Abstract
Background
More than one-quarter of Americans are hypercholesterolemic and/or being treated with cholesterol-lowering medications. Given the systemic nature of hypercholesterolemia and remaining questions regarding its effect on tendons at a local level, we sought to assess the utility of small versus large animal model systems for translational studies by exploring the effect of hypercholesterolemia on supraspinatus tendon elastic mechanical properties in mice, rats, and monkeys. We hypothesized that stiffness and elastic modulus would be increased in tendons across species due to hypercholesterolemia.
Materials and Methods
Supraspinatus tendons from normal (CTL) and high-cholesterol (HC) mice, rats, and monkeys were used in this study. Following dissection, tendons were geometrically measured and tensile tested with tissue strain measured optically.
Results
Overall, HC animals had significantly altered plasma lipid profiles. Biomechanical testing showed a significant increase in stiffness compared to CTL in HC mice and rats, as well as a non-significant trend for HC monkeys. Elastic modulus was also significantly increased in HC mice and monkeys, with HC rats showing a trend.
Conclusions
The consistency of the present findings across species and between small and large animals, combined with the fact that the aged mice were exposed to lifelong hypercholesterolemia (compared with rats and non-human primates which were fed high-cholesterol diets) suggests that these increased properties may be inherent to the effect of hypercholesterolemia on supraspinatus tendon rather than due to an effect of cumulative exposure time to the effects of high cholesterol. Further investigation is needed to confirm this concept.
Keywords: hypercholesterolemia, tendon, biomechanics, rotator cuff, shoulder, animal model
Background
As of 2008, more than one-quarter of the adult U.S. population was either hypercholesterolemic or had been hypercholesterolemic prior to being treated with cholesterol-lowering medications.11 The detrimental effects of elevated plasma levels of total cholesterol, triglycerides, and low density lipoprotein cholesterol (LDL) on cardiovascular health are well-documented13 and from an orthopaedics perspective, they have also been linked to the formation of Achilles tendon xanthomas,6,18 which increase the risk of tendon rupture.21,22 Our own clinical data have correlated hypercholesterolemia and rotator cuff tears in orthopaedic patients presenting with full-thickness tears;1 however, another clinical study suggests no relationship between serum cholesterol and triglycerides and rotator cuff tears.15
The tendons comprising the rotator cuff are routinely subjected to tensile loads and can become pathologic when loads are excessive, repetitive, or otherwise abnormal. In the laboratory setting, it is useful to perform tensile tests on tendons to measure the structural and material properties of these tissues in both their physiologically normal (stiffness/modulus of elasticity) and excessive (maximum load/stress) ranges. Stiffness and elastic modulus are representative of the amount of stretch that a tendon undergoes for an applied load or stress within normal usage. Experimentally, we have shown that native rat supraspinatus tendon stiffness and modulus are increased following exposure to two different high-cholesterol diets for a period of three months.4 This result was surprising based on our previous work in a mouse patellar tendon model, which showed reduced modulus in mice exposed to lifelong hypercholesterolemia.3 Even in the presence of these previous studies, biomechanical data related to high plasma cholesterol in the rotator cuff remains scarce.
Small animal models are critical for a large number of fundamental and basic investigations; however, given the systemic nature of hypercholesterolemia and further questions regarding its effect on tendons at a local level, we sought to compare biomechanical results from small animals to those of a large animal model to assess the utility of the small model systems for further, more translational studies in the rotator cuff. Therefore, the objective of this study was to explore the effect of hypercholesterolemia on supraspinatus tendon mechanical properties in mouse, rat, and non-human primate models. Given our previous findings in the rat,4 we hypothesized that elastic mechanical properties would be increased in supraspinatus tendons across species due to high plasma cholesterol.
Materials and Methods
Mouse
Ten male C57BL/6 control (CTL) mice and ten C57BL/6 mice deficient for apolipoprotein E (APOE) representing a high-cholesterol (HC) group were used with Institutional Animal Care and Use Committee (IACUC) approval. These mice have markedly elevated plasma total cholesterol (TC) levels as well as increased TC-to-high-density lipoprotein cholesterol ratios (TC/HDL).17 All mice were allowed food and water ad libitum and sacrificed at 10 months of age.
Rat
Twenty male Sprague-Dawley rats were obtained at 400–450 grams with IACUC approval. Ten rats received a high-cholesterol diet consisting of 4% cholesterol and 1% sodium cholate (HC) which has been shown by us and others to produce elevated TC values after three months.4,14 The other ten rats received standard chow and served as controls (CTL). All rats were allowed food and water ad libitum for 6 months and were then sacrificed. Blood was drawn immediately following sacrifice and plasma lipid profiles were obtained.
Monkey
Supraspinatus tendons from ten male vervet (African green) monkeys were used as byproducts from a separate study with IACUC approval. The monkeys were fed diets containing 35% of energy as monounsaturated fat supplemented with cholesterol at 0.002 mg/kcal (CTL, n=5) and 0.4 mg/kcal (HC, n=5). At the end of the 2.5-month treatment, all monkeys were sacrificed and plasma lipid profiles were determined.
For all animals, supraspinatus tendons were dissected free from all muscle tissue, while leaving the bony insertion to the humerus intact. For mouse and rat specimens (animal masses on the order of 30 and 700 g, respectively), a stereomicroscope was used to ensure complete removal of peritendinous tissue. There were no noted differences in the amount of extraneous tissue removed from or remaining on tendons from normal and hypercholesterolemic animals. With an average animal mass of 6.7 kg, monkey fine dissections did not require magnification.
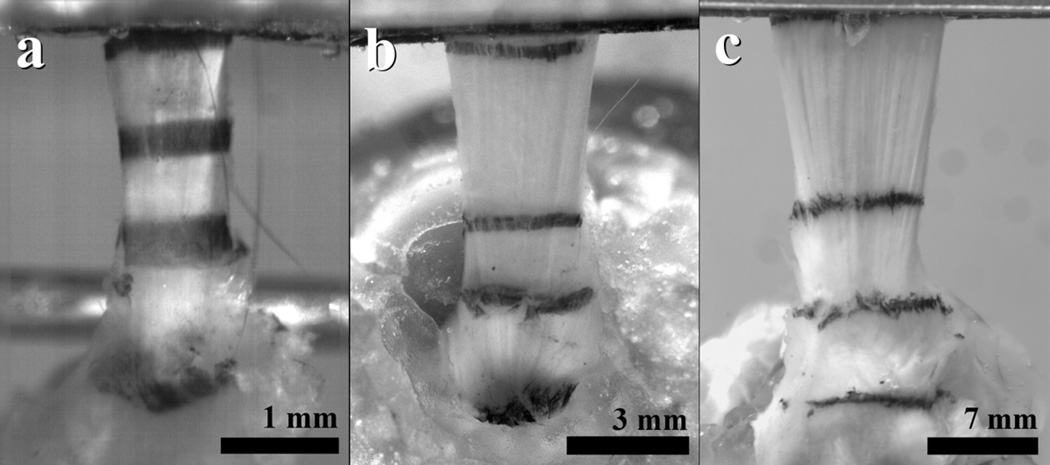
Local tissue strain was optically tracked7 using Verhoeff’s stain lines placed on the tendon at three locations – one at the humeral insertion and two others in the tendon midsubstance10 (Fig. 1). A fourth stain line was used to mark the position of the grip and was used to assess slippage from the grips during testing.
Figure 1.
Supraspinatus tendons with stain lines applied from the (a) mouse, (b) rat, and (c) monkey.
Tendon cross-sectional area was calculated using a custom laser-based device,9 which measured tendon thickness at each stain line by scanning over the tendon using translational stages. Two orthogonal linear variable differential transformers (LVDTs) were used for position measurement and a charge-coupled device laser was used for thickness measurement. Using custom measurement and analysis software, an average cross-sectional area measurement was calculated for the tendon between the insertion site and the third stain line. Reported measurements of overall tendon stiffness and elastic modulus were also calculated between the stain lines at the insertion site and the upper-most stain line in the tendon midsubstance.
The tendon end was fixed between two layers of sandpaper using a cyanoacrylate adhesive and clamped with custom grips. The remaining portion of the humeral diaphysis was potted in poly(methyl) methacrylate with its longitudinal axis aligned with the pot and placed in a base fixture. For all species, the supraspinatus tendon was loaded in the direction on the long axis of the humerus rather than in its physiologic orientation of wrapping around the humeral head. This orientation was chosen based on previous work in our lab showing no differences in rat supraspinatus mechanical properties between these two orientations. In addition, this orientation would allow for comparisons with future data evaluating quantitative polarized light during mechanical testing, which requires light to be able to pass through the tendon.
Specimens were submerged in a 37°C PBS bath and tensile tested to failure in an Instron 5543 mechanical test frame (Instron Corp., Norwood, MA, USA) using a standard protocol. Briefly, tendons were preloaded to remove slack, followed by ten cycles of preconditioning between two species-scaled loads, and a 300 s hold. Next, a stress relaxation test was performed by ramping to 5% strain at 5%/s and relaxing for 600 s. Specimens were then returned to initial gauge length, held for 60 s, and finally ramped to failure at 0.1–0.3%/s. Data from mechanical testing (e.g., stiffness/ modulus, maximum load/stress, percent relaxation) between CTL and HC for each species was evaluated using t-tests. Significance was set at p≤0.05 and trends were noted when p≤0.1.
Results
There were no noted differences in activity level between normal and hypercholesterolemic animals for any species. Plasma lipid analysis for mice (from those used in a previous study17), rats, and monkeys showed that all HC animals had significantly increased TC and TC/HDL cholesterol (Table 1). Overall, no differences were noted in animal mass between groups for any species. Similarly, no differences were noted in tendon size as assessed by cross-sectional area (Table 2).
Table 1.
Plasma lipid results for rats and monkeys used in the current study (means ± standard deviations). Mouse results are from a previous study.16 Both TC and TC/HDL were significantly increased for all species.
| TC (mg/dL) |
HDL (mg/dL) |
TG (mg/dL) |
TC/HDL | ||
|---|---|---|---|---|---|
| Mouse16 | CTL | 116±19 | 54.1±7.7 | 30.9±15.5 | 2.33±0.38 |
| HC | 495±101† | 11.6±11.6† | 58.0±19.3† | 33.33±33.33† | |
| Rat | CTL | 129±39 | 54.3±18.1 | 203.6±85.1 | 2.39±0.15 |
| HC | 595±429† | 88.7±32.1† | 184.0±187.0 | 6.40±2.98† | |
| Monkey | CTL | 212±35 | 125.0±15.4 | 11.5±4.7 | 1.70±0.20 |
| HC | 517±142† | 119.5±28.2 | 5.4±1.5† | 4.45±1.12† | |
denotes statistical significance compared to CTL.
(TC = total cholesterol, HDL = high-density lipoprotein, TG = triglycerides)
Table 2.
Geometrical and biomechanical results for supraspinatus tendons (means ± standard deviations). In general, stiffness and modulus were increased in HC animals across species.
| Area (mm2) |
Peak Load (N) |
Peak Stress (MPa) |
Percent Relaxation |
Stiffness (N/mm) |
Modulus (MPa) |
||
|---|---|---|---|---|---|---|---|
| Mouse | CTL | 0.28±0.04 | 0.93±0.34 | 3.40±1.56 | 34.9±9.57 | 95.1±39.8 | 312.8±127.0 |
| HC | 0.26±0.04 | 0.83±0.27 | 3.06±1.05 | 36.2±6.79 | 43.5±43.6† | 530.8±196.3† | |
| Rat | CTL | 1.56±0.21 | 6.81±1.40 | 4.10±0.87 | 62.8±6.10 | 98.0±20.4 | 325.7±70.9 |
| HC | 1.56±0.19 | 8.00±2.40‡ | 4.99±1.88‡ | 63.9±5.69 | 123.3±39.2† | 416.9±184.0‡ | |
| Monkey | CTL | 10.26±1.80 | 60.7±15.83 | 6.01±1.53 | 34.6±5.58 | 238.8±72.9 | 328.2±66.3 |
| HC | 9.47±1.10 | 64.7±8.93 | 6.06±0.83 | 35.3±4.67 | 312.4±99.8‡ | 417.9±48.0† | |
denotes statistical significance compared to CTL;
denotes a non-significant trend.
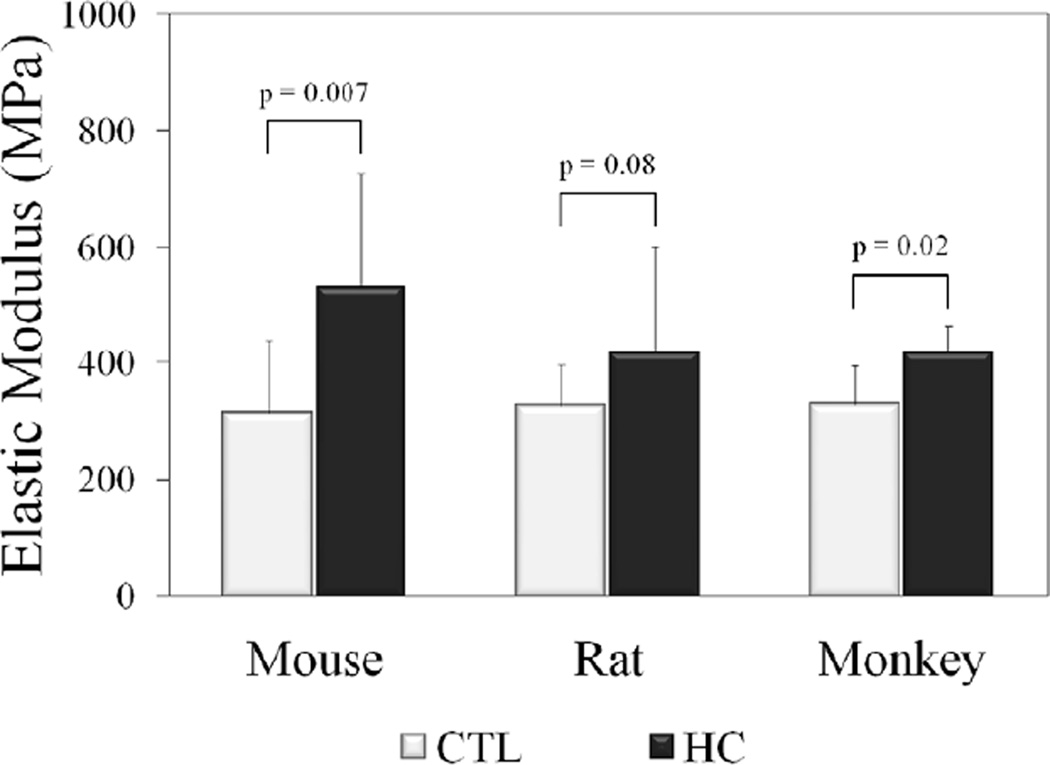
Biomechanical testing showed a significant increase in stiffness compared to CTL in HC mice and rats as well as a trend (p=0.1) in HC monkeys (Table 3). Elastic modulus (Fig. 2) was also increased in HC mice and monkeys, with HC rats exhibiting a trend (p=0.08). From the stress-relaxation test, peak load and peak stress showed a trend toward being increased in the HC rats, but not in other species. There were no differences in equilibrium load/stress or percent relaxation between groups for any species. Since failure occurred by tendon rupture at the grip interface for the majority of specimens, failure properties were not reported.
Table 3.
Overall stiffness and elastic modulus changes due to high plasma cholesterol.
| Mouse | Rat | Monkey | |
|---|---|---|---|
| Stiffness | ⬆ | ⬆ | ↑ |
| Modulus | ⬆ | ↑ | ⬆ |
⬆ = significant increase; ↑ = trend.
Figure 2.
Moduli were increased in HC tendons compared to CTL across species.
Discussion
In this study, we have shown increased stiffness and modulus due to hypercholesterolemia in supraspinatus tendons of three different species of small and large animals, in support of our hypothesis. Previous work in our lab has demonstrated a variety of findings depending on species and tendon type, potentially due to differences in location and function (e.g., intrinsic/extrinsic, intra/extra-synovial, etc.). In particular, high plasma cholesterol was shown to reduce elastic modulus in mouse patellar tendons,3 decrease stiffness and modulus in pig biceps tendons,5 and increase stiffness and modulus in rat supraspinatus tendons.4 Aside from the difference in species, the discrepancy between the shoulder tendons (biceps and supraspinatus) results may be due to the different functions performed by the tendons. As one example, the biceps remains relatively static within the joint during shoulder abduction and rotation, sliding passively within the bicipital groove.2 By contrast, the supraspinatus is subjected to a greater degree of motion as a result of its insertion directly on the humerus and is subjected to a different intrinsic and extrinsic loading environment.20
As mentioned previously, the present study has shown consistent results to our previous rat study4, even after extending the rat diet time course from 3 to 6 months. Additionally, the present results show consistency not only across species, but also between small and large animal models. As an example, elastic modulus values varied less than 5% across species, demonstrating that the native tissue quality is similar. This consistency combined with the fact that the aged mice in our current work were exposed to lifelong hypercholesterolemia (as compared to the rats and monkeys which were subjected to high-cholesterol diets) lends support to the notion that these increased properties may be inherent to the effect of hypercholesterolemia on the supraspinatus tendon rather than due to an effect of length of time exposed to the cumulative effects of high plasma cholesterol. Further investigation is needed to confirm this concept.
The current study found no differences in cross-sectional area comparing supraspinatus tendons from normal and hypercholesterolemic animals. This seems to be in contrast to clinical findings which report increased Achilles tendon thickness and cross-sectional area in patients with familial hypercholesterolemia8 as well as those which have correlated thickening of the Achilles tendon with carotid atherosclerosis in similar patient populations.12 There are no studies, however, demonstrating similar changes in geometry in the supraspinatus tendon.
A limitation of this study is the variations in aspects of the study designs for the different species of animals. For instance, the APOE knockout mice were subjected to lifelong genetic hypercholesterolemia and euthanized at a relatively advanced age, whereas monkeys were fed a high-cholesterol diet for less than three months and euthanized at the end of that treatment. This disparity stemmed from the study being conducted in phases and, in the case of the monkeys, the tissue being obtained as a byproduct from separate study. Although all methods and lengths of treatments resulted in hypercholesterolemic animals, these differences in methods inherently reduced the consistency of the study across species. Nonetheless, the statistical findings were focused on within-species comparisons; relationships across species were made only on a more global level to describe overall consistency in findings.
Another limitation is the degree to which the hypercholesterolemia was achieved in some animals. Mouse plasma lipid panels were not assessed as part of our study; however, from the literature, this strain of APOE knockout mice has been shown to have TC levels between 4.3–6.5 times that of control mice.17,19 Similar increases were seen in the diet-fed rats in this study, while the monkeys saw a 2.4-fold increase in TC. We acknowledge that these levels can be considered to be severely hypercholesterolemic and would correspond to profound cardiovascular risk in a clinical setting. However, it should be noted that the animals on the diet studies were only on the altered diet on the order of months, while clinically, a high-cholesterol diet might be present for much longer such that the relative exposures could be somewhat similar. While a direct clinical parallel could be advantageous in drawing conclusions and expanding results to clinical scenarios, our focus in the current work was at the basic science level to provide data to address our fundamental research question and to give direction for future studies with more explicit clinical relevance.
At present, it is not completely clear how changes in stiffness and/or modulus may affect the overall performance of the tendon based on its particular function. In predominantly load-bearing applications, a higher stiffness would appear to be advantageous not only from an engineering perspective, but also to aid in the prevention of joint laxity. Conversely, an increased stiffness would also allow less stretching of the tendon prior to rupture at its ultimate load. In the case of the rotator cuff, increased stiffness of the individual tendons contributing to overall shoulder stiffness is generally considered to be detrimental to the range of motion and function of the joint overall; however, aiding in joint stability is a primary role of the rotator cuff tendons, which would tend to be improved with increased stiffness.
A large number of orthopaedic patients are unaware of their lipid profile as these patients sometimes present to the orthopaedist with joint complaints prior to ever having had a cholesterol screening by their primary care physician or internist. In fact, one study of patients presenting for Achilles tendon ruptures reported that, of the 83% who were hypercholesterolemic, only 23% were aware of this health condition.16 The correlation of hypercholesterolemia and Achilles tendon ruptures generally agrees with our previous clinical finding relating hypercholesterolemia to rotator cuff tears;1 however, there are other rotator cuff data suggesting no connection.15 The discrepancy between these findings in the rotator cuff could potentially be due to differences in initial screening methodology (e.g., imaging, arthroscopy, etc.). Ultimately, the knowledge that alterations in tendon function are an indication of hypercholesterolemia could provide orthopaedic clinicians with an opportunity to proactively obtain lipid screenings for at-risk patients at the onset of abnormal tendon function and prior to any cardiovascular manifestations. Further clinical and basic science studies will be needed to address the mechanisms governing how the relationships between hypercholesterolemia and tendon mechanical properties uncovered in this study, manifest themselves for ultimate effect on specific joint function.
Conclusions
In summary, we have demonstrated increased supraspinatus stiffness and modulus in three different species of hypercholesterolemic animals. The overall consistency across species lends support to the use of small animal models in addressing future questions in this specific area due to their ease of use and practical considerations for many studies. Concurrent work is actively focusing on the effect of hypercholesterolemia on the healing response to injury in the rat as well as additional immunohistochemical and organizational assays to help uncover mechanisms involved in these findings.
Acknowledgements
The authors thank DA Cromley, L Edelstein, CF Gray, BC Lee, CS Lee, CD Peltz, DJ Rader, IM Stylianou, and JJ Tucker. The mouse component of this work was supported by a grant from the American Orthopaedic Society for Sports Medicine to JA Abboud. The rat component was funded by the Orthopaedic Research and Education Foundation to JA Abboud. Other funding, including support of DP Beason, was provided by the NIH-NIAMS (P30 AR050950 to LJ Soslowsky). Additional support for the non-human primate work was provided by a pathway to independence grant from the NIH-NHLBI (K99/R00-HL088528 to RE Temel). AL McDaniel was supported by a training grant from the NIH-NHLBI (T32-HL091797).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: No financial biases exist for any author. This work was approved by the Institutional Animal Care and Use Committees of the University of Pennsylvania (Protocols: 801616, 802634, and 803246) and Wake Forest University (Protocol: A10-024).
References
- 1.Abboud JA, Kim JS. The effect of hypercholesterolemia on rotator cuff disease. Clin Orthop Relat Res. 2010;468:1493–1497. doi: 10.1007/s11999-009-1151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahrens PM, Boileau P. The long head of biceps and associated tendinopathy. J Bone Joint Surg Br. 2007;89:1001–1009. doi: 10.1302/0301-620X.89B8.19278. [DOI] [PubMed] [Google Scholar]
- 3.Beason DP, Abboud JA, Kuntz AF, Bassora R, Soslowsky LJ. Cumulative effects of hypercholesterolemia on tendon biomechanics in a mouse model. J Orthop Res. 2011;29:380–383. doi: 10.1002/jor.21255. [DOI] [PubMed] [Google Scholar]
- 4.Beason DP, Hsu JE, Edelstein L, Lee CS, Tucker JJ, Abboud JA, et al. Effect of Diet-Induced Hypercholesterolemia on Rotator Cuff Tendon Mechanics in a Rat Model. Trans Orthop Res Soc. 2011;36:223. [Google Scholar]
- 5.Beason DP, Kuntz AF, Hamamdzic D, Wilensky RL, Mohler ERI, Abboud JA, et al. High Cholesterol Adversely Affects Biceps Tendon Mechanical Properties in a Porcine Model. Trans Orthop Res Soc. 2009;34:184. [Google Scholar]
- 6.Beeharry D, Coupe B, Benbow EW, Morgan J, Kwok S, Charlton-Menys V, et al. Familial hypercholesterolaemia commonly presents with Achilles tenosynovitis. Ann Rheum Dis. 2006;65:312–315. doi: 10.1136/ard.2005.040766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derwin KA, Soslowsky LJ, Green WD, Elder SH. A new optical system for the determination of deformations and strains: calibration characteristics and experimental results. Journal of Biomechanics. 1994;27:1277–1285. doi: 10.1016/0021-9290(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 8.Descamps OS, Leysen X, Van Leuven F, Heller FR. The use of Achilles tendon ultrasonography for the diagnosis of familial hypercholesterolemia. Atherosclerosis. 2001;157:514–518. doi: 10.1016/s0021-9150(01)00533-0. [DOI] [PubMed] [Google Scholar]
- 9.Favata M. PhD Dissertation. Philadelphia: University of Pennsylvania; 2006. Scarless Healing in the Fetus: Implications and Strategies for Postnatal Tendon Repair. [Google Scholar]
- 10.Gimbel JA, Van Kleunen JP, Lake SP, Williams GR, Soslowsky LJ. The role of repair tension on tendon to bone healing in an animal model of chronic rotator cuff tears. J Biomech. 2007;40:561–568. doi: 10.1016/j.jbiomech.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Health, United States, 2010: With Special Feature on Death and Dying. Hyattsville: National Center for Health Statistics; 2011. [PubMed] [Google Scholar]
- 12.Kiortsis DN, Argyropoulou MI, Tsouli SG, Xydis V, Elisaf MS. Correlation of Achilles tendon thickness evaluated by ultrasonography with carotid intima-media thickness in patients with familial hypercholesterolemia. Atherosclerosis. 2006;186:228–229. doi: 10.1016/j.atherosclerosis.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Langer T, Strober W, Levy RI. The metabolism of low density lipoprotein in familial type II hyperlipoproteinemia. J Clin Invest. 1972;51:1528–1536. doi: 10.1172/JCI106949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee M, Ryu JK, Piao S, Choi MJ, Kim HA, Zhang LW, et al. Efficient gene expression system using the RTP801 promoter in the corpus cavernosum of high-cholesterol diet-induced erectile dysfunction rats for gene therapy. J Sex Med. 2008;5:1355–1364. doi: 10.1111/j.1743-6109.2008.00771.x. [DOI] [PubMed] [Google Scholar]
- 15.Longo UG, Franceschi F, Spiezia F, Forriol F, Maffulli N, Denaro V. Triglycerides and total serum cholesterol in rotator cuff tears: do they matter? Br J Sports Med. 2010;44:948–951. doi: 10.1136/bjsm.2008.056440. [DOI] [PubMed] [Google Scholar]
- 16.Mathiak G, Wening JV, Mathiak M, Neville LF, Jungbluth KH. Serum cholesterol is elevated in patients with Achilles tendon ruptures. Archives of Orthopaedic and Trauma Surgery. 1999;119:280–284. doi: 10.1007/s004020050410. [DOI] [PubMed] [Google Scholar]
- 17.Moghadasian MH, McManus BM, Nguyen LB, Shefer S, Nadji M, Godin DV, et al. Pathophysiology of apolipoprotein E deficiency in mice: relevance to apo E-related disorders in humans. FASEB J. 2001;15:2623–2630. doi: 10.1096/fj.01-0463com. [DOI] [PubMed] [Google Scholar]
- 18.Ozgurtas T, Yildiz C, Serdar M, Atesalp S, Kutluay T. Is high concentration of serum lipids a risk factor for Achilles tendon rupture? Clinica Chimica Acta. 2003;331:25–28. doi: 10.1016/s0009-8981(03)00075-5. [DOI] [PubMed] [Google Scholar]
- 19.Pászty C, Maeda N, Verstuyft J, Rubin EM. Apolipoprotein AI transgene corrects apolipoprotein E deficiency-induced atherosclerosis in mice. J Clin Invest. 1994;94:899–903. doi: 10.1172/JCI117412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedowitz DI, Beason DP, Soslowsky LJ. Biomechanics of the Shoulder and Elbow Complex. In: Nordin M, Andersson GBJ, Pope MH, editors. Musculoskeletal Disorders in the Workplace: Principles and Practice. Mosby: St. Louis; 2006. pp. 155–166. [Google Scholar]
- 21.Tsouli SG, Kiortsis DN, Argyropoulou MI, Mikhailidis DP, Elisaf MS. Pathogenesis, detection and treatment of Achilles tendon xanthomas. European Journal of Clinical Investigation. 2005;35:236–244. doi: 10.1111/j.1365-2362.2005.01484.x. [DOI] [PubMed] [Google Scholar]
- 22.von Bahr S, Movin T, Papadogiannakis N, Pikuleva I, Ronnow P, Diczfalusy U, et al. Mechanism of Accumulation of Cholesterol and Cholestanol in Tendons and the Role of Sterol 27-hydroxylase (CYP27A1) Arterioscler Thromb Vasc Biol. 2002;22:1129–1135. doi: 10.1161/01.atv.0000022600.61391.a5. [DOI] [PubMed] [Google Scholar]