Abstract
Schizophrenia is a complex constellation of positive, negative and cognitive symptoms. Acute administration of the non-competitive antagonist of the N-methyl D-aspartate receptor (NMDAR) dizocilpine (MK801) in rats is one of few preclinical animal models of this disorder that has both face and/or construct validity for these multiple at-risk behavioral domains and predictive power for the efficacy of therapeutic drugs in treating them. This study asked whether and to what extent the rat NMDAR hypofunction model also embodies the sex differences that distinguish the symptoms of schizophrenia and their treatment. Thus, we compared the effects of acute MK801, with and without pretreatment with haloperidol or clozapine, on seven discrete spontaneous open field activities in adult male and female rats. These analyses revealed that MK801 was more effective in stimulating ataxia and locomotion and inhibiting stationary behavior in females while more potently stimulating stereotypy and thigmotaxis and inhibiting rearing and grooming in males. Haloperidol and clozapine pretreatments had markedly different efficacies in terms of behaviors but strong similarities in their effectiveness in male and female subjects. These results bear intriguing relationships with the complex male/female differences that characterize the symptoms of schizophrenia and suggest possible applications for acute NMDAR hypofunction as a preclinical model for investigating the neurobiology that underlies them.
Keywords: schizophrenia, clozapine, haloperidol, dizocilpine, negative symptoms, positive symptoms
INTRODUCTION
Schizophrenia’s symptoms are divided into three categories: positive symptoms which include hallucinations, delusions, cognitive disturbances and other interjected behaviors not seen in healthy subjects; negative symptoms including flattened affect, avolition, social withdrawal and other disruptions/decreases in normal behavior; and cognitive symptoms that include deficits in executive, mnemonic and attentional operations (Nasrallah et al., 2011). Several disease models recapitulate one or another of these symptom classes (Castner et al., 2004, Featherstone et al., 2007, Barak, 2009, Lodge and Grace, 2009, van den Buuse, 2010, Jones et al., 2011). However, a recent review highlights acute administration of non-competitive antagonists of the N-methyl D-aspartate receptor (NMDA-R) such as phencyclidine, ketamine or dizocilpine (MK801) as one of a very few capable of modeling several of schizophrenia’s major at-risk behavioral domains(Javitt and Zukin, 1991, Krystal et al., 1994, Jentsch and Roth, 1999, Krystal et al., 2002, Jones et al., 2011) These NMDAR hypofunction models also have predictive power for the clinical effectiveness of both established, e.g., atypical, typical neuroleptics (Behrens and Gattaz, 1992, Hoffman, 1992, Gattaz et al., 1994, Corbett, 1995, Malhotra et al., 1997, Ninan and Kulkarni, 1998, Gaisler-Salomon and Weiner, 2003) and emerging therapeutics(Moghaddam and Adams, 1998, Javitt et al., 1999). Certainly there are limits of acute drug treatments in modeling a disorder that is chronic, derived in part from developmental origins, and most responsive to repeated drug treatments. Nonetheless, NMDAR hypofunction models have been successfully used to make key contributions to understanding the neurobiological and neurochemical bases for schizophrenia and other psychoses (Ellison, 1995, Olney et al., 1999, Adell et al., 2012). The purpose of this study was to explore the potential of the NMDAR hypofunction model in rats for also understanding the sex differences that sharply distinguish schizophrenia’s positive, negative and cognitive symptoms and their effective treatment.
Sex differences in schizophrenia include clear male/female differences in incidence of birth complications, age of onset and in the presence or extent of brain abnormalities(Leung and Chue, 2000). There are also consistent findings that males are more vulnerable to schizophrenia’s negative and cognitive symptoms, whereas females are more often afflicted with positive symptoms, show more co-morbid anxiety or depression and tend to respond more quickly and to lower doses of typical and atypical neuroleptic medications (Leung and Chue, 2000, Seeman, 2006, Canuso and Pandina, 2007, Cotton et al., 2009, Ochoa et al., 2012) (Szymanski et al., 1996, Goldstein et al., 2002, Seeman, 2006, Usall et al., 2007, Seeman, 2012). These etiological findings, the significant relationships found between circulating hormone levels and symptom severity in both sexes(Shirayama et al., 2002, Taherianfard and Shariaty, 2004, Ko et al., 2007, Kulkarni et al., 2012, Seeman, 2012) and recent indications of the potential benefits of hormone augmentation as adjuncts to conventional neuroleptic treatment(Elias and Kumar, 2007, Ko et al., 2008, Kulkarni et al., 2012, Torrey and Davis, 2012) give strong impetus to better understand the bases for sex differences in schizophrenia, other psychoses and their treatment. What is lacking is, however, a well-validated animal model in which to conduct this research.
There is some evidence that NMDA-R hypofunction models are suitable platforms to pursue questions of sex differences in schizophrenia. These include sex differences identified in the mnemonic effects of ketamine in healthy human subjects (Morgan et al., 2006) and in the psychostimulant effects of MK801 and other non-competitive NMDAR blockers in rodents (Honack and Loscher, 1993, Andine et al., 1999, Frantz and Van Hartesveldt, 1999, Devaud et al., 2002, Devaud, 2003). However, a clear and more complete view of the model’s embodiment of the sex differences observed in disease is hampered by a paucity of comparative data and their dispersion across studies where key experimental and/or analytical variables differ. Further there are few studies in which behavioral testing fully avoids time windows when sex differences in hepatic metabolism are known to yield significant female over male differences in brain drug levels (Nabeshima et al., 1984, Vezzani et al., 1989, Schwartz and Wasterlain, 1993, Andine et al., 1999, Wegener et al., 2011), or in which potentially confounding activational hormone effects of the estrus cycle in females are strictly controlled. Taking these points into consideration, the studies presented here approached the question of behavioral sex differences in NMDAR hypofunction models by comparing the effects of acute MK801-- one of the most selective and potent of the non-competitive NMDAR blockers(Zukin and Zukin, 1979, Reynolds et al., 1987, Lodge and Johnson, 1990) on seven measures of novelty-induced, spontaneous open field activity in adult male rats and female rats. These behaviors ranged from motor, e.g., locomotion, stereotypy, to motivational, e.g., thigmotaxis, self-grooming and included endpoints modeling schizophrenia’s sexually dimorphic positive and negative symptoms. Each behavior was also assessed with and without pretreatment with haloperidol or clozapine administered at doses matching the high and low end of the human therapeutic range(Kapur et al., 2003) and within 30 minutes of intraperitoneal injection of MK801 injection. Finally, for the females behavioral observations were made only when subjects were confirmed by vaginal cytology to be in the proestrus phase of the four-day rat estrus cycle-- when circulating estrogens are highest (Everett, 1989). As discussed below, the findings obtained revealed both strengths and weaknesses in the extent to which acute NMDA hypofunction effectively models sex differences in schizophrenia’s positive and negative symptoms.
EXPERIMENTAL PROCEDURES
Animal Subjects
A total of 64 adult male and 64 adult female Sprague-Dawley rats that were between 5 and 6 months of age (Taconic Farms, Hudson, NY) were used. Animals were housed under conditions that are SPF for all known rat pathogens in same-sex pairs under a 12:12 hour light/dark cycle (lights on 7 AM). Ground corn cob bedding is used (Bed O’ Cobs, Anderson) and food (Purina LabDiet: ProLab RMH 3000) and water were available ad libitum. All procedures involving rats were approved by the Institutional Animal Care and Use Committee of SUNY Stony Brook and were designed to minimize animal use and discomfort.
Determination of Estrus Cycle Stage in Females and Yoking in Males
To minimize inter-subject variance caused by the dramatic fluctuations in circulating estrogens and progestins across the rats four-day estrus cycle, (Everett, 1989), prior to behavioral testing females underwent daily vaginal lavage for at least 10 days and vaginal cytology was used to determine estrus cycle regularity and stage (Marcondes et al., 2002, Goldman et al., 2007). Lavage was performed between 11 AM and 2 PM by gently wrapping rats in a thick towel and briefly holding them supine. To minimize the potential for handling bias, male rats were randomly paired with a female subject and handled at the same times in similar ways.
Lavage samples were collected in saline using a fire-polished, sterile glass pipette. The sampled fluid was immediately placed on a slide, a coverslip was gently placed over it and cytology was evaluated using light microscopy, differential interference contrast (DIC) optics and a 20 × objective. After tracking over 2 cycles, regularly cycling females found to be in proestrus (by the abundance of nucleated, non-cornified epithelial cells) and the male rats paired with them were assigned to the same experimental group (below) and tested that day.
Drugs and Drug Treatments
All drugs were purchased from Sigma-Aldrich (St. Louis, MO). Each rat received two injections prior to testing; all injections were adjusted to final volumes of 0.5cc. Rats first received a subcutaneous injection of haloperidol (0.04 or 0.08 mg/kg in saline acidified with 0.025% acetic acid), clozapine (5 or 10 mg/kg in acidified saline) or acidified saline alone. Fifteen minutes later, rats were injected intraperitoneally with either MK801 (0.05, 0.1 or 0.2 mg/kg in saline) or saline alone; this injection route assists in minimizing the potential for sex differences in brain concentration of drug by avoiding first pass metabolism. Eight female rats and the 8 paired males were randomly assigned to one of the following eight groups: vehicle/vehicle; vehicle/0.05, 0.1 or 0.2 mg/kg MK801; 0.04 or 0.08 mg/kg haloperidol/0.2 mg/kg MK801; or 5 or 10 mg/kg clozapine/0.2 mg/kg MK801.
Open Field Apparatus and Behavioral Observation
Behavioral observation was 15 min in duration and took place 15 minutes after the second injection (MK801, above) in a sound attenuated room. This timeframe placed behavioral observations during periods when brain concentrations of MK801 are expected to be dose-dependently peaking in all subjects (Vezzani et al., 1989, Schwartz and Wasterlain, 1993, Andine et al., 1999, Wegener et al., 2011) but prior to times when sex differences in clearance rates cause them to diverge (Andine et al., 1999).
The open field arena used was an opaque plastic enclosure measuring 80 × 30 cm, with walls 30 cm high and a floor that was marked off in 10 × 10 cm squares. The arena was completely surrounded by a white curtain to minimize extra-maze cues. A video camera (Apple iSight) suspended one meter above the arena floor was used to archive animals’ activities. On the day of observation, rats were injected as above, transferred to a holding cage and brought to the testing room for the first time. After 5 minutes of acclimation, rats were placed in a central start position in the open arena and allowed to explore for 15 minutes. The investigator left the room during the session, the arena was cleaned with 70% ethanol after each session and individual rats were tested only once.
Behavioral Assessment
Behavior was analyzed off-line from videos by a single observer (IF); although it was not always strictly possible to remain blind to animal sex, videos were identified to the observer only by codes that did not reveal information about animal sex or group. Tapes were scored in sequential series of one-minute epochs using event-capture software (JWatcher 1.0) for the following behaviors:
Locomotion: defined as sustained forward motion of front and hind paws and measured as number of line crosses made by rats’ midsection.
Thigmotaxis: defined as maintaining whisker, head or body contact with arena walls during locomotion. It was measured as percent of time spent engaged in thigmotaxis relative to total locomotion.
Rearing: defined as upward motion either with forepaws in contact with the wall or freestanding and measured as number of attempts. Failed attempts to rear were not included (see Ataxia).
Grooming: defined as licking or preening any part of the body and measured as time spent engaged in grooming.
Stationary Behavior: defined and measured as the sum of time rats spent sitting still (includes sniffing).
-
Stereotypy: measured as the summed time spent on the following:
Head-weaving: defined as repeated (> 2 times) side-to-side motion of the proximal end of the rat with the head deviating more than 30 degrees from the body axis.
Circling: defined as a tight, closed, loop of locomotion.
Axial turning: defined as circling movements specifically driven by the forepaws with the haunches remaining still.
-
Ataxia: defined and measured as numbers of instances of the following:
Falling: defined as all four feet losing contact with the floor.
Failed rearing: defined as an attempt to rear that results in a failure to fully elevate.
Analyses and Statistics
Behaviors were scored separately over consecutive 1-minute epochs and summed over the 15-minute trials. Statistical comparisons used only the summed values and SPSS software (IBM, version 19). Behaviors were first compared across all groups using two-way analyses of variance (ANOVA) to identify overall main effects of sex and/or drug treatment. Allowed follow-up analyses were performed using one-way ANOVAs that compared data within treatment group across sex and within sex across treatment group to separately evaluate these factors. Post-hoc testing was applied to analyses of group, which had more than two levels. These comparisons used the Fisher’s protected least significant difference (PLSD) test. Observations were considered significant at p<0.05.
RESULTS
Locomotion
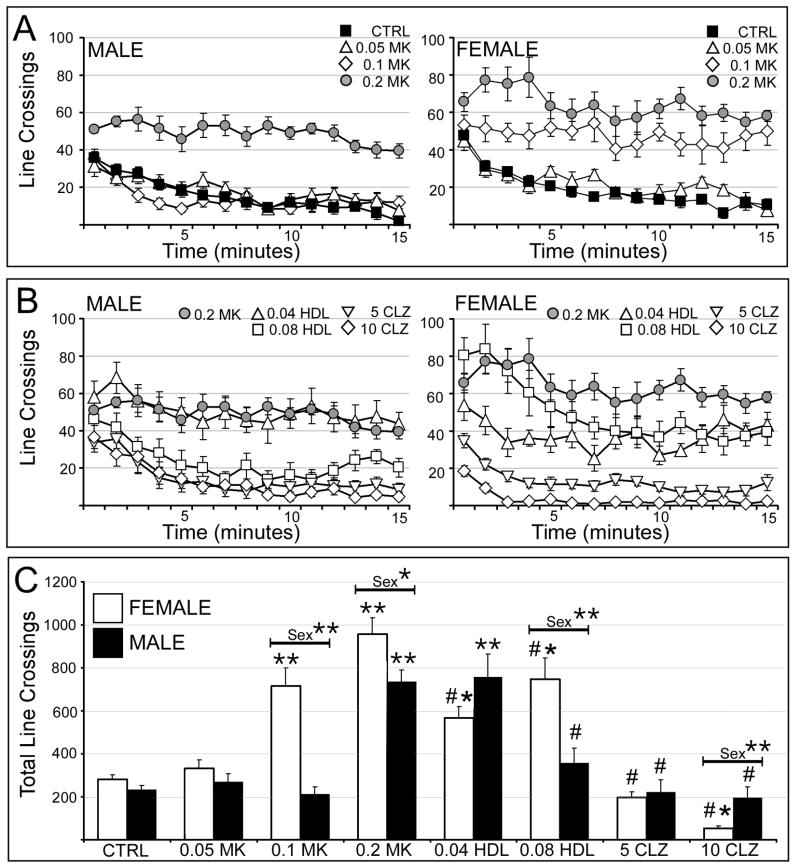
Vehicle injected male and female rats showed similar initial rates of locomotion and similar steady decrementS in locomotion over the 15 min testing period (Fig. 1A). In males, MK801at doses of 0.2 mg/kg MK801, but not 0.05 or 0.1 mg/kg markedly increased the initial rate of locomotion and largely eliminated its decrement over time (Fig. 1A, left). Pretreatment with haloperidol had no discernable effect on MK801-induced hyperlocomotion at 0.04 mg/kg, while haloperidol at 0.08 mg/kg and clozapine at both 5 and 10 mg/kg clearly reduced this behavior (Fig. 1B, left, right). In females, MK801 had no effect on locomotion at 0.05 mg/kg but dose-dependently increased locomotion and reduced its decrement over time at 0.1 and 0.2 mg/kg (Fig. 1A, right). These effects were partially attenuated by pretreatment with 0.04 and 0.08 mg/kg haloperidol, were more fully attenuated by pretreatment with 5 mg/kg clozapine, and were depressed to below baseline levels by 10 mg/kg clozapine (Fig. 1B, right). Statistical comparisons were performed on total locomotor scores (Fig. 1C). These identified significant overall main effects of drug treatment and sex as well as significant interactions between the two (Table 1). Follow-up, within-group, across-sex comparisons also confirmed that the hyperlocomotion induced by 0.1 mg/kg and 0.2 mg/kg MK801 was significantly greater in females than in males. Finally, within-sex, across-group comparisons identified significant main effects of drug treatment in both sexes (Table 2). The allowed post-hoc testing further showed that in males only 0.2 mg/kg MK801 significantly increased locomotion relative to control and that pretreatment with the high dose of haloperidol and both doses of clozapine significantly decreased MK801-induced hyperlocomotion to levels that were indistinguishable from control (Fig. 1C). In contrast, in females, the corresponding comparisons showed that MK801 significantly increased locomotion relative to control at 0.1 mg/kg and at 0.2 mg/kg, that both haloperidol pretreatments significantly albeit partially decreased locomotion and that hyperlocomotion was significantly decreased by clozapine at 5 mg/kg to control levels and decreased to levels that were significantly lower than control at 10 mg/kg (Fig. 1C).
Figure 1.
Effects of MK801 and neuroleptic pretreatments on locomotion. All data are expressed as group means ± SEM. A: Line graphs showing the number of line crosses made per minute during the 15 minute testing session by male (right side) and female (left side) rats that were either vehicle-injected (CTRL, black squares) or treated with 0.05 mg/kg MK801 (0.05 MK, open triangles), 0.1 mg/kg MK801 (0.1 MK, open diamonds) or with 0.2 mg/kg MK801 (0.2 MK, grey circles). B: Line graphs showing the mean number of lines crossed per minute during the 15 minute testing session by male (right side) and female (left side) rats that were treated with 0.2 mg/kg MK801 (0.2 MK, grey circles), or were pretreated with 0.04 mg/kg haloperidol (0.04 HDL, open triangles), 0.08 mg/kg haloperidol (0.08 HDL, open squares), 5 mg/kg clozapine (5 CLZ, inverted triangles) or with 10 mg/kg clozapine (10 CLZ, open diamonds) followed by 0.2 mg/kg MK801. C: Bar graphs showing the total number of line crosses for the 15 minute testing session by female (white bars) and male (black bars) in all treatment groups. Single asterisks (*) indicate a significant difference of p<0.05 compared to control, double asterisks (**) indicate a significant difference of p<0.001 compared to control; pound signs (#) indicate a significant difference of p<0.001 compared to MK801. Significant sex differences are indicated by horizontal bars; single asterisks indicate a significant difference of p<0.05, double asterisks indicate a significant difference of p<0.001.
TABLE 1.
Outcomes from iniital two-way ANOVAs testing for overall main effects sex, group (drug treatment) and for interactions between the two for each of seven discrete open field behaviors observed. Non-significant effects are not included.
| BEHAVIOR | Main effects of SEX | Main effects of GROUP | SEX x GROUP Interaction |
|---|---|---|---|
| Locomotion | F(1,111) = 13.24, p<0.001 | F(7,111) = 34.50, p<0.001 | F(7,111) = 8.69, p<0.001 |
| Thigmotaxis | ------------- | F(7,111) = 12.33, p<0.001 | F(1,111) = 3.67, p<0.001 |
| Rearing | ------------- | F(7,111) = 37.18, p<0.001 | F(1,111) = 3.68, p<0.001 |
| Grooming | ------------- | F(7,111) = 35.50, p<0.001 | F(1,111) = 2.74, p<0.001 |
| Stationary | F(1,111) = 6.35, p<0.05 | F(7,111) = 25.54, p<0.001 | F(1,111) = 2.41, p<0.05 |
| Stereotypy | ------------- | F(7,111) = 14.64, p<0.001 | ------------- |
| Ataxia | ------------- | F(7,111) = 29.65, p<0.001 | ------------- |
TABLE 2.
Outcomes from within-sex, one-way ANOVAs testing for overall main effects of group (drug treatment) for each of seven discrete behaviors observed. The results of the allowed post-hoc tests that were subsequently conducted to these comparisons are depicted by symbols in graphs of summed behaviors (panel C, Figures 1–7).
| BEHAVIOR | Main effects of GROUP: Male | Main effects of GROUP: Female |
|---|---|---|
| Locomotion | F(7,62) = 15.70, p<0.001 | F(7,63) = 27.52, p<0.001 |
| Thigmotaxis | F(7,62) = 11.49, p<0.001 | F(7,63) = 4.42, p<0.001 |
| Rearing | F(7,62) = 16.00, p<0.001 | F(7,63) = 24.58, p<0.001 |
| Grooming | F(7,62) = 25.76, p<0.001 | F(7,63) = 15.53, p<0.001 |
| Stationary | F(7,62) = 11.88, p<0.001 | F(7,63) = 16.19, p<0.001 |
| Stereotypy | F(7,62) = 10.40, p<0.001 | F(7,63) = 6.04, p<0.001 |
| Ataxia | F(7,62) = 19.66, p<0.001 | F(7,63) = 14.99, p<0.001 |
Thigmotaxis
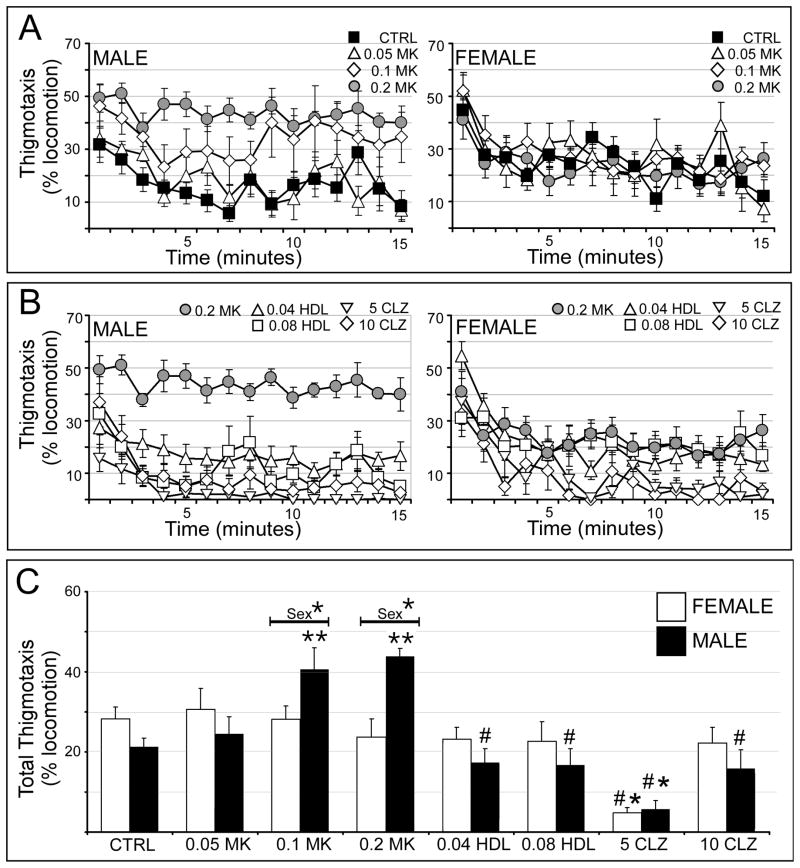
Vehicle-injected male and female rats spent similar amounts of time engaged in thigmotaxis and both exhibited progressively less thigmotaxis as testing went on (Fig. 2A). In males, MK801 had no effect on thigmotaxis at 0.05 mg/kg but nearly doubled initial rates of thigmotaxis and virtually eliminated its decrement over time at 0.1 and 0.2 mg/kg (Fig. 2A, left). Pretreatment with both doses of haloperidol and the higher dose of clozapine (10 mg/kg) attenuated these effects, while clozapine at 5 mg/kg reduced thigmotaxis to below-baseline levels (Fig. 2B, left). In females, none of the doses of MK801 used had discernable effects on thigmotaxis. Pretreatments with haloperidol (both doses) and the higher dose of clozapine were also without effect (Figs. 2A, B right) while pretreatment with 5 mg/kg clozapine decreased thigmotaxis (Fig. 2B, right). Statistical analyses of total (percent of locomotion) times spent in thigmotaxis (Fig. 2C) identified a significant overall main effect of drug treatment and a significant interaction between drug treatment and sex (Table 1). Subsequent within-group, across-sex comparisons further showed that the stimulating effects of MK801 on thigmotaxis were significantly greater in the males at 0.1 and 0.2 mg/kg (Fig. 2C). Within-sex, across-group comparisons next identified significant main effects of drug treatment in both sexes (Table 2) and the allowed post-hoc tests showed that in males MK801 significantly increased thigmotaxis relative to control at 0.1 mg/kg and 0.2 mg/kg and that pretreatment with both doses of haloperidol and the high dose of clozapine significantly decreased 0.2 mg/kg MK801-stimulated thigmotaxis to levels that were indistinguishable from control (Fig. 2C). Post-hoc testing for the females on the other hand confirmed that none of the doses of MK801 used, neither of the pretreatments with haloperidol and pretreatment with 10 mg/kg clozapine significantly affected thigmotaxis (Fig. 2C). However, as in males pretreatment with 5 mg/kg clozapine reduced thigmotaxis to levels that were significantly lower than control (Fig. 2C).
Figure 2.
Effects of MK801 and neuroleptic pretreatments on thigmotaxis. All data are expressed as group means ± SEM. A: Line graphs showing the percentages of locomotor time spent in thigmotaxis per minute during the 15 minute testing session by male (right side) and female (left side) rats that were either vehicle-injected (CTRL, black squares) or treated with 0.05 mg/kg MK801 (0.05 MK, open triangles), 0.1 mg/kg MK801 (0.1 MK, open diamonds) or with 0.2 mg/kg MK801 (0.2 MK, grey circles). B: Line graphs showing the percentages of locomotor time spent in thigmotaxis per minute during the 15 minute testing session by male (right side) and female (left side) rats that were treated with 0.2 mg/kg MK801 (0.2 MK, grey circles), or were pretreated with 0.04 mg/kg haloperidol (0.04 HDL, open triangles), 0.08 mg/kg haloperidol (0.08 HDL, open squares), 5 mg/kg clozapine (5 CLZ, inverted triangles) or with 10 mg/kg clozapine (10 CLZ, open diamonds) followed by 0.2 mg/kg MK801. C: Bar graphs showing the total percent time of locomotion spent in thigmotaxis for the 15 minute testing session by female (white bars) and male (black bars) in all treatment groups. Single asterisks (*) indicate a significant difference of p<0.05 compared to control, double asterisks (**) indicate a significant difference of p<0.001 compared to control; pound signs (#) indicate a significant difference of p<0.001 compared to MK801. Significant sex differences are indicated by horizontal bars; single asterisks indicate a significant difference of p<0.05, double asterisks indicate a significant difference of p<0.001.
Rearing
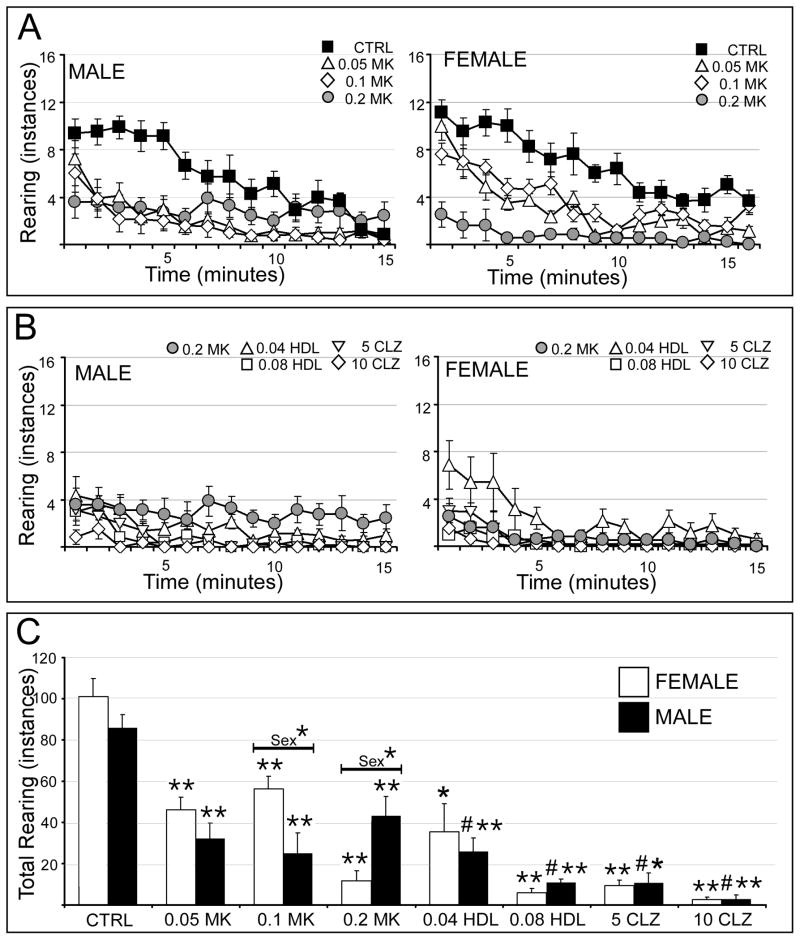
Vehicle injected male and female rats showed similar initial rates of rearing and both reared less frequently as testing proceeded (Fig. 3A). In males, MK801 at 0.05 mg/kg and 0.1 mg/kg dose-dependently decreased rearing; at 0.2 mg/kg MK801 also decreased rearing, albeit noticeably less so than the lower two doses (Fig. 3A, left, Fig. 3C). All pretreatments with haloperidol and clozapine reduced rearing even further (Fig. 3B, left). In females, MK801 decreased rearing according to an inverted U-shaped dose-response curve. (Fig. 3A, right). Further, pretreatments 0.08 mg/kg haloperidol and both doses of clozapine depressed rearing further while the lower dose of haloperidol increased it (Fig. 3B, right). Statistical comparisons of total numbers of rears (Fig. 3C) identified a significant overall main effect of and a significant interaction between treatment and sex (Table 1). Subsequent within group, across-sex comparisons showed that rearing was reduced significantly more by 0.1 mg/kg MK801 in males compared to females and was reduced significantly more by 0.2 mg/kg MK801 in females compared to males (Fig. 3C). Within-sex, across group comparisons further identified significant main effects of drug treatment on rearing in both sexes (Table 2). The allowed post-hoc tests showed that in both sexes, all doses of MK801 significantly reduced rearing and that all neuroleptic pretreatments in males further reduced rearing to levels that were significantly lower than MK801-treated rats and controls (Fig. 3C). Findings in the females only differed in so far as a significant increase in rearing induced by 0.04 mg/kg haloperidol relative to MK801 treated rats (Fig. 3C). This unexpected effect was found to be driven by aberrantly high behavioral scores in two animals.
Figure 3.
Effects of MK801 and neuroleptic pretreatment on rearing. All data are expressed as group means ± SEM. A: Line graphs showing the number of rears made per minute during the 15 minute testing session by male (right side) and female (left side) rats that were either vehicle-injected (CTRL, black squares) or treated with 0.05 mg/kg MK801 (0.05 MK, open triangles), 0.1 mg/kg MK801 (0.1 MK, open diamonds) or with 0.2 mg/kg MK801 (0.2 MK, grey circles). B: Line graphs showing the mean number of rears made per minute during the 15 minute testing session by male (right side) and female (left side) rats that were treated with 0.2 mg/kg MK801 (0.2 MK, grey circles), or were pretreated with 0.04 mg/kg haloperidol (0.04 HDL, open triangles), 0.08 mg/kg haloperidol (0.08 HDL, open squares), 5 mg/kg clozapine (5 CLZ, inverted triangles) or with 10 mg/kg clozapine (10 CLZ, open diamonds) followed by 0.2 mg/kg MK801. C: Bar graphs showing the total number of rears made during the 15 minute testing session by female (white bars) and male (black bars) in all treatment groups. Single asterisks (*) indicate a significant difference of p<0.05 compared to control, double asterisks (**) indicate a significant difference of p<0.001 compared to control; pound signs (#) indicate a significant difference of p<0.001 compared to MK801. Significant sex differences are indicated by horizontal bars; single asterisks indicate a significant difference of p<0.05, double asterisks indicate a significant difference of p<0.001.
Grooming
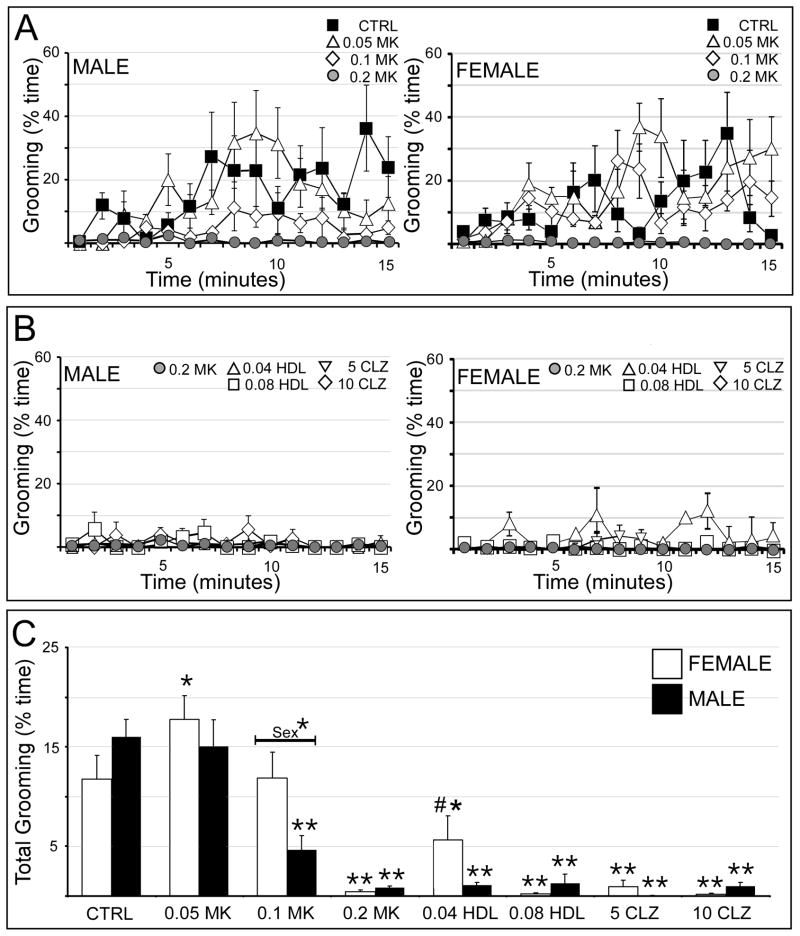
Vehicle-injected rats of both sexes groomed in periodic bouts across the testing period and both sexes exhibited progressively more grooming as testing went on (Fig. 4A). In males, 0.05 mg/kg MK801 had no effect on grooming, 0.1 mg/kg MK801 delayed the onset of grooming and 0.2mg/kg MK801 eliminated grooming (Fig. 4A, left). None of the neuroleptic pretreatments restored this behavior (Fig. 4B, left). In females, drug effects on grooming were more complex. Thus, grooming was increased slightly by 0.05 mg/kg MK801, was unaffected by 0.1 mg/kg MK801, and was eliminated by 0.2 mg/kg MK801 (Fig. 4A, right). Three of the four neuroleptic pretreatments used had no impact on MK801-depressed grooming; only 0.04 mg/kg haloperidol increased this behavior (Fig. 4B, right). Statistical comparisons of total time spent grooming (Fig. 4C) identified a significant overall main effect of drug treatment and a significant interaction between drug treatment and sex (Table 1). Subsequent within-group, across-sex comparisons confirmed that males treated with 0.1 mg/kg MK801 spent significantly less time grooming than females (Fig. 4C). Within-sex, across group comparisons further identified significant main effects of drug treatment in both sexes (Table 2). The allowed post-hoc tests showed that in males 0.05 mg/kg MK801 had no effect on grooming; that both 0.1 and 0.2 mg/kg significantly reduced grooming relative to control; and that none of the neuroleptic pretreatments impacted grooming activity (Fig. 4C). In contrast, in females 0.05 mg/kg MK801 significantly increased grooming relative to control, 0.1 mg/kg MK801 had no effect on grooming, and 0.2 mg/kg MK801 significantly reduced grooming relative to control (Fig. 4C). Finally, although pretreatment with 0.04 mg/kg haloperidol significantly increase grooming relative to MK801-treated rats, this outcome was determined to be entirely dependent on extremely high grooming scores in two subjects. None of the other neuroleptic pretreatments had any effects of MK801-depressed grooming in this sex (Fig. 4C).
Figure 4.
Effects of MK801 and neuroleptic pretreatment on grooming. All data are expressed as group means ± SEM. A: Line graphs showing the percent time spent grooming per minute during the 15 minute testing session by male (right side) and female (left side) rats that were either vehicle-injected (CTRL, black squares) or treated with 0.05 mg/kg MK801 (0.05 MK, open triangles), 0.1 mg/kg MK801 (0.1 MK, open diamonds) or with 0.2 mg/kg MK801 (0.2 MK, grey circles). B: Line graphs showing the percent time spent grooming per minute during the 15 minute testing session by male (right side) and female (left side) rats that were treated with 0.2 mg/kg MK801 (0.2 MK, grey circles), or were pretreated with 0.04 mg/kg haloperidol (0.04 HDL, open triangles), 0.08 mg/kg haloperidol (0.08 HDL, open squares), 5 mg/kg clozapine (5 CLZ, inverted triangles) or with 10 mg/kg clozapine (10 CLZ, open diamonds) followed by 0.2 mg/kg MK801. C: Bar graphs showing the total percent time spent grooming during the 15 minute testing session by female (white bars) and male (black bars) in all treatment groups. Single asterisks (*) indicate a significant difference of p<0.05 compared to control, double asterisks (**) indicate a significant difference of p<0.001 compared to control; pound signs (#) indicate a significant difference of p<0.001 compared to MK801. Significant sex differences are indicated by horizontal bars; single asterisks indicate a significant difference of p<0.05, double asterisks indicate a significant difference of p<0.001.
Stationary Behavior
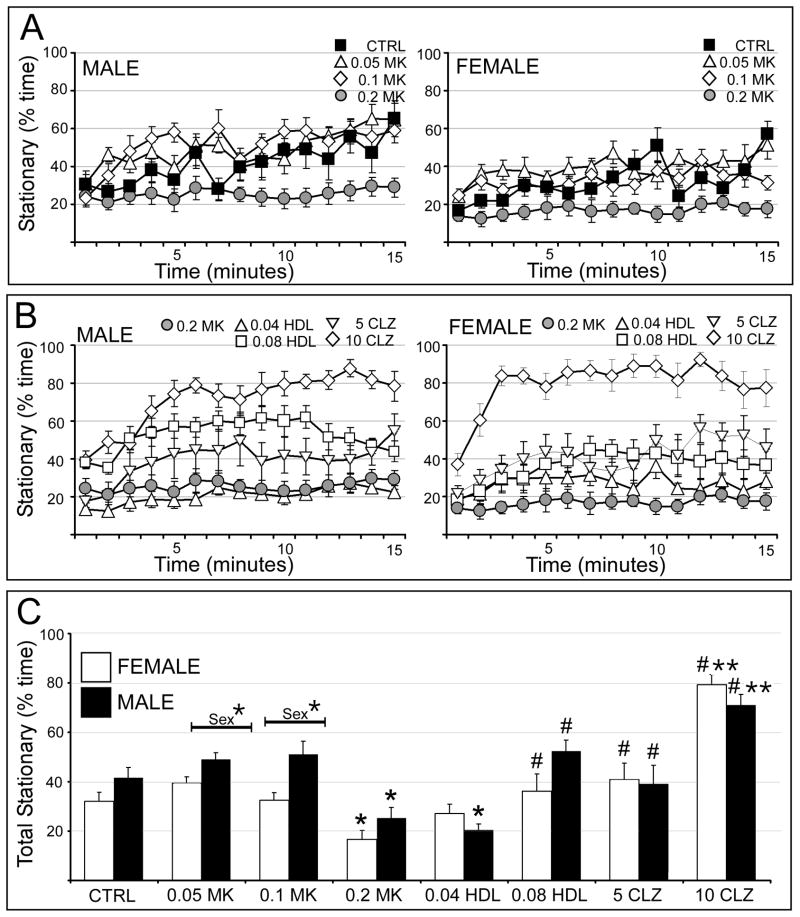
Vehicle injected rats of both sexes spent similar, minimal amounts of time still at the beginning of testing trials and progressively more time stationary as testing proceeded (Fig. 5A). Both 0.05 and 0.1 mg/kg MK801 had no appreciable effects on stationary behavior in either sex (Fig. 5A). However, in both sexes 0.2 mg/kg MK801 decreased the amounts of time animals spent standing still (Fig. 5A). In males, 0.04 mg/kg haloperidol had no effect on MK801-suppression of stationary behavior, 0.08 mg/kg haloperidol and 5 mg/kg clozapine reinstated more normal levels of stationary behavior, and 10 mg/kg clozapine increased time spent stationary to levels well above those of controls (Fig. 5B, left). Similar effects were noted in females with the exception of a greater relative potency of 0.04 mg/kg haloperidol (Fig. 5B, right). Statistical comparisons of total times spent stationary (Fig. 5C) identified significant overall main effects of drug treatment and of sex and a significant interaction between the two on this behavior (Table 1). Follow up within-group, across-sex comparisons further confirmed that females given 0.05 mg/kg and 0.1 mg/kg MK801 spent significantly less time stationary than males (Fig. 5C). Within-sex, across-group comparisons also identified significant main effects of drug treatment in both sexes (Table 2). The allowed post-hoc tests also confirmed that in both sexes only 0.2 mg/kg MK801 significantly decreased stationary time relative to control; that 0.04 mg/kg haloperidol had no impact on MK801-depressed stationary behavior; that 0.08 mg/kg haloperidol and 5 mg/kg clozapine significantly increased stationary behavior relative to MK801-treated rats to levels indistinguishable from control; and that 10 mg/kg clozapine increased stationary behavior to levels that were significantly higher than control (Fig. 5C).
Figure 5.
Effects of MK801 and neuroleptic pretreatment on stationary behavior. All data are expressed as group means ± SEM. A: Line graphs showing the percent time spent stationary per minute during the 15 minute testing session by male (right side) and female (left side) rats that were either vehicle-injected (CTRL, black squares) or treated with 0.05 mg/kg MK801 (0.05 MK, open triangles), 0.1 mg/kg MK801 (0.1 MK, open diamonds) or with 0.2 mg/kg MK801 (0.2 MK, grey circles). B: Line graphs showing the percent time spent stationary per minute during the 15 minute testing session by male (right side) and female (left side) rats that were treated with 0.2 mg/kg MK801 (0.2 MK, grey circles), or were pretreated with 0.04 mg/kg haloperidol (0.04 HDL, open triangles), 0.08 mg/kg haloperidol (0.08 HDL, open squares), 5 mg/kg clozapine (5 CLZ, inverted triangles) or with 10 mg/kg clozapine (10 CLZ, open diamonds) followed by 0.2 mg/kg MK801. C: Bar graphs showing the total percent time spent stationary during the 15 minute testing session by female (white bars) and male (black bars) in all treatment groups. Single asterisks (*) indicate a significant difference of p<0.05 compared to control, double asterisks (**) indicate a significant difference of p<0.001 compared to control; pound signs (#) indicate a significant difference of p<0.001 compared to MK801. Significant sex differences are indicated by horizontal bars; single asterisks indicate a significant difference of p<0.05, double asterisks indicate a significant difference of p<0.001.
Stereotypy
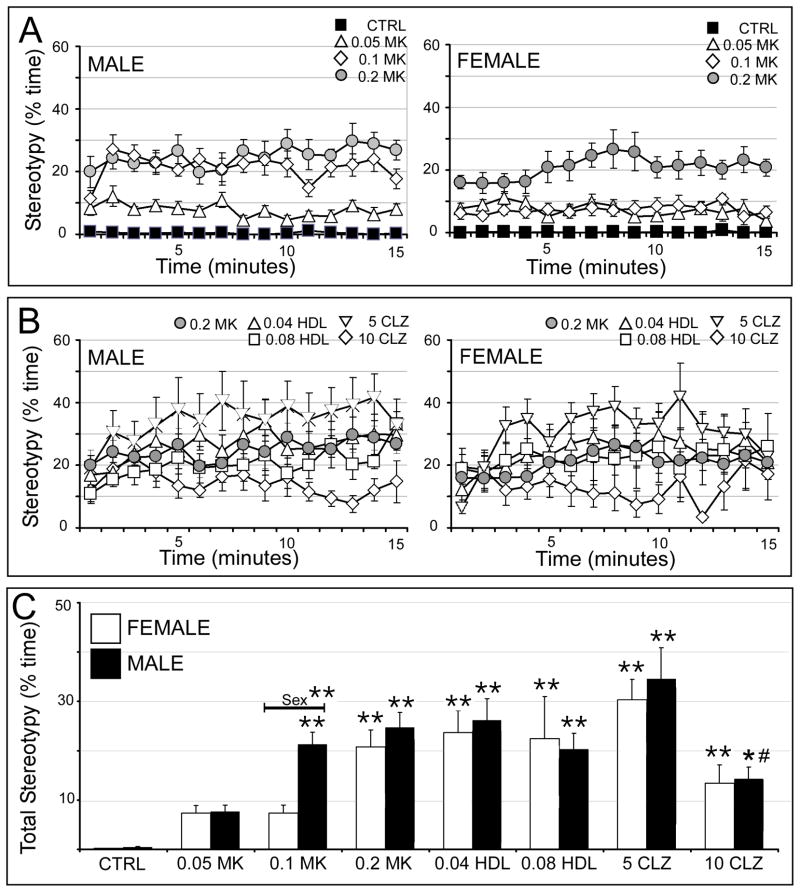
Stereotypy was not observed in vehicle-treated rats (Fig. 6A). However, MK801 at all three doses elicited clear examples of these abnormal, repetitive behaviors in both sexes (Fig. 6A). For males, 0.05 mg/kg MK801 produced a relatively modest expression of stereotypy relative to the more potent and similar effects of drug at 0.1 and 0.2 mg/kg (Fig. 6A, left). For females, both 0.05 and 0.1 mg/kg MK801 produced modest increases in stereotypy, whereas 0.2 mg/kg MK801 showed greater potency in eliciting these behaviors (Fig. 6A, right). In both sexes, pretreatments with haloperidol (both doses) and with 5 mg/kg clozapine failed to reduce stereotypy and 10 mg/kg clozapine diminished but did not eliminate these aberrant behaviors (Fig. 6B). Statistical comparisons of total time spent in stereotypy (Fig. 6C) identified a significant overall main effect of drug treatment but no significant effects or interactions with sex on this outcome measure (Table 1). Subsequent across-sex, within-group comparisons further confirmed that treatment with 0.1 mg/kg had significantly greater effects on stereotypy in males than in females (Fig. 6C). Within-sex, across-group comparisons further identified significant main effects of drug treatment in both sexes (Table 2). Allowed post-hoc testing in males showed that both 0.1 mg/kg and 0.2 mg/kg MK801 significantly increased stereotypy relative to control; that haloperidol (both doses) and 5 mg/kg clozapine had no effect on MK801-induced stereotypy; and that pretreatment with 10 mg/kg clozapine significantly reduced stereotypy relative to MK801, albeit not to control levels (Fig. 6C). In contrast, in females, only 0.2 mg/kg MK801 significantly increased stereotypy relative to control and none of the neuroleptic pretreatments had significant effects on this metric (Fig. 6C).
Figure 6.
Effects of MK801 and neuroleptic pretreatment on stereotypic behavior. All data are expressed as group means ± SEM. A: Line graphs showing the percent time engaged in stereotypy per minute during the 15 minute testing session by male (right side) and female (left side) rats that were either vehicle-injected (CTRL, black squares) or treated with 0.05 mg/kg MK801 (0.05 MK, open triangles), 0.1 mg/kg MK801 (0.1 MK, open diamonds) or with 0.2 mg/kg MK801 (0.2 MK, grey circles). B: Line graphs showing the percent time engaged in stereotypy per minute during the 15 minute testing session by male (right side) and female (left side) rats that were treated with 0.2 mg/kg MK801 (0.2 MK, grey circles), or were pretreated with 0.04 mg/kg haloperidol (0.04 HDL, open triangles), 0.08 mg/kg haloperidol (0.08 HDL, open squares), 5 mg/kg clozapine (5 CLZ, inverted triangles) or with 10 mg/kg clozapine (10 CLZ, open diamonds) followed by 0.2 mg/kg MK801. C: Bar graphs showing the total percent time engaged in stereotypy during the 15 minute testing session by female (white bars) and male (black bars) in all treatment groups. Single asterisks (*) indicate a significant difference of p<0.05 compared to control, double asterisks (**) indicate a significant difference of p<0.001 compared to control; pound signs (#) indicate a significant difference of p<0.001 compared to MK801. Significant sex differences are indicated by horizontal bars; single asterisks indicate a significant difference of p<0.05, double asterisks indicate a significant difference of p<0.001.
Ataxia
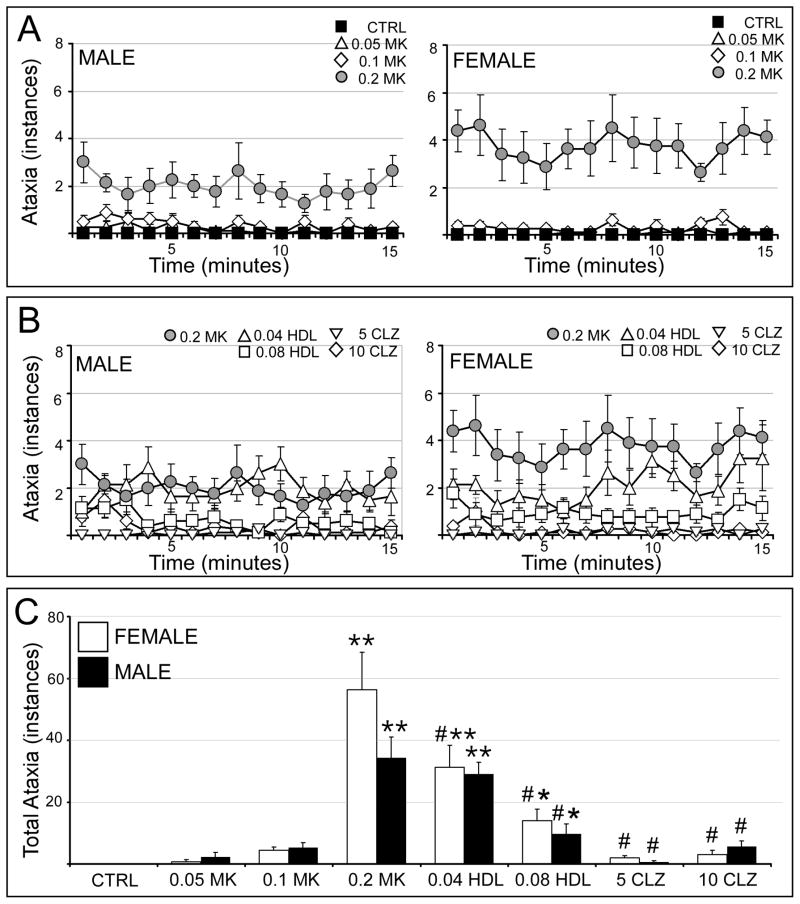
Ataxia was not observed in vehicle-injected rats and was rare in rats of both sexes given 0.05 or 0.1 mg/kg MK801 (Fig. 7A). However, following 0.2 mg/kg MK801, males displayed numerous instances of ataxia (Fig. 7A, left) and females even more so (Fig. 7A, right). In males, pretreatment with 0.08 mg/kg but not 0.04 mg/kg haloperidol diminished ataxia, while pretreatments with clozapine (both doses) more effectively did so (Fig. 7B, left). In females, haloperidol dose dependently reduced ataxia but was less effective overall than clozapine (both doses) in diminishing these abnormal signs (Fig. 7B, right). Statistical comparisons of total ataxia scores (Fig. 7C) identified a significant overall main effect of treatment but no significant effects or interactions with sex (Table 1). Subsequent across-sex, within-group comparisons further confirmed that there were no significant sex differences in ataxia among vehicle or drug-treated groups (Fig. 7C). Within-sex, across group comparisons next identified significant main effects of drug treatment in both sexes (Table 2). Allowed post hoc testing revealed that in males and females alike, 0.05 mg/kg and 0.1 mg/kg MK801 had no significant effects on ataxia; that 0.2 mg/kg MK801 significantly increased ataxia relative to control; and that both doses of clozapine significantly reduced ataxia to levels that were statistically indistinguishable from control (Fig. 7C). Only haloperidol was found to have sex-specific effects; while both doses of haloperidol significantly reduced ataxia in females, only 0.08 mg/kg haloperidol significantly diminished ataxia in males (Fig. 7C).
Figure 7.
Effects of MK801 and neuroleptic pretreatment on ataxia. All data are expressed as group means ± SEM. A: Line graphs showing the number of ataxic events occurring per minute during the 15 minute testing session by male (right side) and female (left side) rats that were either vehicle-injected (CTRL, black squares) or treated with 0.05 mg/kg MK801 (0.05 MK, open triangles), 0.1 mg/kg MK801 (0.1 MK, open diamonds) or with 0.2 mg/kg MK801 (0.2 MK, grey circles). B: Line graphs showing the mean number of ataxic events occurring per minute during the 15 minute testing session by male (right side) and female (left side) rats that were treated with 0.2 mg/kg MK801 (0.2 MK, grey circles), or were pretreated with 0.04 mg/kg haloperidol (0.04 HDL, open triangles), 0.08 mg/kg haloperidol (0.08 HDL, open squares), 5 mg/kg clozapine (5 CLZ, inverted triangles) or with 10 mg/kg clozapine (10 CLZ, open diamonds) followed by 0.2 mg/kg MK801. C: Bar graphs showing the total number of ataxic events occurring during the 15 minute testing session by female (white bars) and male (black bars) in all treatment groups. Single asterisks (*) indicate a significant difference of p<0.05 compared to control, double asterisks (**) indicate a significant difference of p<0.001 compared to control; pound signs (#) indicate a significant difference of p<0.001 compared to MK801. Significant sex differences are indicated by horizontal bars; single asterisks indicate a significant difference of p<0.05, double asterisks indicate a significant difference of p<0.001.
DISCUSSION
This study explored sex differences in MK801-induced spontaneous open field behaviors and their attenuation by pretreatments with typical and atypical neuroleptics in adult male and female rats. While previous studies have described female over male differences in the ability of acute administration of ketamine, phencyclidine and MK801 to stimulate motor behaviors and abnormalities in rodents (Honack and Loscher, 1993, Andine et al., 1999, Frantz and Van Hartesveldt, 1999). (Becker et al., 1982, Nabeshima et al., 1984, Wilson et al., 2007), we expanded on these in several ways. First, we examined seven discrete behaviors, thus going beyond the hyperlocomotion, stereotypy and ataxia that have been mainly to exclusively studied in the past. Further, all behaviors were quantified as continuous variables (rather than rating scales) within 30 minutes of drug injection. This placed all analyses well within a period when IP injection of MK801 at the same doses and in male and female rats of the same strain and approximate size has been shown to produce similar, peaking brain drug levels in both sexes-- and well before the hours long period when sex differences in hepatic metabolism/clearance yield brain drug levels that are several fold higher in females (Nabeshima et al., 1984, Andine et al., 1999). Finally, among all studies using female rats, this is the only one to document and strictly control hormone status. To both minimize within-group variance and maximize across-sex differences in circulating estrogen and progestin levels, all female subjects in this study were tested during the proestrus phase of the estrus cycle when circulating ovarian hormones are at their highest. By combining these strategies with both dose-response and neuroleptic pretreatment challenges, new and comprehensive evidence has been obtained for greater male responses to the effects of MK801 on some behaviors, for greater female responses to drug in others and for similarities across sex in the abilities of haloperidol and clozapine to attenuate them. Below, these findings are placed in contexts of the extant basic science literature. Given the differences noted in NMDAR hypofunction induced by ketamine, PCP, MK801 and other NMDAR antagonists (Danysz et al., 1994, Ogren and Goldstein, 1994, Ellison, 1995), these discussions center mainly on studies using MK801. The data are also considered in relation to the clinical literature describing sex differences in schizophrenia and its treatment and the strengths and limitations of acute NMDAR hypofunction as models for these are discussed.
Sex Differences in the Effects of MK801 on Motor and Other Open Field Behaviors: Comparisons to Previous Studies
Our studies revealed no significant sex differences among vehicle-treated rats in any of the seven open field behaviors measured. However, each behavior differed significantly across sex in the MK801-treated groups. In sum, MK801 more potently stimulated locomotion and ataxia and more strongly inhibited stationary behavior in females compared to males, while more potently stimulating stereotypy and thigmotaxis and more strongly inhibiting rearing and grooming in males compared to females.
The findings obtained with respect to hyperlocomotion and ataxia are consistent and confirmatory of a substantial literature in male rats (Dunn et al., 1989, Honack and Loscher, 1993, Danysz et al., 1994, Carey et al., 1998, Andine et al., 1999, Frantz and Van Hartesveldt, 1999, Devaud et al., 2002, Devaud, 2003, Bubenikova-Valesova et al., 2007, Scorza et al., 2008, Wegener et al., 2011) and of the smaller literature including females in the analysis(Loscher and Honack, 1992, Honack and Loscher, 1993, Andine et al., 1999, Frantz and Van Hartesveldt, 1999, Devaud et al., 2002, Devaud, 2003, Le Pen et al., 2011). However, findings with regard to stereotypy, the third of a triad of behaviors defining MK801’s so-called motor syndrome, are less concordant. For example, some studies in males identified doses of MK801 of 0.2 mg/kg or higher as necessary to stimulate qualifying repetitive movements(Honack and Loscher, 1993, Andine et al., 1999). However, others including the present study observed stereotypy at doses as low as 0.05 mg/kg (Dall’Olio et al., 1996, Scorza et al., 2008). The source of these disparities is unclear as findings do not readily parse with study-to-study differences in the inclusivity/exclusivity of defining behaviors or in the use of rating scales vs. continuous variables to quantify them. Further, for females there is good agreement that MK801 dose-dependently elicits stereotypy in the 0.05 mg/kg–0.2 mg/kg range (Honack and Loscher, 1993, Andine et al., 1999). However, previous studies report that stereotypy in this sex was more pronounced than in males (Honack and Loscher, 1993, Andine et al., 1999, Devaud et al., 2002, Devaud, 2003). This contrasts with data from this study showing the opposite. Although there are methodological differences among these studies, it is unclear that these can account for what are in essence their ratiometric male-to-female differences. However, what may be relevant are findings showing that MK801-induced stereotypy in females significantly outlasts what are more transient effects on this behavior in males (Honack and Loscher, 1993). Thus, the significantly longer periods of time over which behavior was summed in the prior studies included periods when stereotypy had diminished in males but not females. This could explain the overall female over male differences observed. Our use of narrower time windows to evaluate behavior avoided such contributions to sex the differences observed.
The literature describing MK801’s open field effects on behaviors beyond the motor syndrome is much more limited. For example, depressive actions of MK801 on rearing, grooming and stationary behavior here been reported, albeit only in studies in males and these are often limited to qualitative observations (Danysz et al., 1994, Carey et al., 1998, Andine et al., 1999, Devaud et al., 2002, Devaud, 2003). The present study helps bring quantitative consensus to these data. Thus across studies, there is growing agreement that MK801 in the 0.05–0.1 mg/kg range suppresses grooming and rearing but that higher doses (0.2 mg/kg or higher) are needed to decrease stationary behavior (Dall’Olio et al., 1996, Scorza et al., 2008, Dubiela et al., 2011). This study also provides the first assessments of these behaviors in females and the new findings of a markedly lower potency of MK801 in diminishing rearing, grooming and stationary behavior in this sex. Finally, this study is also the first to quantify the effects of MK801 on open-field thigmotaxis. This endpoint proved to be the most strikingly sexually dimorphic. Whereas thigmotaxis was unchanged by MK801 in females it was dose dependently increased by MK801 in the 0.1 mg/kg to 0.2 mg/kg range in males.
Sex Differences in Neuroleptic Attenuation of MK801 Effects on Open Field Behaviors: Comparisons to Previous Studies
The attenuation of MK801 effects on spontaneous open field activities by pretreatments with the typical neuroleptic haloperidol and the atypical neuroleptic clozapine was also assessed. Both drugs are in current clinical use, and for each the two doses that were used corresponded to the high and low ends of their human therapeutic range(Kapur et al., 2003). Overall, the effectiveness of haloperidol and clozapine to ameliorate MK801 effects varied with behavior and often differed from one another. However, there was little to no difference noted in the efficacy of either drug at either dose across sex.
The results obtained with respect to the MK801 motor syndrome were largely in keeping with a modest existing literature in male and/or female rats. Thus, all studies including the present agree that haloperidol dose-dependently reduces MK801-induced hyperlocomotion and axtaxia and that clozapine is more effective in doing so in both male (Hoffman, 1992, Gattaz et al., 1994, Ninan and Kulkarni, 1998, Scorza et al., 2008) and female rats (Loscher and Honack, 1992, Andine et al., 1999, Le Pen et al., 2011). However, while previous studies have also shown that both typical and atypical neuroleptics can attenuate MK801-induced stereotypy (Tiedtke et al., 1990, Behrens and Gattaz, 1992, Loscher and Honack, 1992, Gattaz et al., 1994, Scorza et al., 2008, Le Pen et al., 2011) in this study, only the higher (10 mg/kg) dose of clozapine was able to reduce these abnormal repetitive behaviors. Finally, there is only one paper that we are aware of that investigated neuroleptic attenuation of MK801 effects on open field behaviors beyond those of the motor syndrome. This study found no impact of 1.0 mg/kg clozapine on MK801-depressed rearing in male rats (Scorza et al., 2008). In this study, we found that neither haloperidol nor clozapine restored MK801-diminished rearing or grooming in either sex. However, clozapine was more potent than haloperidol- again in both sexes, in restoring stationary behavior while both drugs were similarly effective in diminishing the MK801-induced increase in thigmotaxis that was seen only in males.
The doses of haloperidol and clozapine used in this study were selected to model human therapeutics. However, in evaluating outcomes it is important to consider the sedating properties that haloperidol and clozapine have in rodents. While obscured by basement effects associated with MK801’s inhibition of grooming in both sexes and of rearing in females, it was evident that the higher doses of both haloperidol and clozapine used exacerbated MK801 depression of rearing in males and increased time spent stationary in both sexes to levels exceeding those of drug naïve controls. The seemingly sedating actions of 0.8 mg/kg haloperidol were unexpected since previous studies report no evidence that doses similar to and higher than this produce overt sedation in drug naïve adult Sprague Dawley rats (Salamone et al., 1996). However, for clozapine sedation has been shown at the 10 mg/kg dose in drug-free animals (Salamone et al., 1996) and was evident in this study in measures of MK801-affected stationary behavior and locomotion. Thus, at the higher doses used, the ability haloperidol and clozapine to attenuate MK801 effects via neurochemically specific mechanisms cannot be readily divorced from sedation. Nonetheless, comparisons across sex revealed remarkably similar effects in males and females of both drugs at both doses used for all behaviors. There were no indications of the significantly greater drug responses noted in schizophrenia among female patient populations(Szymanski et al., 1996, Goldstein et al., 2002, Seeman, 2006, Usall et al., 2007, Cotton et al., 2009, Seeman, 2012). Thus, the predictive power established for the acute MK801/NMDAR hyopfunction model may not extend to the area of sex-specific therapeutics. Given that the more rapid time course and/or increased neuroleptic sensitivities that have been described clinically in fact occur over days to weeks (Goldstein et al., 2002, Usall et al., 2007, Cotton et al., 2009) it is likely that chronic models that incorporate differences in drug metabolism or fat storage may be needed to unravel the basis for sex differences in the treatment of schizophrenia and related psychoses.
The NMDAR Hypofunction Model and Sex Differences in Schizophrenia
Sex differences have been a part of the clinical profile of schizophrenia since its initial description by Kraeplin as a thought disorder in males. Although findings do not always agree, observations of sex differences in schizophrenia and first episode psychoses can be distilled into a set of principal findings for which there is wide consensus. These include etiologic data indicating that males suffer more often and/or more severely from negative symptoms and display more disorganization/disorientation of thought and behavior; females on the other hand more frequently display co-morbid symptoms of anxiety or depression and have been noted to suffer more positive symptoms (Leung and Chue, 2000, Seeman, 2006, Canuso and Pandina, 2007, Elias and Kumar, 2007, Ochoa et al., 2012).
Although sex differences are important features of schizophrenia, there is little understanding of what causes them. This is due in part to the current lack of preclinical animal models that are validated for studies of this aspect of disease. A recent critical appraisal of the NMDAR hypofunction model reaffirmed its applicability for investigating multiple at risk behavioral domains in this complex mental illness(Adell et al., 2012). The present study showing that the effects of MK801 on discrete open field behaviors not only differ across sex but do so in behaviorally specific ways suggest that this model also meets minimum criterion of producing complex patterns of male/female vulnerability that are potentially commensurate with those seen across symptoms and symptom clusters in organic disease. More specific parallels can also be appreciated by considering the clinical dimensions that given endpoints measured in open field testing are thought to represent. Thus, as behaviors that are exaggerated or not normally observed, the elements of MK801’s motor syndrome (hyperlocomotion, stereotypy, ataxia) have been collectively considered as models for schizophrenia’s positive symptoms while the absence of rearing and grooming meet criteria for correlates of schizophrenia’s negative signs(Tiedtke et al., 1990, Andine et al., 1999, Powell et al., 2009). Within these broad interpretive frameworks there is evidence that acute MK801 models both the especial vulnerability of females to schizophrenia’s positive symptoms, i.e., MK801 induces greater hyperlocomotion and ataxia in female rats, and the increased succeptibility of males to schizophrenia’s negative signs, i.e., MK801 more potently inhibits rearing in grooming in male rats. However, unlike the effects of MK801 on prepulse inhibition that map closely to the sensorimotor gating deficits demonstrated in schizophrenic patients (Swerdlow et al., 2000, Geyer et al., 2001, Geyer, 2006, Gururajan et al., 2010), there are clear qualitative differences in the open field behaviors that are induced and inhibited by MK801 in rodents and those that embody schizophrenia’s positive and negative symptoms. For example, while hyperlocomotion has argued face validity for positive signs such as psychotic agitation (Tiedtke et al., 1990, Andine et al., 1999, Powell et al., 2009), MK801 induced ataxia more more closely resembles the cerebellar disturbances seen in schizophrenia which include abnormalities in posture and in the sequencing movements (Andreasen and Pierson, 2008, Kasparek et al., 2012). Because there are currently no clinical data regarding sex differences in these cerebellar signs, it is unclear how this interpretation of MK801’s effects bears on the question of its validity for modeling sex differences in disease. However, arguments for the purposeless, repetitive movements that define MK801-induced stereotypy as modeling the disorganized behaviors associated with schizophrenia (Ochoa et al., 2012) potentially supports the model as the male over female differences in MK801-induced stereotypy observed in this study parallel the predominance of disorganized behavior in schizophrenic males. Finally, decrements in the exploratory activity of rearing and in self grooming can occur for several reasons. However, because these deficits emerge at MK801 doses lower than those producing significant ataxia or hyperlocomotion, their diminution/absence is unlikely to be due to motor confounds and could as previously suggested be an index animals’ avolition or amotivation(Scorza et al., 2008). These are two common manifestations of the male-predominating negative symptoms of schizophrenia (Messinger et al., 2011). That we found rearing and grooming were both more potently inhibited by MK801 in male than in females rats is thus in keeping with this acute NMDAR hypofunction model as providing a good match for the most consistent of all sex differences noted in schizophrenia--the predominance of negative signs in males(Elias and Kumar, 2007, Ko et al., 2007).
In sum, there are a number of intriguing relationships between the disparate effects of acute MK801 on spontaneous open field behaviors in male and female rats and consensus views about the sex differences that distinguish specific sets of symptoms in schizophrenia. It should be noted, however, that there also are notable exceptions. For example, we found that MK801 stimulation of thigmotaxis, an accepted index of anxiety in rodents (Treit and Fundytus, 1988) was unique to male rats. This is the opposite of what is observed in schizophrenia, where female patients are significantly more likely than males to exhibit co-morbid signs of pathological anxiety or depression(Leung and Chue, 2000, Canuso and Pandina, 2007, Ochoa et al., 2012). Nonetheless, despite certain caveats and limitations, the present results suggest a fruitfulness of further query, e.g., using additional paradigms and measures of behavior, to explore sex differences in the acute NMDAR hypofunction model and define its validity for investigating the impact that biological sex and sex hormones have on the clinical profile of schizophrenia and the behavioral constructs that are at risk in this disorder.
Highlights.
MK801 has sex-specific effects on seven open field behaviors in rats.
The sex-specific effects of MK801 parallel some sex differences in schizophrenia
Neuroleptics have similar effects on MK801-induced behaviors in male and female rats
NMDAR hypofunction models may be useful in studying sex differences in psychosis
Acknowledgments
Sources of support: The work was supported by an RO1 award from NINDS/NIH to MFK (RO1 NS 041966).
The authors thank Ms. Lisa Servilio and Ms. Aiying Liu for their valued technical assistance with these studies. The work was supported by an RO1 award from NINDS/NIH to MFK (RO1 NS 041966).
Footnotes
There are no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Adell A, Jimenez-Sanchez L, Lopez-Gil X, Romon T. Is the acute NMDA receptor hypofunction a valid model of schizophrenia? Schizophr Bull. 2012;38:9–14. doi: 10.1093/schbul/sbr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andine P, Widermark N, Axelsson R, Nyberg G, Olofsson U, Martensson E, Sandberg M. Characterization of MK-801-induced behavior as a putative rat model of psychosis. J Pharmacol Exp Ther. 1999;290:1393–1408. [PubMed] [Google Scholar]
- Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S. Modeling cholinergic aspects of schizophrenia: focus on the antimuscarinic syndrome. Behav Brain Res. 2009;204:335–351. doi: 10.1016/j.bbr.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Becker JB, Robinson TE, Lorenz KA. Sex differences and estrous cycle variations in amphetamine-elicited rotational behavior. Eur J Pharmacol. 1982;80:65–72. doi: 10.1016/0014-2999(82)90178-9. [DOI] [PubMed] [Google Scholar]
- Behrens S, Gattaz WF. MK-801 induced stereotypies in rats are decreased by haloperidol and increased by diazepam. J Neural Transm Gen Sect. 1992;90:219–224. doi: 10.1007/BF01250962. [DOI] [PubMed] [Google Scholar]
- Bubenikova-Valesova V, Votava M, Palenicek T, Horacek J. The opposite effect of a low and a high dose of serotonin-1A agonist on behavior induced by MK-801. Neuropharmacology. 2007;52:1071–1078. doi: 10.1016/j.neuropharm.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Canuso CM, Pandina G. Gender and schizophrenia. Psychopharmacol Bull. 2007;40:178–190. [PubMed] [Google Scholar]
- Carey RJ, Dai H, Gui J. Effects of dizocilpine (MK-801) on motor activity and memory. Psychopharmacology (Berl) 1998;137:241–246. doi: 10.1007/s002130050616. [DOI] [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS, Williams GV. Animal models of working memory: insights for targeting cognitive dysfunction in schizophrenia. Psychopharmacology (Berl) 2004;174:111–125. doi: 10.1007/s00213-003-1710-9. [DOI] [PubMed] [Google Scholar]
- Corbett R. Clozapine but not haloperidol antagonizes an MK-801 discriminative stimulus cue. Pharmacol Biochem Behav. 1995;51:561–564. doi: 10.1016/0091-3057(95)00072-5. [DOI] [PubMed] [Google Scholar]
- Cotton SM, Lambert M, Schimmelmann BG, Foley DL, Morley KI, McGorry PD, Conus P. Gender differences in premorbid, entry, treatment, and outcome characteristics in a treated epidemiological sample of 661 patients with first episode psychosis. Schizophr Res. 2009;114:17–24. doi: 10.1016/j.schres.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Dall’Olio R, Rimondini R, Gandolfi O. Effect of NMDA receptor antagonists on D1, D2 and D1/D2 mediated behaviors in intact rats. Psychopharmacology (Berl) 1996;123:187–190. doi: 10.1007/BF02246176. [DOI] [PubMed] [Google Scholar]
- Danysz W, Essmann U, Bresink I, Wilke R. Glutamate antagonists have different effects on spontaneous locomotor activity in rats. Pharmacol Biochem Behav. 1994;48:111–118. doi: 10.1016/0091-3057(94)90506-1. [DOI] [PubMed] [Google Scholar]
- Devaud LL. Effects of ethanol or rimcazole on dizocilpine maleate-induced behaviors in male and female rats. Alcohol. 2003;29:69–81. doi: 10.1016/s0741-8329(02)00326-9. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Bartoo G, Malthankar G. Altered responses to dizocilpine maleate administration in ethanol-withdrawn male and female rats. Alcohol. 2002;28:83–93. doi: 10.1016/s0741-8329(02)00239-2. [DOI] [PubMed] [Google Scholar]
- Dubiela FP, Messias MF, Moreira KD, Zanlorenci LH, Grassl C, Filho RF, Nobrega JN, Tufik S, Hipolide DC. Reciprocal interactions between MK-801, sleep deprivation and recovery in modulating rat behaviour. Behav Brain Res. 2011;216:180–185. doi: 10.1016/j.bbr.2010.07.031. [DOI] [PubMed] [Google Scholar]
- Dunn RW, Corbett R, Fielding S. Effects of 5-HT1A receptor agonists and NMDA receptor antagonists in the social interaction test and the elevated plus maze. Eur J Pharmacol. 1989;169:1–10. doi: 10.1016/0014-2999(89)90811-x. [DOI] [PubMed] [Google Scholar]
- Elias A, Kumar A. Testosterone for schizophrenia. Cochrane Database Syst Rev. 2007:CD006197. doi: 10.1002/14651858.CD006197.pub2. [DOI] [PubMed] [Google Scholar]
- Ellison G. The N-methyl-D-aspartate antagonists phencyclidine, ketamine and dizocilpine as both behavioral and anatomical models of the dementias. Brain Res Brain Res Rev. 1995;20:250–267. doi: 10.1016/0165-0173(94)00014-g. [DOI] [PubMed] [Google Scholar]
- Everett JW. Neurobiology of reproduction in the female rat. A fifty-year perspective. Monogr Endocrinol. 1989;32:1–133. [PubMed] [Google Scholar]
- Featherstone RE, Kapur S, Fletcher PJ. The amphetamine-induced sensitized state as a model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1556–1571. doi: 10.1016/j.pnpbp.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Frantz K, Van Hartesveldt C. Locomotion elicited by MK801 in developing and adult rats: temporal, environmental, and gender effects. Eur J Pharmacol. 1999;369:145–157. doi: 10.1016/s0014-2999(99)00070-9. [DOI] [PubMed] [Google Scholar]
- Gaisler-Salomon I, Weiner I. Systemic administration of MK-801 produces an abnormally persistent latent inhibition which is reversed by clozapine but not haloperidol. Psychopharmacology (Berl) 2003;166:333–342. doi: 10.1007/s00213-002-1311-z. [DOI] [PubMed] [Google Scholar]
- Gattaz WF, Schummer B, Behrens S. Effects of zotepine, haloperidol and clozapine on MK-801-induced stereotypy and locomotion in rats. J Neural Transm Gen Sect. 1994;96:227–232. doi: 10.1007/BF01294789. [DOI] [PubMed] [Google Scholar]
- Geyer MA. The family of sensorimotor gating disorders: comorbidities or diagnostic overlaps? Neurotox Res. 2006;10:211–220. doi: 10.1007/BF03033358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth defects research Part B, Developmental and reproductive toxicology. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Cohen LS, Horton NJ, Lee H, Andersen S, Tohen M, Crawford A, Tollefson G. Sex differences in clinical response to olanzapine compared with haloperidol. Psychiatry Res. 2002;110:27–37. doi: 10.1016/s0165-1781(02)00028-8. [DOI] [PubMed] [Google Scholar]
- Gururajan A, Taylor DA, Malone DT. Effect of testing conditions on the propsychotic action of MK-801 on prepulse inhibition, social behaviour and locomotor activity. Physiol Behav. 2010;99:131–138. doi: 10.1016/j.physbeh.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Hoffman DC. Typical and atypical neuroleptics antagonize MK-801-induced locomotion and stereotypy in rats. J Neural Transm Gen Sect. 1992;89:1–10. doi: 10.1007/BF01245347. [DOI] [PubMed] [Google Scholar]
- Honack D, Loscher W. Sex differences in NMDA receptor mediated responses in rats. Brain Res. 1993;620:167–170. doi: 10.1016/0006-8993(93)90287-w. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Balla A, Sershen H, Lajtha A. A.E. Bennett Research Award. Reversal of phencyclidine-induced effects by glycine and glycine transport inhibitors. Biol Psychiatry. 1999;45:668–679. doi: 10.1016/s0006-3223(98)00237-6. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Jones CA, Watson DJ, Fone KC. Animal models of schizophrenia. Br J Pharmacol. 2011;164:1162–1194. doi: 10.1111/j.1476-5381.2011.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther. 2003;305:625–631. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- Kasparek T, Rehulova J, Kerkovsky M, Sprlakova A, Mechl M, Mikl M. Cortico-cerebellar functional connectivity and sequencing of movements in schizophrenia. BMC Psychiatry. 2012;12:17. doi: 10.1186/1471-244X-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko YH, Jung SW, Joe SH, Lee CH, Jung HG, Jung IK, Kim SH, Lee MS. Association between serum testosterone levels and the severity of negative symptoms in male patients with chronic schizophrenia. Psychoneuroendocrinology. 2007;32:385–391. doi: 10.1016/j.psyneuen.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Ko YH, Lew YM, Jung SW, Joe SH, Lee CH, Jung HG, Lee MS. Short-term testosterone augmentation in male schizophrenics: a randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2008;28:375–383. doi: 10.1097/JCP.0b013e31817d5912. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Anand A, Moghaddam B. Effects of NMDA receptor antagonists: implications for the pathophysiology of schizophrenia. Arch Gen Psychiatry. 2002;59:663–664. doi: 10.1001/archpsyc.59.7.663. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Kulkarni J, Hayes E, Gavrilidis E. Hormones and schizophrenia. Curr Opin Psychiatry. 2012;25:89–95. doi: 10.1097/YCO.0b013e328350360e. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Jay TM, Krebs MO. Effect of antipsychotics on spontaneous hyperactivity and hypersensitivity to MK-801-induced hyperactivity in rats prenatally exposed to methylazoxymethanol. J Psychopharmacol. 2011;25:822–835. doi: 10.1177/0269881110387839. [DOI] [PubMed] [Google Scholar]
- Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl. 2000;401:3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x. [DOI] [PubMed] [Google Scholar]
- Lodge D, Johnson KM. Noncompetitive excitatory amino acid receptor antagonists. Trends Pharmacol Sci. 1990;11:81–86. doi: 10.1016/0165-6147(90)90323-z. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Gestational methylazoxymethanol acetate administration: a developmental disruption model of schizophrenia. Behav Brain Res. 2009;204:306–312. doi: 10.1016/j.bbr.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W, Honack D. The behavioural effects of MK-801 in rats: involvement of dopaminergic, serotonergic and noradrenergic systems. Eur J Pharmacol. 1992;215:199–208. doi: 10.1016/0014-2999(92)90029-4. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Adler CM, Kennison SD, Elman I, Pickar D, Breier A. Clozapine blunts N-methyl-D-aspartate antagonist-induced psychosis: a study with ketamine. Biol Psychiatry. 1997;42:664–668. doi: 10.1016/s0006-3223(96)00546-x. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- Messinger JW, Tremeau F, Antonius D, Mendelsohn E, Prudent V, Stanford AD, Malaspina D. Avolition and expressive deficits capture negative symptom phenomenology: implications for DSM-5 and schizophrenia research. Clin Psychol Rev. 2011;31:161–168. doi: 10.1016/j.cpr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Perry EB, Cho HS, Krystal JH, D’Souza DC. Greater vulnerability to the amnestic effects of ketamine in males. Psychopharmacology (Berl) 2006;187:405–414. doi: 10.1007/s00213-006-0409-0. [DOI] [PubMed] [Google Scholar]
- Nabeshima T, Yamaguchi K, Furukawa H, Kameyama T. Role of sex hormones in sex-dependent differences in phencyclidine-induced stereotyped behaviors in rats. Eur J Pharmacol. 1984;105:197–206. doi: 10.1016/0014-2999(84)90610-1. [DOI] [PubMed] [Google Scholar]
- Nasrallah H, Tandon R, Keshavan M. Beyond the facts in schizophrenia: closing the gaps in diagnosis, pathophysiology, and treatment. Epidemiol Psychiatr Sci. 2011;20:317–327. doi: 10.1017/s204579601100062x. [DOI] [PubMed] [Google Scholar]
- Ninan I, Kulkarni SK. Preferential blockade by clozapine of hyperlocomotion induced by non-competitive NMDA antagonist MK-801. Indian J Physiol Pharmacol. 1998;42:375–382. [PubMed] [Google Scholar]
- Ochoa S, Usall J, Cobo J, Labad X, Kulkarni J. Gender differences in schizophrenia and first-episode psychosis: A comprehensive literature review. Schizophrenia Research and Treatment. 2012;2012:1–9. doi: 10.1155/2012/916198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren SO, Goldstein M. Phencyclidine- and dizocilpine-induced hyperlocomotion are differentially mediated. Neuropsychopharmacology. 1994;11:167–177. doi: 10.1038/sj.npp.1380103. [DOI] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Powell SB, Zhou X, Geyer MA. Prepulse inhibition and genetic mouse models of schizophrenia. Behav Brain Res. 2009;204:282–294. doi: 10.1016/j.bbr.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds IJ, Murphy SN, Miller RJ. 3H-labeled MK-801 binding to the excitatory amino acid receptor complex from rat brain is enhanced by glycine. Proc Natl Acad Sci U S A. 1987;84:7744–7748. doi: 10.1073/pnas.84.21.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Maio C, Champion M, Turski T, Kovach J. Different behavioral effects of haloperidol, clozapine and thioridazine in a concurrent lever pressing and feeding procedure. Psychopharmacology (Berl) 1996;125:105–112. doi: 10.1007/BF02249408. [DOI] [PubMed] [Google Scholar]
- Schwartz PH, Wasterlain CG. Determination of serum and brain concentrations of neuroprotective and non-neuroprotective doses of MK-801. J Neurol Sci. 1993;115:26–31. doi: 10.1016/0022-510x(93)90063-5. [DOI] [PubMed] [Google Scholar]
- Scorza MC, Meikle MN, Hill XL, Richeri A, Lorenzo D, Artigas F. Prefrontal cortex lesions cause only minor effects on the hyperlocomotion induced by MK-801 and its reversal by clozapine. Int J Neuropsychopharmacol. 2008;11:519–532. doi: 10.1017/S1461145708008432. [DOI] [PubMed] [Google Scholar]
- Seeman MV. Estrogen, schizophrenia and neurodevelopment. Womens Health (Lond Engl) 2006;2:571–576. doi: 10.2217/17455057.2.4.571. [DOI] [PubMed] [Google Scholar]
- Seeman MV. Menstrual exacerbation of schizophrenia symptoms. Acta Psychiatr Scand. 2012;125:363–371. doi: 10.1111/j.1600-0447.2011.01822.x. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Hashimoto K, Suzuki Y, Higuchi T. Correlation of plasma neurosteroid levels to the severity of negative symptoms in male patients with schizophrenia. Schizophr Res. 2002;58:69–74. doi: 10.1016/s0920-9964(01)00367-x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon. Behav Pharmacol. 2000;11:185–204. doi: 10.1097/00008877-200006000-00002. [DOI] [PubMed] [Google Scholar]
- Szymanski S, Lieberman J, Pollack S, Kane JM, Safferman A, Munne R, Umbricht D, Woerner M, Masiar S, Kronig M. Gender differences in neuroleptic nonresponsive clozapine-treated schizophrenics. Biol Psychiatry. 1996;39:249–254. doi: 10.1016/0006-3223(95)00138-7. [DOI] [PubMed] [Google Scholar]
- Taherianfard M, Shariaty M. Evaluation of serum steroid hormones in schizophrenic patients. Indian J Med Sci. 2004;58:3–9. [PubMed] [Google Scholar]
- Tiedtke PI, Bischoff C, Schmidt WJ. MK-801-induced stereotypy and its antagonism by neuroleptic drugs. J Neural Transm Gen Sect. 1990;81:173–182. doi: 10.1007/BF01245040. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Davis JM. Adjunct treatments for schizophrenia and bipolar disorder: what to try when you are out of ideas. Clin Schizophr Relat Psychoses. 2012;5:208–216. doi: 10.3371/CSRP.5.4.5. [DOI] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Usall J, Suarez D, Haro JM. Gender differences in response to antipsychotic treatment in outpatients with schizophrenia. Psychiatry Res. 2007;153:225–231. doi: 10.1016/j.psychres.2006.09.016. [DOI] [PubMed] [Google Scholar]
- van den Buuse M. Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophr Bull. 2010;36:246–270. doi: 10.1093/schbul/sbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Serafini R, Stasi MA, Caccia S, Conti I, Tridico RV, Samanin R. Kinetics of MK-801 and its effect on quinolinic acid-induced seizures and neurotoxicity in rats. J Pharmacol Exp Ther. 1989;249:278–283. [PubMed] [Google Scholar]
- Wegener N, Nagel J, Gross R, Chambon C, Greco S, Pietraszek M, Gravius A, Danysz W. Evaluation of brain pharmacokinetics of (+)MK-801 in relation to behaviour. Neurosci Lett. 2011;503:68–72. doi: 10.1016/j.neulet.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Wilson C, Kercher M, Quinn B, Murphy A, Fiegel C, McLaurin A. Effects of age and sex on ketamine-induced hyperactivity in rats. Physiol Behav. 2007;91:202–207. doi: 10.1016/j.physbeh.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Zukin SR, Zukin RS. Specific [3H]phencyclidine binding in rat central nervous system. Proc Natl Acad Sci U S A. 1979;76:5372–5376. doi: 10.1073/pnas.76.10.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]