammation in white adipose tissue of C57 mice (n=8) and FVB mice (n=8) at 9 weeks of age on a chow diet. * P<0.05.
A) The expression of genes of markers for macrophage and recruitment of macrophage
B) Different polarization of macrophage in white adipose tissue of C57 mice and FVB mice. M1 and M2 mean the type of macrophage polarization. M1-type macrophages are more involved in pro-inflammation and recruitment of macrophage. M2-type macrophages are involved in tissue remodeling.
* P<0.05. Arg1, arginase; Ccl2, chemokine (C-C motif) ligand 2; Ccl3, chemokine (C-C motif) ligand 3; Ccr2, chemokine (C-C motif) receptor 2; Cxcl2, chemokine (C-X-C motif) ligand 2; Cd68, CD68 antigen; Emr1, egf-like module containing, mucin-like, hormone receptor-like 1; Ifng, interferon gamma; Il1b, interleukin 1, beta; Il6, interleukin-6; Itgax, integrin, alpha X (complement component 3 receptor 4 subunit); Jag1, jagged 1; Mgl2, macrophage galactose N-acetyl-galactosamine specific lectin 2; Tgfb, transforming growth factor, beta 1; Tnf, tumor necrosis factor
Discussion
The goal of these studies was to identify potential factors that might contribute to the differences observed in weight gain among animals exposed to a HFD. Long-term maintenance on a HFD increased body weight and adiposity of C57 mice whereas those of FVB mice were not significantly increased. The energy surplus in C57 mice was likely the result of both increased energy intake (Figure 1) and decreased energy expenditure.15, 16 This is consistent with previous data on these strains of mice (Figure 1).16 However, the difference of body weight between these strains at a younger age was not consistent with what was observed after 8 weeks on the HFD. Specifically, the body weight and adiposity were significantly lower in C57 mice relative to FVB mice at 9 weeks of age before exposure to a HFD. Consistent with the lower adiposity, leptin gene expression was lower in WAT of C57 mice relative to FVB mice and their adipocyte size was smaller. In addition, the amount of WAT correlated with expression of genes linked to lipid metabolism, including lower Lpl, Cd36, and Fasn, and higher Adrb3 in C57 compared to FVB mice.
The key finding in this study is that young C57 mice have an expression profile consistent with greater inflammation and angiogenesis in WAT despite having less body fat than young FVB mice. Such data suggest that young C57 mice may already have a higher capacity for WAT remodeling.43, 44 The number of AT macrophages is positively correlated with increased size of individual adipocytes.12 Interestingly, C57 mice have increased expression of inflammatory markers in WAT despite having reduced adipocyte size. WAT remodeling dynamically occurs both after acute weight loss or when WAT rapidly grows such as occurs in C57 mice exposed to a HFD.43 Since expression of genes associated with WAT lipid metabolism correlated with the increased adiposity of young FVB mice (Figure 2G), neither fatty acid metabolism nor adipocyte death appears to contribute to increased macrophage and proinflammatory cytokine markers in young C57 mice.43, 45
Numerous cell types in WAT, including immune cells, ECs, preadipocytes, and mature adipocytes, are involved in the process of tissue remodeling since WAT expansion and adipocyte differentiation require an adjusted vascular network and angiogenesis precedesadipogenesis.3, 5, 8, 44, 46 In addition, angiogenesis accompanies inflammation, and increased macrophage infiltration has been hypothesized to promote angiogenesis in WAT.8, 47 During this process, hypoxia is a critical factor to initiate angiogenesis and it induces inflammation in WAT.43 Larger adipocytes suffer from lack of oxygen and nutrient transfer because oxygen exchange usually occurs by diffusion through adipocytes.5 Given the smaller adipocytes of C57 mice relative to FVB mice, it is an interesting finding that levels of hypoxia evidenced by increased pimonidamole adduct staining and gene expression of Hif-2alpha are increased in WAT of C57 mice consistent with higher expression of pro-angiogenic and pro-inflammatory genes. WAT hypoxia in obesity has been observed in previous studies.48 Those studies suggest that the increased WAT hypoxia in young DIO mice is important in initiating increased angiogenesis and the mild inflammation that contributes to the necessary tissue remodeling that allows C57 mice to gain more weight when exposed to a HFD.
In this study, several important pro-angiogenic factors including Vegfa, Fgf1, Egf, Hgf, Igf1, Figf1 and Angpt1 were highly expressed and anti-angiogenic factors such as Thbs1, Thbs2, Col18a1 and Col4a3 were lower in WAT of young C57 mice, suggesting an important difference in angiogenic potential between C57 and FVB mice. For example, Vegfa is a key target for HIF-1 alpha and plays a critical role in the angiogenic response to hypoxia. Vegfa stimulates angiogenesis in WAT and VEGF receptor-2 signaling contributes to both WAT angiogenesis and adipogenesis.3, 24 HGF is another WAT pro-angiogenic factor that does not directly promote adipocyte differentiation.49 Both VEGF and HGF synergistically stimulate neovascularization, endothelial migration and proliferation.50 ANGPT1, which stabilizes vessels, also plays a role in vascular remodeling by promoting angiogenesis in conjunction with VEGF.51
Of note, the gene expression level of Fgf1 was dramatically upregulated in young C57 mice relative to young FVB mice while there was no difference of Fgf2, a potent and common angiogenic stimulator. FGF1 simulates adipogenesis in WAT of humans,52 and FGFs are well known to stimulate angiogenesis in tumors by increasing VEGF-driven neovascularization.25, 26 Although a role of each of these genes in angiogenesis has been reported, many of these gene products have also been reported to regulate inflammation and adipogenesis.23, 43
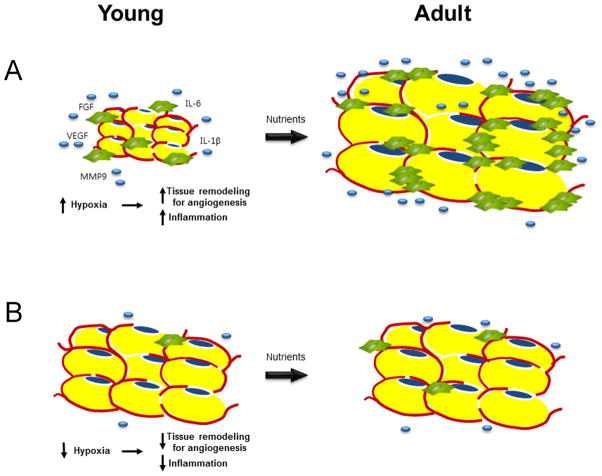
Given the growing prevalence of obesity, an important question is why individual’s susceptibility to obesity varies. The present study used C57 mice and FVB mice to explore differences in WAT that may contribute to this variable response to obesogenic environments. While energy balance clearly involves CNS processes that regulate both food intake and energy expenditure, growing evidence points to an important role in signals from WAT that are related to the status of WAT vasculature.6 Consequently, the one way to view the current data is that C57 and FVB mice differ in the capacity of their WAT to expand and remodel and that this contributes to the propensity for increased intake and weight gain when mice are exposed to a HFD. Further work will be needed to assess the causal relationships between adipose tissue remodeling and energy balance (Figure 6).
Figure 6. “Potential” hypothesis of increased inflammation and pro-angiogenesis presumably caused by adipose tissue hypoxia despite smaller adipocytes and lower adiposity in young mice.
A) The increase in capacity for tissue remodeling resulting from hypoxia may determine the development of obesity in the future. Reduced vascular function responding to dynamic change of nutrients or gas exchange surrounding adipocytes may result in increased hypoxia and subsequent pro-angiogenesis in white adipose tissue despite smaller adipocytes. Inflammation is accompanied by angiogenesis.
B) The adequate ability of vasculature to meet the requirements may cause resistance to obesity. Reactivity of vasculature responding to the environments may be enough to compensate for the rapid increase of need for supplying nutrients or gas exchange. Therefore, adipocytes do not need the tissue remodeling for angiogenesis.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant DK093848 and Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012-0003537).
Footnotes
Supplementary information is available at IJO’s website.
Conflict of Interest
Dr. Seeley has served as a paid consultant for Novo Nordisk, Eli Lilly, Angiochem, Novartis, and Ethicon Endo-Surgery. Dr. Seeley has served as a paid speaker for Novo Nordisk, Pfizer, and Ethicon Endo-Surgery. Dr. Seeley has received research support from Novo Nordisk, Pfizer, Mannkind, Ablaris and Ethicon Endo-Surgery.
References
- 1.Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 2010;11 (4):263–7. doi: 10.1016/j.cmet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin BE. Developmental gene x environment interactions affecting systems regulating energy homeostasis and obesity. Front Neuroendocrinol. 2010;31(3):270–83. doi: 10.1016/j.yfrne.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, et al. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res. 2003;93 (9):e88–97. doi: 10.1161/01.RES.0000099243.20096.FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, et al. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A. 2002;99(16):10730–5. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crandall DL, Hausman GJ, Kral JG. A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation. 1997;4(2):211–32. doi: 10.3109/10739689709146786. [DOI] [PubMed] [Google Scholar]
- 6.Kim DH, Woods SC, Seeley RJ. Peptide designed to elicit apoptosis in adipose tissue endothelium reduces food intake and body weight. Diabetes. 2010;59(4):907–15. doi: 10.2337/db09-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat Med. 2004;10(6):625–32. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- 8.Cho CH, Koh YJ, Han J, Sung HK, Jong Lee H, Morisada T, et al. Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circulation Research. 2007;100(4):e47–57. doi: 10.1161/01.RES.0000259564.92792.93. [DOI] [PubMed] [Google Scholar]
- 9.Voros G, Maquoi E, Demeulemeester D, Clerx N, Collen D, Lijnen HR. Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology. 2005;146(10):4545–54. doi: 10.1210/en.2005-0532. [DOI] [PubMed] [Google Scholar]
- 10.Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes (Lond) 2005;29(11):1308–14. doi: 10.1038/sj.ijo.0802987. [DOI] [PubMed] [Google Scholar]
- 11.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim DH, Sandoval D, Reed JA, Matter EK, Tolod EG, Woods SC, et al. The role of GM-CSF in adipose tissue inflammation. Am J Physiol Endocrinol Metab. 2008;295(5):E1038–46. doi: 10.1152/ajpendo.00061.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu CC, Qing K, Chen Y. Diet-induced changes in stearoyl-CoA desaturase 1 expression in obesity-prone and -resistant mice. Obes Res. 2004;12(8):1264–70. doi: 10.1038/oby.2004.160. [DOI] [PubMed] [Google Scholar]
- 16.Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, et al. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput Biol. 2009;5(3):e1000324. doi: 10.1371/journal.pcbi.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajri T, Han XX, Bonen A, Abumrad NA. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest. 2002;109(10):1381–9. doi: 10.1172/JCI14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coburn CT, Knapp FF, Jr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275(42):32523–9. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- 19.Galitzky J, Reverte M, Portillo M, Carpene C, Lafontan M, Berlan M. Coexistence of β1-, β2-, and β3-adrenoceptors in dog fat cells and their differential activation by catecholamines. American Journal of Physiology - Endocrinology and Metabolism. 1993;264(3 27–3):E403–E412. doi: 10.1152/ajpendo.1993.264.3.E403. [DOI] [PubMed] [Google Scholar]
- 20.Van Liefde I, Van Witzenburg A, Vauquelin G. Multiple beta adrenergic receptor subclasses mediate the l-isoproterenol- induced lipolytic response in rat adipocytes. Journal of Pharmacology and Experimental Therapeutics. 1992;262(2):552–558. [PubMed] [Google Scholar]
- 21.Collins S, Daniel KW, Rohlfs EM, Ramkumar V, Taylor IL, Gettys TW. Impaired expression and functional activity of the β3- and β1- adrenergic receptors in adipose tissue of congenitally obese (C57BL/6J ob/ob) mice. Molecular Endocrinology. 1994;8 (4):518–527. doi: 10.1210/mend.8.4.7914350. [DOI] [PubMed] [Google Scholar]
- 22.Shi H, Akunuru S, Bierman JC, Hodge KM, Mitchell MC, Foster MT, et al. Diet-induced obese mice are leptin insufficient after weight reduction. Obesity (Silver Spring) 2009;17(9):1702–9. doi: 10.1038/oby.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117(9):2362–8. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8(6):464–78. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 25.Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282(5):C947–70. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- 26.Christiaens V, Lijnen HR. Angiogenesis and development of adipose tissue. Molecular and Cellular Endocrinology. 2010;318(1–2):2–9. doi: 10.1016/j.mce.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441(7092):437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 28.Kuiper EJ, Roestenberg P, Ehlken C, Lambert V, van Treslong-de Groot HB, Lyons KM, et al. Angiogenesis is not impaired in connective tissue growth factor (CTGF) knock-out mice. J Histochem Cytochem. 2007;55(11):1139–47. doi: 10.1369/jhc.7A7258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoki I, Shiomi T, Hashimoto G, Enomoto H, Nakamura H, Makino K, et al. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J. 2002;16(2):219–21. doi: 10.1096/fj.01-0332fje. [DOI] [PubMed] [Google Scholar]
- 30.Shikada Y, Yonemitsu Y, Koga T, Onimaru M, Nakano T, Okano S, et al. Platelet-derived growth factor-AA is an essential and autocrine regulator of vascular endothelial growth factor expression in non-small cell lung carcinomas. Cancer Res. 2005;65(16):7241–8. doi: 10.1158/0008-5472.CAN-04-4171. [DOI] [PubMed] [Google Scholar]
- 31.Yang X, Castilla LH, Xu X, Li C, Gotay J, Weinstein M, et al. Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development. 1999;126(8):1571–80. doi: 10.1242/dev.126.8.1571. [DOI] [PubMed] [Google Scholar]
- 32.Takeda N, Maemura K, Imai Y, Harada T, Kawanami D, Nojiri T, et al. Endothelial PAS domain protein 1 gene promotes angiogenesis through the transactivation of both vascular endothelial growth factor and its receptor, Flt-1. Circ Res. 2004;95(2):146–53. doi: 10.1161/01.RES.0000134920.10128.b4. [DOI] [PubMed] [Google Scholar]
- 33.Naiche LA, Papaioannou VE. Loss of Tbx4 blocks hindlimb development and affects vascularization and fusion of the allantois. Development. 2003;130(12):2681–2693. doi: 10.1242/dev.00504. [DOI] [PubMed] [Google Scholar]
- 34.Brown CB, Wenning JM, Lu MM, Epstein DJ, Meyers EN, Epstein JA. Cre-mediated excision of Fgf8 in the Tbx1 expression domain reveals a critical role for Fgf8 in cardiovascular development in the mouse. Developmental biology. 2004;267(1):190–202. doi: 10.1016/j.ydbio.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 35.Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, et al. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell. 2003;3(6):589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kzhyshkowska J, Workman G, Cardo-Vila M, Arap W, Pasqualini R, Gratchev A, et al. Novel function of alternatively activated macrophages: stabilin-1-mediated clearance of SPARC. J Immunol. 2006;176(10):5825–32. doi: 10.4049/jimmunol.176.10.5825. [DOI] [PubMed] [Google Scholar]
- 37.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93(5):705–16. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 38.Kerber M, Reiss Y, Wickersheim A, Jugold M, Kiessling F, Heil M, et al. Flt-1 signaling in macrophages promotes glioma growth in vivo. Cancer Res. 2008;68(18):7342–51. doi: 10.1158/0008-5472.CAN-07-6241. [DOI] [PubMed] [Google Scholar]
- 39.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376(6535):62–6. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 40.Pedram A, Razandi M, Levin ER. Natriuretic peptides suppress vascular endothelial cell growth factor signaling to angiogenesis. Endocrinology. 2001;142(4):1578–1586. doi: 10.1210/endo.142.4.8099. [DOI] [PubMed] [Google Scholar]
- 41.Shukunami C, Iyama KI, Inoue H, Hiraki Y. Spatiotemporal pattern of the mouse chondromodulin-I gene expression and its regulatory role in vascular invasion into cartilage during endochondral bone formation. International Journal of Developmental Biology. 1999;43(1):39–49. [PubMed] [Google Scholar]
- 42.Yoshioka M, Yuasa S, Matsumura K, Kimura K, Shiomi T, Kimura N, et al. Chondromodulin-I maintains cardiac valvular function by preventing angiogenesis. Nature Medicine. 2006;12(10):1151–1159. doi: 10.1038/nm1476. [DOI] [PubMed] [Google Scholar]
- 43.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121(6):2094–101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han J, Lee JE, Jin J, Lim JS, Oh N, Kim K, et al. The spatiotemporal development of adipose tissue. Development. 2011;138(22):5027–37. doi: 10.1242/dev.067686. [DOI] [PubMed] [Google Scholar]
- 45.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120(10):3466–79. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56(6):1517–26. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 47.Pang C, Gao Z, Yin J, Zhang J, Jia W, Ye J. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am J Physiol Endocrinol Metab. 2008;295(2):E313–22. doi: 10.1152/ajpendo.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56(4):901–11. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 49.Bell LN, Cai L, Johnstone BH, Traktuev DO, March KL, Considine RV. A central role for hepatocyte growth factor in adipose tissue angiogenesis. Am J Physiol Endocrinol Metab. 2008;294(2):E336–44. doi: 10.1152/ajpendo.00272.2007. [DOI] [PubMed] [Google Scholar]
- 50.Xin X, Yang S, Ingle G, Zlot C, Rangell L, Kowalski J, et al. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am J Pathol. 2001;158(3):1111–20. doi: 10.1016/S0002-9440(10)64058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, et al. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282(5388):468–471. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- 52.Hutley L, Shurety W, Newell F, McGeary R, Pelton N, Grant J, et al. Fibroblast growth factor 1: a key regulator of human adipogenesis. Diabetes. 2004;53(12):3097–106. doi: 10.2337/diabetes.53.12.3097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.