Abstract
Widening of the electrocardiographic [ECG] spatial QRS-T angle has been predictive of cardiovascular disease [CVD] events in the general population. However, its prognostic significance in HIV-infected individuals remains unknown. Spatial QRS-T angle was derived from the baseline ECG of 4453 HIV-infected patients aged 43.5 (SD 9.3) years from the Strategies for Management of Antiretroviral Therapy [SMART] trial. CVD events were identified during a median follow up of 28.7 months. Quartiles of spatial QRS-T angle was calculated for males and females separately, and values in the upper quartile were considered as widened angle (values above 74° for women and 93° for men). Multivariable Cox proportional hazards analysis was used to examine the association between widened baseline spatial QRS-T angle and incident CVD events. During 11965 person-years of follow-up, 152 CVD events occurred at a rate of 1.27 events per 100 person-years. The rate of CVD events in individuals with widened spatial QRS-T angle was almost double the rate in those with normal spatial QRS-T angle [Rate ratio (95% CI) of 1.94 (1.40, 2.69); p <0.001]. In a model adjusted for study treatment arm, demographics, CVD risk factors, HIV characteristics, inflammatory markers and other ECG abnormalities, widened spatial QRS-T angle was associated with more than 50% increased risk of CVD events compared to normal spatial QRS-T angle [Hazard ratio (95% CI): 1.53 (1.07, 2.17); p= 0.02]. There was no interaction by SMART trial arms [p-value for interaction 0.37] or by gender [p-value for interaction 0.84]. In Conclusion, widened spatial QRS-T angle is independently predictive of CVD events in HIV-infected patients on antiretroviral therapy. This highlights the potential role of routine ECG as a simple non-invasive CVD risk-screening tool in HIV-infected individuals.
Keywords: Spatial QRS-T angle, Electrocardiogram, HIV/AIDS
INTRODUCTION
Developing a cost-effective strategy for prevention and treatment of CVD in HIV-infected population requires identifying simple non-invasive tools for screening and risk stratification. Accordingly, the 12-lead resting electrocardiogram (ECG) can play an important role. We have previously shown that presence of a major ECG abnormality defined as major ventricular conduction defects, major Q/QS, ST-T, or AV conduction abnormalities, left ventricular hypertrophy, atrial fibrillation/flutter, or major QT prolongation as identified by Minnesota ECG Code Classification is a predicator of incident CVD events in HIV-infected individuals [1]. Nevertheless, ECG classification by Minnesota Coding [2] is better conducted at an ECG core laboratory, and hence searching for novel ECG predictors that could be used in clinical settings is warranted. Widening of the spatial QRS-T angle, defined as the angle between the mean QRS and T vector on the 12-lead ECG, has been a strong independent predictor of incident CVD and total mortality in the general population [3-10]. With the widespread availability of automated ECG interpretation machines nowadays, a spatial QRS-T angle could easily be calculated and incorporated into the output measurements matrix [11]. Whether spatial QRS-T angle is predictive of CVD events in HIV-infected population, in whom the virus and ART may be added risk factors, is currently unknown. We sought to examine the prognostic significance of spatial QRS-T angle for prediction of CVD events in HIV-infected individuals enrolled in the Strategies for Management of Antiretroviral Therapy [SMART] trial.
METHODS
Detailed descriptions of the design and aims of the SMART trial have been published elsewhere [12, 13]. Briefly, SMART was an open-label, randomized trial comparing two antiretroviral treatment strategies. Viral Suppression [VS] strategy, the control arm, was defined to be consistent with the guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents [14]; that is, available antiretroviral regimens were to be used in an uninterrupted manner with the goal of maximal and continuous suppression of HIV replication. The experimental Drug Conservation [DC] strategy entailed the episodic use of ART according to CD4+ T cell count thresholds; that is, the use of ART was deferred until the CD4+ T cell count decreased to less than 250 cells per cubic millimeter [cells/mm3], at which time ART was to be initiated [or reinitiated] and continued until the CD4+ T cell count increased to more than 350 cells/mm3. On January 10, 2006, the data and safety monitoring board recommended stopping enrollment in the SMART trial because of a safety risk in the DC group. After this change in protocol, all patients were advised to receive continuous ART and were followed up for an additional 1.5 years. This analysis describes findings through July 11, 2007.
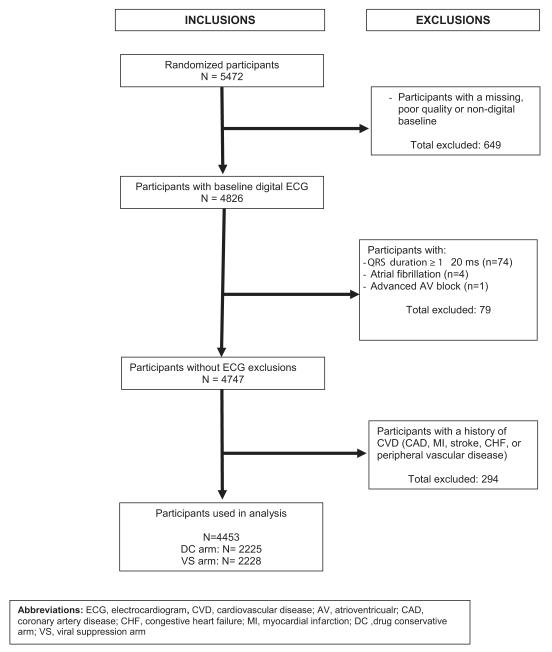
SMART recruited individuals infected with HIV who were older than 13 years and were not pregnant or breast-feeding. Participants were eligible for inclusion in the SMART study if their CD4+ T cell count exceeded 350 cells/mm3 and they were willing to participate. All participants in the trial were considered eligible for the present analysis except those with missing or poor-quality ECG data, ECG conditions that interfere with calculation of spatial QRS-T angle, and/or known CVD at baseline. After all exclusions, 4453 participants were included in this analysis [Figure 1].
Figure 1.
Inclusion and Exclusion Criteria
Before randomization [baseline], an ART and medical history were obtained, CD4+ T cell count and plasma HIV RNA levels were measured, and a 12-lead ECG was obtained. Follow-up visits were scheduled monthly for the first 2 months, every 2 months thereafter for the first year, and every 4 months in the second and subsequent years. At each visit, a history was taken, an examination was conducted and the CD4+ T cell count and plasma HIV RNA level were measured.
At the baseline visit and at each annual visit, a 12-lead ECG was obtained. Detailed description of ECG recording in SMART has been published elsewhere [1]. Briefly, identical electrocardiographs [GE MAC 1200 models, GE Milwaukee, WI] were used in all of the study clinical sites, and standard 12-lead ECGs were recorded in all participants by strictly standardized procedures. The digital ECG tracings stored in the electrocardiographs were transmitted regularly over analog phone lines to the SMART ECG Reading Center, EPICARE, located at Wake Forest School of Medicine, Winston-Salem, NC for analysis. ECGs were evaluated blinded to treatment group and ART use. After being visually checked for quality, the study ECGs were automatically processed using the 2001 version of the GE Marquette 12-SL program [GE, Milwaukee, WI]. ECG abnormalities were classified using the Minnesota ECG Classification [10]. Spatial QRS-T angle was defined as the angle between the mean QRS vector and T vector. Mean spatial QRS and T vectors were automatically calculated from quasi-orthogonal X, Y, and Z leads reconstructed from standard ECG leads using a matrix transformation method [15].
Using pre-established criteria, an independent end point review committee reviewed major clinical events, including CVD events [16]. The endpoint review committee classified the underlying cause of death using the Coding of Death in HIV Project classification system [17]. A composite CVD outcome [incident myocardial infarction, coronary artery disease, congestive heart failure, peripheral vascular disease, stroke, sudden death, or CVD death] was used in this analysis.
Quartiles of spatial QRS-T angle were calculated for males and females separately because of the reported gender differences in the distribution of spatial QRS-T angle [18], and participant characteristics were compared across these quartiles. Incidence rates of CVD events per 100 person-years in each quartile and overall were calculated. Given the observed similar rates of CVD events in the lower three quartiles, these quartiles were combined into a single reference group and compared to the fourth quartile. Cox proportional hazard analysis was used to assess the risk of CVD events associated with the upper quartile QRS-T angle [referred to as widened spatial QRS-T angle in the rest of this report] versus the lower three quartiles combined [referred to as normal QRS-T angle in the rest of this report]. Five models were created: Model 1, adjusted for the trial treatment groups; Model 2, adjusted for treatment group plus demographics [age, sex, and race/ethnicity]; Model 3, adjusted for model 2 covariates plus common CVD risk factors [smoking status, total/high-density lipoprotein [HDL] cholesterol ratio, body mass index, diabetes, and blood pressure and lipid-lowering drugs] and HIV characteristics [time since HIV diagnosis, baseline CD4+ T cell count, and plasma HIV RNA level/ART status]; and Model 4, adjusted for model 3 covariates plus inflammatory markers [interleukin 6 (IL-6) and high sensitivity C-reactive protein (hs-CRP)], and presence of any minor or major ECG abnormalities as defined by the Minnesota ECG Code Classification and a previous report [1, 2]. The interaction with treatment group and gender, separately, was examined. In an additional analysis we also used spatial QRS-T angle in the models as a time dependent variable to take into account changes in the levels of spatial QRS-T angle during the follow-up visit. Follow-up time was measured from baseline to first CVD event, non-CVD death, lost to follow-up, or end of study in July 11, 2007. All reported P values are two sided, and P <.05 was considered statistically significant. SAS, version 9.1 [SAS Institute, Inc, Cary, NC] was used in all analyses.
RESULTS
This analysis included 4453 SMART participants [46% non-whites, 28% women] who were 43.5 ± 9.3 years at the time of enrollment. The mean [SD] of the spatial QRS-T angle was 70.3 [29.1] degrees. Among men the mean was 74.53 and the 25th, 50th and 75th percentiles were 54.95, 73.35 and 93.30; for women the mean was 58.81 and the corresponding percentile cut points were 38.68, 55.79, and 74.06. Table 1 shows the baseline characteristics of the participants stratified by gender-specific quartiles of spatial QRS-T angle. As shown, across spatial QRS-T angle quartiles there were significant differences in age, race/ethnicity, smoking status, HDL-cholesterol, triglycerides, total/HDL cholesterol ratio, body mass index, diabetes, use of blood pressure lowering drugs, proportion of participants with plasma HIV RNA < 400 copies/mL, HIV-RNA level/ART status, and any major or minor ECG abnormalities [p <0.001 for all except smoking p=0.03; and triglycerides p=0.003].
Table 1.
Participant Characteristics by Quartiles of QRS-T Spatial Angle at Baseline in the SMART Study
| Characteristic | Spatial QRS-T angle a |
p-value | |||
|---|---|---|---|---|---|
| Q1 N=1113 |
Q2 N=1113 |
Q3 N=1113 |
Q4 N=1114 |
||
| Age (year) | 42.9 (9.5) | 42.9 (9.3) | 43.1 (9.1) | 44.8 (9.0) | <0.001 |
| Female sex | 318 (28.6%) | 318 (28.6%) | 318 (28.6%) | 318 (28.5%) | N/A b |
| Race/ethnicity | <0.001 | ||||
| Black | 321 (28.8%) | 295 (26.5%) | 295 (26.5%) | 383 (34.4%) | |
| White | 554 (49.8%) | 628 (56.4%) | 640 (57.5%) | 590 (53.0%) | |
| Asian | 76 (6.8%) | 61 (5.5%) | 50 (4.5%) | 21 (1.9%) | |
| Other | 162 (14.6%) | 129 (11.6%) | 128 (11.5%) | 120 (10.8%) | |
| Smoker | 0.03 | ||||
| Current | 408 (36.7%) | 431 (38.7%) | 478 (42.9%) | 458 (41.1%) | |
| Former | 280 (25.2%) | 264 (23.7%) | 241 (21.7%) | 282 (25.3%) | |
| Never | 425 (38.2%) | 418 (37.6%) | 394 (35.4%) | 374 (33.6%) | |
| Total Cholesterol (mg/dL) | 194.6 (44.4) | 196.1 (50.4) | 195.3 (47.0) | 196.8 (49.5) | 0.72 |
| LDL Cholesterol (mg/dL) | 114.0 (34.1) | 114.2 (35.0) | 114.6 (35.8) | 115.0 (36.4) | 0.90 |
| HDL Cholesterol( mg/dL) | 44.7 (14.7) | 43.6 (14.4) | 43.1 (14.9) | 42.2 (14.9) | <0.001 |
| Triglycerides (mg/dL) | 203.2 (201.6) | 215.1 (218.3) | 213.3 (180.9) | 236.8 (260.9) | 0.003 |
| Total Cholesterol/HDL | 4.8 (2.2) | 5.0 (2.6) | 5.0 (2.4) | 5.2 (2.4) | <0.001 |
| Body mass index (kg/m2) | 25.7 (5.1) | 25.7 (5.5) | 25.9 (5.5) | 26.5 (5.6) | <0.001 |
| Diabetes mellitus | 44 (4.0%) | 51 (4.6%) | 76 (6.8%) | 108 (9.7%) | <0.001 |
| BP-lowering drugs | 144 (12.9%) | 147 (13.2%) | 164 (14.7%) | 248 (22.3%) | <0.001 |
| Lipid lowering drugs | 140 (12.6%) | 158 (14.2%) | 136 (12.2%) | 167 (15.0%) | 0.18 |
| CD4+ T cells (cells/mm3) | 656.0 (242.6) | 657.3 (261.1) | 661.3 (256.3) | 656.5 (262.6) | 0.96 |
| HIV RNA (% ≤ 400 copies/mL) | 829 (74.6%) | 814 (73.5%) | 785 (70.7%) | 747 (67.4%) | <0.001 |
| HIV-RNA and ART Status | <0.001 | ||||
| Off ART | 161 (14.5%) | 171 (15.4%) | 197 (17.7%) | 190 (17.1%) | |
| On ART, HIV-RNA ≤ 400 | 144 (12.9%) | 146 (13.2%) | 149 (13.4%) | 200 (18.0%) | |
| On ART, HIV-RNA > 400 | 807 (72.6%) | 793 (71.4%) | 765 (68.9%) | 720 (64.9%) | |
| hs-CRP (mg/L) | 3.6 (6.5) | 3.6 (6.5) | 3.7 (7.3) | 3.5 (5.7) | 0.99 |
| Interleukin-6 (pg/mL) | 2.9 (9.2) | 2.3 (3.4) | 2.8 (4.2) | 4.3 (47.0) | 0.28 |
| ECG abnormalities c | 560 (50.3%) | 491 (44.1%) | 526 (47.3%) | 683 (61.3%) | <0.001 |
Values expressed as N (%) or mean (SD); p-value for differences across quartiles calculated by chi-square tests for proportions and F-tests for means
Quartiles are calculated for females and males separately with values as follows: Q1< 54.94, 54.94 <= Q2 < 73.35, 73.35 <= Q3 < 93.30 and Q4>= 93.30 in males while Q1 < 38.68, 38.68 <= Q2 < 55.79, 55.79 <= Q3 < 74.06, Q4 >= 74.06 in females.
By desing females have simialr number across quartiles
Defined as presence of minor or major ECG abnormalities by Minnesota ECG classification (see reference 9)
Abbreviations: BP, blood pressure; CVD, cardiovascular disease; ART, antiretroviral therapy; hsCRP, high sensitivity C-reactive protein
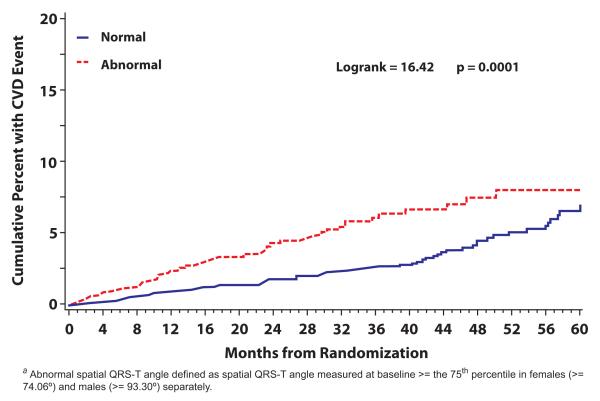
During 11965 person-years of follow-up, 152 CVD events occurred at a rate of 1.27 events per 100 person-years. There was about 11% increased risk with each 10 degrees increase in the spatial QRS-T angle (p<0.001) (Table 2). When the CVD events were examined across spatial QRS-T angle quartiles, it was noticeable that the number of events was almost identical in the first 3 quartiles of spatial QRS-T angle [32 events in Q1 and 30 events in each of Q2 and Q3] while double that number [60 events] was observed in Q4. As shown in Table 2, the event rate ratio in the upper quartile [i.e. widened spatial QRS-T angle] versus the lower three quartiles combined [i.e. normal QRS-T angle] was 1.94 with a 95% confidence interval between 1.07 and 1.47 and p-value <0.001. A Kaplan-Meier estimates of the cumulative percent experiencing CVD for those with widened vs. normal spatial QRS-T is shown in Figure 2. As shown, the risk of CVD associated with widened spatial QRS-T angle on the ECG persists during the follow-up period [P> .99 for proportional hazards].
Table 2.
Rates of Cardiovascular Events by Quartile of Spatial QRS-T Angle
| Spatial QRS-T angle | Participants N |
Events N |
Person years N |
Events per 100 Person Years Rate (95% CI) |
Rate Ratio (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Q1 [Ref] a | 1113 | 32 | 2910 | 1.10 (0.72, 1.48) | 1.0 | -- |
| Q2 | 1113 | 30 | 3012 | 1.00 (0.64, 1.35) | 0.91 (0.55, 1.49) | 0.70 |
| Q3 | 1113 | 30 | 3036 | 0.99 (0.63, 1.34) | 0.90 (0.55, 1.48) | 0.67 |
| Q4 | 1114 | 60 | 3006 | 2.00 (1.49, 2.50) | 1.82 (1.18, 2.79) | 0.006 |
|
| ||||||
| Q1-Q3 [Ref] | 3339 | 92 | 8959 | 1.03 (0.82, 1.24) | 1.0 | -- |
| Q4 [Widened QRS-T] | 1114 | 60 | 3006 | 2.00 (1.49, 2.50) | 1.94 (1.40, 2.69) | <0.001 |
|
| ||||||
| All population | 4453 | 152 | 11965 | 1.27 (1.07, 1.47) | 1.11 (1.05, 1.17)b | <0.001 |
Quartiles are calculated for females and males separately with values as follows: Q1< 54.94 °, 54.94 ° <= Q2 < 73.35 °, 73.35 ° <= Q3 < 93.30 ° and Q4>= 93.30° in males while Q1 < 38.68 °, 38.68 ° <= Q2 < 55.79 °, 55.79 ° <= Q3 < 74.06 °, Q4 >= 74.06 ° in females.
Hazard Ratio (95% CI) per 10° increase
Figure 2.
Kaplan-Meier Estimates of the Cumulative Risk of Cardiovascular Events for Normal (Q1-Q3) and Widened (Q4) Spatial QRS-T angle a
Table 3 shows the results of the multivariable adjusted Cox proportional hazards models for the association between widened baseline spatial QRS-T angle and incident CVD events. As shown, baseline widened spatial QRS-T angle [compared to normal] was associated with significantly higher risk of CVD events across all models. This includes the final model which was adjusted for study treatment arm (DC/VS), demographics, CVD risk factors, HIV-characteristics, inflammatory markers and other ECG abnormalities [hazard ratio (95% CI): 1.53 (1.07, 2.17); p= 0.02]. Similar results were observed when spatial QRS-T angle was used in the models as a time dependent variable that takes into account changes of QRS-T angle during follow-up visits [hazard ratio (95% CI): 1.69 (1.19, 2.40); p= 0.003]. There was no significant interaction by SMART treatment group [p-value for interaction 0.37] or by gender [p-value 0.84].
Table 3.
Multivariable Adjusted Associations between Baseline Spatial QRS-T Angle and Incident Cardiovascular Events
| Model 1 adjusted for treatment group |
Model 2 Adjusted for Model 1 plus age, gender and race |
Model 3 adjusted for Model 2 plus smoking status, total cholesterol/HDL ratio, BMI, diabetes, lipid lowering drug use, duration of HIV diagnosis, CD4, HIV-RNA and ART status |
Model 4 adjusted for Model 4 plus the presence of any ECG abnormalities , IL-6 and hs-CRP |
|||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Factors | HR [95% CI] | P | HR [95% CI] | P | HR [95% CI] | P | HR [95% CI] | P |
| Widened spatial QRS-T angle a | 1.92 (1.39, 2.66) | <0.001 | 1.75 (1.26, 2.43) | <0.001 | 1.60 (1.14, 2.25) | 0.007 | 1.53 (1.07, 2.17) | 0.02 |
| Treatment Group (DC vs. VS) | 1.45 (1.05, 2.01) | 0.02 | 1.46 (1.05, 2.01) | 0.02 | 1.44 (1.03, 2.01) | 0.03 | 1.41 (1.01, 1.98) | 0.05 |
| Age (year) | 1.06 (1.04, 1.08) | <0.001 | 1.05 (1.03, 1.07) | <0.001 | 1.04 (1.02, 1.06) | <0.001 | ||
| Sex (Female vs. Male) | 0.55 (0.36, 0.86) | 0.008 | 0.58 (0.36, 0.92) | 0.02 | 0.56 (0.35, 0.90) | 0.02 | ||
| Race/ethnicity | ||||||||
| Black (vs. White) | 1.25 (0.88, 1.78) | 0.21 | 1.04 (0.70, 1.52) | 0.86 | 1.00 (0.67, 1.49) | 0.99 | ||
| Asian (vs. White) | 0.38 (0.05, 2.74) | 0.34 | 0.49 (0.07, 3.55) | 0.48 | 0.99 (0.14, 7.21) | >0.99 | ||
| Other Races (vs. White) | 0.76 (0.42, 1.38) | 0.37 | 0.81 (0.44, 1.49) | 0.50 | 0.79 (0.42, 1.48) | 0.46 | ||
| Smoker | ||||||||
| Current (vs. Never) | 2.04 (1.32, 3.16) | 0.001 | 1.58 (1.01, 2.47) | 0.05 | ||||
| Past (vs. Never) | 1.33 (0.83, 2.15) | 0.24 | 1.19 (0.73, 1.93) | 0.48 | ||||
| Total/HDL Cholesterol Ratio | 1.04 (1.00, 1.09) | 0.04 | 1.03 (0.98, 1.08) | 0.21 | ||||
| Baseline BMI | 1.01 (0.97, 1.04) | 0.72 | 0.99 (0.96, 1.02) | 0.49 | ||||
| Diabetes mellitus (Y vs. N) | 1.87 (1.18, 2.95) | 0.008 | 1.92 (1.21, 3.04) | 0.006 | ||||
| Use of BP-lowering drugs | 1.85 (1.27, 2.68) | 0.001 | 1.75 (1.20, 2.56) | 0.003 | ||||
| Use of lipid lowering drugs | 1.02 (0.67, 1.55) | 0.93 | 1.05 (0.68, 1.63) | 0.81 | ||||
| Years since HIV diagnosis | 1.00 (0.97, 1.04) | 0.95 1.00 | (0.97, 1.04) | 0.91 | ||||
| Baseline CD4+ T cells (per 100) | 1.00 (1.00, 1.00) | 0.31 | 1.00 (1.00, 1.00) | 0.22 | ||||
| Baseline HIV-RNA and ART Status | ||||||||
| Off ART (vs. On ART, HIV-RNA > 400 | 1.24 (0.72, 2.12) | 0.44 | 1.20 (0.69, 2.10) | 0.52 | ||||
| On ART, HIV-RNA ≤ 400 (vs. On ART, HIV-RNA > 400 | 1.00 (0.65, 1.56) | 0.99 | 0.97 (0.62, 1.52) | 0.88 | ||||
| Interleukin-6 (per log2 unit) | 1.32 (1.17, 1.50) | <0.001 | ||||||
| Hs CRP (per log2 unit) | 1.21 (1.09, 1.34) | <0.001 | ||||||
| Any ECG abnormalities (Y vs. N) | 1.21 (0.84, 1.73) | 0.30 | ||||||
Defined as spatial QRS-T angle >=the 75th percentile in females (>= 74.06° )and males (>= 93.30°) separately.
Abbreviations: BP, blood pressure; CVD, cardiovascular disease; ART, antiretroviral therapy; hsCRP, high sensitivity C-reactive protein; DC ,drug conservative arm; VS, viral suppression arm
DISCUSSION
We showed that widened spatial QRS-T angle in the resting 12-lead ECG is associated with future CVD events in a demographically diverse HIV-infected population initially free of CVD. This association is independent of common CVD risk factors, HIV characteristics and other ECG abnormalities. These findings suggest that important predictive information can be derived from spatial QRS-T angle in HIV-infected individuals similar to what previously reported in the general population [3-10].
The spatial QRS-T angle, the angle between the QRS axis and T axis in the plane that these axes derived form, is considered as a global ECG descriptor of cardiac repolarization and its relation to the preceding depolarization. The concept of the spatial QRS-T angle stems from vectorcardiography, but today its calculation is made from routine standard 12-lead ECGs [18]. In healthy subjects, spatial direction of the initial repolarization, dominated largely by repolarization of the left ventricular lateral wall, is predominantly reverse to the direction of depolarization. Terminal repolarization, in contrast, is predominantly concordant with depolarization sequence. Correspondingly, the initial QRS and T wave polarities tend to be concordant and terminal QRS and T wave polarities discordant [19]. At the cellular level, an abnormally wide angle indicates pathophysiologic changes in the gating mechanism of ionic channels in the myocardium, which in turn alters the regional sequence of ventricular repolarization [20-22].
There are no widely accepted reference values for spatial QRS-T angle, but overall, the wider the angle, the higher the probability of ongoing pathological changes either clinical or subclinical. In the general population, there have been some suggested normal values for spatial QRS-T angle [23, 24]. However, these values were defined based on the normal distribution of spatial QRS-T angle in the general population, not based on how much they predict future outcomes, which is more important. In our study, CVD events among participants in the upper quartile of spatial QRS-T angle were almost double the events in any of the lower quartiles. Accordingly, focusing extensive CVD risk management and follow up on those in the upper quartile of spatial QRS-T angle (i.e. spatial QRST angle >= 93° in males and 74° females) would provide the highest cost-benefit ratio. Notably, the 75th percentile of spatial QRS-T angle in our study population of HIV-infected individuals enrolled in the SMART study was similar to that in the general population of apparently healthy individuals enrolled in the ARIC study [25] [93 ° in males in both SMART and ARIC, and 77° and 74° in females in ARIC and SMART respectively].
Based on our findings in this study and what we have reported previously on other utilities of ECG in HIV-infected patients [1, 26], it may be useful to introduce the ECG in the routine care of HIV-infected patients. The ECG is the least expensive and most available clinical tool for evaluation of CVD, and therefore it can fit any healthcare system regardless of the economic status whether in developed or developing countries.
Our results and their application should be read in the context of few considerations. This analysis was conducted on individuals who chose to enter a clinical trial of HIV treatment strategies. Thus, it is possible that the results are not generalizable to all HIV-infected individuals. Further, although spatial QRS-T angle could be simply incorporated in automated ECG reports by modern digital ECG machines [11], these machines may not be available in places where the majority of HIV-infected individuals resides (e.g. Sub-Saharan Africa), and many clinicians are not yet familiar with this measurement. Hence, updating the ECG recording equipment and educating clinicians treating HIV-infected patients may be needed to take full advantage of incorporating ECG in routine care of HIV infection. Nevertheless, updating the ECG recording equipment at a large scale may be challenged by the regulations controlling manufacturing of ECG machines and the economic status of some countries. However, it is possible to reasonably calculate the mean spatial QRS-T angle by using a simple set of manual measurements of peak QRS and T waves from 3 ECG leads recorded with any non-digital ECG machine. QRS-T angle could then by determined by a pocket calculator [27]
Our study has several strengths that warrant highlighting. This analysis was conducted on a well-defined population of HIV-infected patients free of CVD. The key exposure variable [spatial QRS-T angle] and the outcome [incident CVD] were carefully ascertained; that is, ECG recordings were obtained by trained technicians using a standardized protocol, and the events were adjudicated by an independent adjudication committee.
Acknowledgment
The authors would like to thank the SMART study investigators and participants. For a complete list of SMART investigators, see N Engl J Med 2006; 355(22):2283-2296 Support provided by the National Institute of Allergy and Infectious Diseases (NIAID); NIH grants U01AI042170, U01AI46362, U01AI068641.
Funding: The SMART study was sponsored by the National Institute of Allergy and Infectious Disease, National Institutes of Health (grants U01AI042170, U01AI046362, and U01AI068641).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
Trial Registration: ClinicalTrials.gov (NCT00027352)
References
- 1.Soliman EZ, Prineas RJ, Roediger MP, Duprez DA, Boccara F, Boesecke C, Stephan C, Hodder S, Stein JH, Lundgren JD, Neaton JD. Prevalence and prognostic significance of ECG abnormalities in HIV-infected patients: results from the Strategies for Management of Antiretroviral Therapy study. J Electrocardiol. 2011;44:779–785. doi: 10.1016/j.jelectrocard.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prineas RJ, Crow RS, Blackburn H. The Minnesota Code manual of electrocardiographic findings. John Wright–PSG; Littleton, MA: 1982. [Google Scholar]
- 3.Zabel M, Malik M, Hnatkova K, Papademetriou V, Pittaras A, Fletcher RD, Franz MR. Analysis of T wave morphology from the 12-lead electrocardiogram for prediction of long term prognosis in male US veterans. Circulation. 2002;105:1066–1070. doi: 10.1161/hc0902.104598. [DOI] [PubMed] [Google Scholar]
- 4.Kors JA, Kardys I, van der Meer IM, van Herpen G, Hofman A, van der Kuip DA, Witteman JC. Spatial QRS-T Angle as a Risk Indicator of Cardiac Death in an Elderly Population. J Electrocardiol. 2003;36:113–114. doi: 10.1016/j.jelectrocard.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 5.Kardys I, Kors JA, van der Meer IM, Hofman A, van der Kuip DA, Witteman JC. Spatial QRS/T angle predicts cardiac death in a general population. Eur Heart J. 2003;24:1357–1364. doi: 10.1016/s0195-668x(03)00203-3. [DOI] [PubMed] [Google Scholar]
- 6.de Torbal A, Kors JA, van Herpen G, Meij S, Nelwan S, Simoons ML, Boersma E. The electrical T-axis and the spatial QRS-T angle are independent predictors of long-term mortality in patients admitted with acute ischemic chest pain. Cardiology. 2004;101:199–207. doi: 10.1159/000076697. [DOI] [PubMed] [Google Scholar]
- 7.Rautaharju PM, Ge S, Nelson JC, Marino Larsen EK, Psaty BM, Furberg CD, Zhang ZM, Robbins J, Gottdiener JS, Chaves PH. Comparison of mortality risk for electrocardiographic abnormalities in men and women with and without coronary heart disease (from the Cardiovascular Health Study) Am J Cardiol. 2006;97:309–315. doi: 10.1016/j.amjcard.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 8.Rautaharju PM, Kooperberg C, Larson JC, LaCroix A. Electrocardiographic abnormalities that predict coronary heart disease events and mortality in postmenopausal women: then Women’s Health Initiative. Circulation. 2006;113:473–480. doi: 10.1161/CIRCULATIONAHA.104.496091. [DOI] [PubMed] [Google Scholar]
- 9.Rautaharju PM, Kooperberg C, Larson JC, LaCroix A. Electrocardiographic predictors of incident congestive heart failure and all-cause mortality in postmenopausal women: the Women’s Health Initiative. Circulation. 2006;113:481–489. doi: 10.1161/CIRCULATIONAHA.105.537415. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki T, Froelicher VF, Myers J, Chun S, Wang P. Spatial QRS-T angle predicts cardiac death in a clinical population. Heart Rhythm. 2005;2:73–78. doi: 10.1016/j.hrthm.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 11.Macfarlane PW. The frontal plane QRS-T angle. Europace. 2012;14:773–775. doi: 10.1093/europace/eus057. [DOI] [PubMed] [Google Scholar]
- 12.The SMART Study Group CD4+ count-guided interruption of antiretroviral therapy. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 13.SMART Study Group Risk for opportunistic disease and death after reinitiating continuous antiretroviral therapy in patients with HIV previously receiving ePIodic therapy, a randomized trial. Ann Int Med. 2008;149:289–299. doi: 10.7326/0003-4819-149-5-200809020-00003. [DOI] [PubMed] [Google Scholar]
- 14. [Accessed March 13, 2012];Panel on Clinical Practices for Treatment of HIV Infection. Guidelines for the use of antiretroviral agents in HIV-1–infected adults and adolescents. http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 15.Edenbrandt L, Pahlm O. Vector cardiogram synthesized from a 12-lead ECG: superiority of the inverse Dower matrix. J Electrocardiol. 1988;21:361–367. doi: 10.1016/0022-0736(88)90113-6. [DOI] [PubMed] [Google Scholar]
- 16.Lifson AR, INSIGHT Endpoint Review Committee Writing Group. Belloso WH, Davey RT, Duprez D, Gatell JM, Hoy JF, Krum EA, Nelson R, Pedersen C, Perez G, Price RW, Prineas RJ, Rhame FS, Sampson JH, Worley J, INSIGHT Study Group Development of Diagnostic Criteria for Serious Non-Aids Events in HIV Clinical Trials. The Insight Study Group. HIV Clin Trials. 2010;11:205–219. doi: 10.1310/hct1104-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lifson AR, INSIGHT Cause of Death Writing Group. Belloso WH, Carey C, Davey RT, Duprez D, El-Sadr WM, Gatell JM, Gey DC, Hoy JF, Krum EA, Nelson R, Nixon DE, Paton N, Pedersen C, Perez G, Price RW, Prineas RJ, Rhame FS, Sampson J, Worley J. Determination of the underlying cause of death in three multicenter international HIV clinical trials. HIV Clin Trials. 2008;9:177–1785. doi: 10.1310/hct0903-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreurs CA, Algra AM, Man SC, Cannegieter SC, van der Wall EE, Schalij MJ, Kors JA, Swenne CA. The spatial QRS-T angle in the Frank vectorcardiogram: accuracy of estimates derived from the 12-lead electrocardiogram. J Electrocardiol. 2010;43:294–301. doi: 10.1016/j.jelectrocard.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Rautaharju PM, Zhou SH, Gregg RE, Startt-Selvester RH. Heart rate, gender differences, and presence versus absence of diagnostic ST elevation as determinants of spatial QRS|T angle widening in acute coronary syndrome. Am J Cardiol. 2011;15:107–1744. doi: 10.1016/j.amjcard.2011.02.333. [DOI] [PubMed] [Google Scholar]
- 20.van Huysduynen BH, Swenne CA, Bax JJ, Bleeker GB, Draisma HH, van Erven L, Molhoek SG, van de Vooren H, van der Wall EE, Schalij MJ. Dispersion of repolarization in cardiac resynchronization therapy. Heart Rhythm. 2005;2:1286–1293. doi: 10.1016/j.hrthm.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 21.Dilaveris P, Roussos D, Giannopoulos G, Katinakis S, Maragiannis D, Raftopoulos L, Arsenos P, Gatzoulis K, Stefanadis C. Clinical determinants of electrocardiographic and spatial vectorcardiographic descriptors of ventricular repolarization in healthy children. Ann Noninvasive Electrocardiol. 2011;16:49–55. doi: 10.1111/j.1542-474X.2010.00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dilaveris P, Pantazis A, Gialafos E, Triposkiadis F, Gialafos J. Determinants of electrocardiographic and spatial vectorcardiographic descriptors of ventricular repolarization in normal subjects. Am J Cardiol. 2001;88:912–4. A9. doi: 10.1016/s0002-9149(01)01907-5. [DOI] [PubMed] [Google Scholar]
- 23.Scherptong RW, Henkens IR, Man SC, Le Cessie S, Vliegen HW, Draisma HH, Mean AC, Schalij MJ, Swenne CA. Normal limits of the spatial QRS-T angle and ventricular gradient in 12-lead electrocardiograms of young adults. J Electrocardiol. 2008;41:648–655. doi: 10.1016/j.jelectrocard.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Rautaharju PM, Zhou SH, Gregg RE, Startt-Selvester RH. Electrocardiographic estimates of action potential durations and transmural repolarization time gradients in healthy subjects and in acute coronary syndrome patients—profound differences by sex and by presence vs absence of diagnostic ST elevation. J Electrocardiol. 2011;44:309–319. doi: 10.1016/j.jelectrocard.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Zhang ZM, Prineas RJ, Case D, Soliman EZ, Rautaharju PM, ARIC Research Group Comparison of the prognostic significance of the electrocardiographic QRS/T angles in predicting incident coronary heart disease and total mortality (from the atherosclerosis risk in communities study) Am J Cardiol. 2007;100:844–849. doi: 10.1016/j.amjcard.2007.03.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soliman EZ, Lundgren JD, Roediger MP, Duprez DA, Temesgen Z, Bickel M, Shlay JC, Somboonwit C, Reiss P, Stein JH, Neaton JD, INSIGHT SMART Study Group Associations between ritonavir boosted protease inhibitors with QTc and PR interval durations in the Strategies for Management of Antiretroviral Therapy (SMART) Trial. AIDS. 2011;25:367–377. doi: 10.1097/QAD.0b013e328341dcc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rautaharju PM, Prineas RJ, Zhang ZM. A simple procedure for estimation of the spatial QRS/T angle from the standard 12-lead electrocardiogram. J Electrocardiol. 2007;40:300–304. doi: 10.1016/j.jelectrocard.2006.11.003. [DOI] [PubMed] [Google Scholar]