Abstract
Background
Previous evidence suggests that 25-hydroxyvitamin D3 [25(OH)D3] protects against several cancers. However, little is known regarding urothelial bladder cancer (UBC). We analyzed the association between plasma 25(OH)D3 and overall risk of UBC, as well as according to stage and FGFR3 molecular subphenotypes.
Methods
Plasma concentrations of 25(OH)D3 in 1125 cases with UBC and 1028 control subjects were determined by a chemiluminescence immunoassay. FGFR3 mutational status and expression in tumor tissue were assessed. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by logistic regression adjusting for potential confounders. Analyses were further stratified by tumor invasiveness and grade, FGFR3 expression, and smoking status. Cell proliferation was measured in human UBC cell lines cultured with 1α,25-dihydroxyvitamin D3.
Results
A statistically significantly increased risk of UBC was observed among subjects presenting the lowest concentrations of 25(OH)D3 (ORadj = 1.83; 95% CI = 1.19 to 2.82; P = .006), showing a dose–response effect (P trend = .004). The association was stronger for patients with muscle-invasive tumors, especially among low-FGFR3 expressers (ORadj = 5.94; 95% CI = 1.72 to 20.45; P = .005). The biological plausibility of these associations is supported by the fact that, in vitro, 1α,25-dihydroxyvitamin D3 upregulates FGFR3 expression in UBC cell lines with low levels of wild-type FGFR3.
Conclusion
These findings support a role of vitamin D in the pathogenesis of UBC and show that 25(OH)D3 levels are associated with FGFR3 expression in the tumor. Because FGFR3 mutation and overexpression are markers of better outcome, our findings suggest that individuals with low levels of plasma 25(OH)D3 may be at high risk of more aggressive forms of UBC.
Vitamin D, or cholecalciferol, is a prohormone involved in bone biology that may also protect against a variety of cancers (1–3). The most active vitamin D metabolite, 1α,25dihydroxyvitamin D3 [1α,25(OH)2D3, calcitriol], can regulate proliferation, apoptosis, and cell adhesion at the tumor cell level and it can also affect tumor interaction with the microenvironment through modulation of angiogenesis, invasion, and metastasis [reviewed in (1)]. In addition, it decreases oxidative DNA damage (4). Epidemiologic evidence shows that vitamin D insufficiency, as defined by low levels of 25-hydroxyvitamin D3 [25(OH)D3, calcidiol], which is the major circulating and most stable form of vitamin D, is associated with an increased risk of colorectal and breast cancers (5,6). As for other cancers, including pancreatic and prostate cancers, evidence of association with 25(OH)D3 is null or controversial (7–10). A recent paper on prostate cancer reported a statistically significant association with lethal disease only (11).
Urothelial bladder cancer (UBC) is an important public health issue because of its high incidence in most developed countries and the high costs to society. Spain presents one of the highest UBC incidence rates worldwide, with a male-to-female ratio of seven (12,13). The main established risk factors for UBC are smoking, occupational exposure to aromatic amines, and high levels of arsenic intake (12). Smoking accounts for a large proportion of the etiology of UBC, whereas the other factors contribute only to specific risk groups; yet, an important fraction of the disease remains unexplained. Little is known about the contribution of vitamin D to UBC, and only two studies have examined the association between 25(OH)D3 plasma levels and the risk of this disease. Whereas, in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention–nested case–control study of male smokers, low levels of 25(OH)D3 were associated with increased risk of UBC (14), in the PLCO nested case–control study, no association was found (15). 1α,25(OH)2D3 has been shown to inhibit proliferation and induce apoptosis of human bladder tumor cells in vitro and to reduce tumorigenesis in an N-methylnitrosourea-induced model of bladder cancer in rats (16). Expression of vitamin D receptor has also been detected in human urothelium (17).
UBC is a heterogeneous disease at the clinical, pathological, and genetic levels and at least two major progression pathways have been identified: papillary, low-grade, non-muscle-invasive bladder cancer (NMIBC) harbors FGFR3 mutations in approximately 60% of cases and displays low levels of genomic instability; high-grade NMIBC and muscle-invasive bladder cancers (MIBC) display a low prevalence of FGFR3 mutations and frequent alterations in the p53 and Rb pathways (18). Overall, FGFR3 mutations and FGFR3 protein overexpression characterize a large subgroup of NMIBC with good prognosis (19), but the molecular mechanisms underlying this association are not well established.
We aimed to assess the association between plasma 25(OH)D3 levels and risk of UBC in the Spanish Bladder Cancer/EPICURO Study (SBC/EPICURO Study) and explore the molecular mechanisms involved therein. We found that treatment with 1α,25(OH)2D3 leads to the upregulation of FGFR3 messenger RNA and protein in cultured UBC cells with low basal expression levels; these observations led to an analysis of the association of plasma 25(OH)D3 levels with UBC subphenotypes defined according to tumor invasiveness and grade and FGFR3 mutational and expression status.
Methods
Study Participants
Subjects came from the SBC/EPICURO Study, a hospital-based, case–control study conducted in 18 hospitals from five areas in Spain (20). Briefly, cases were patients newly diagnosed with histologically confirmed UBC in 1998 to 2001. A panel of expert pathologists classified homogeneously all cases according to the invasiveness and grade (21,22). Control subjects were selected from patients admitted to participating hospitals for diagnoses believed to be unrelated to the exposures of interest and were individually matched to the cases on age, sex, ethnic origin, and region. Written informed consent was obtained from all subjects, and the study was approved by the local institutional review boards and the US National Cancer Institute. Information on known or potential cancer risk factors and blood samples were obtained during the inpatient hospital stay for both cases and control subjects. A total of 1219 cases (84% eligible) and 1271 control subjects (88% eligible) agreed to participate in the study and were interviewed. Plasma from blood samples collected at diagnostic time was available from 1130 cases and 1038 control subjects.
Experimental Procedures
Details on the quantification of 25(OH)D3, cell proliferation, and FGFR3 expression assays using UBC cell lines and FGFR3 expression and mutational status analyses in tumoral tissue are specified in the Supplementary Methods (available online).
Statistical Analysis
Student’s t test was applied to analyze the effects of vitamin D on proliferation and expression of p21, p27, and FGFR3 in UBC cells. Mann–Whitney U test was used to assess differences between cases and control subjects regarding median plasma concentrations of 25(OH)D3. For the analysis of association between 25(OH)D3 and UBC, logistic regression was applied to estimate odds ratios (ORs) and their 95% confidence intervals (CIs), comparing each category of low plasma 25(OH)D3 concentration (20–29.99, 15–19.99, 10–14.99, and <10ng/mL) with the reference category (≥30ng/mL). A basic model was adjusted for age at interview, sex, region, smoking status, and season of blood draw. Further adjustments were made for body mass index, alcohol and calcium intake as previously associated with vitamin D (23–26), and occupational exposure to aromatic amines and toenail arsenic. Tests for linear trend were computed with the median of each category of the plasma 25(OH)D3 concentration treated as a continuous variable. Because almost a quarter of the control subjects were admitted to the hospital with bone fractures, a sensitivity analysis was carried out excluding these control subjects. The risk of UBC was further evaluated according to smoking status, with the “ever smoker” category created by collapsing the categories of occasional, former, and current smokers. Statistical interaction between smoking and 25(OH)D3 was assessed by including an interaction term as the product of the median of each category of plasma 25(OH)D3 and the never smoker or ever smoker categories. This was repeated by adjusting for duration of cigarette smoking, cigarettes per day, and pack-years among the ever smokers. The association with 25(OH)D3 was also examined by type of tobacco (blond or black) among the ever smokers. The analysis was also stratified by season of blood draw, and the interaction between season and plasma 25(OH)D3 was assessed.
Polytomous logistic regression models were applied to analyze the association between 25(OH)D3 and risk of low-grade NMIBC, high-grade NMIBC, and MIBC. Adjustment was performed for the same variables included in the logistic regression models. Difference in odds ratios between case groups was tested using a likelihood ratio test comparing models with and without the odds ratio constrained to be equal for the corresponding case groups. The polytomous logistic regression models were also applied to the stratified analysis for low and high FGFR3 expression and the presence or absence of mutations in FGFR3 in tumor tissue, adjusting for the same variables as before. All statistical tests were two-sided, and results were considered significant when P was less than or equal to .05. Statistical analyses were performed using STATA/SE version 10.1. This study conforms to the guidelines of Strengthening the Reporting of Observational Studies in Epidemiology Statement for observational studies.
Results
Effects of 1α,25(OH)2D3 on Cultured UBC
For this study, four UBC cell lines with distinct features were selected. Under basal conditions, two of them show an epithelial adhesive phenotype and form compact colonies: RT112 displays high levels of constitutively active wild-type FGFR3, whereas MGH-U3 harbors constitutively active mutant FGFR3. In contrast, the other two lines show a less epithelial phenotype: J82 cells have a mutated FGFR3 but lack expression both at the RNA and protein levels, whereas MGH-U4 cells express low levels of wild-type FGFR3 (27).
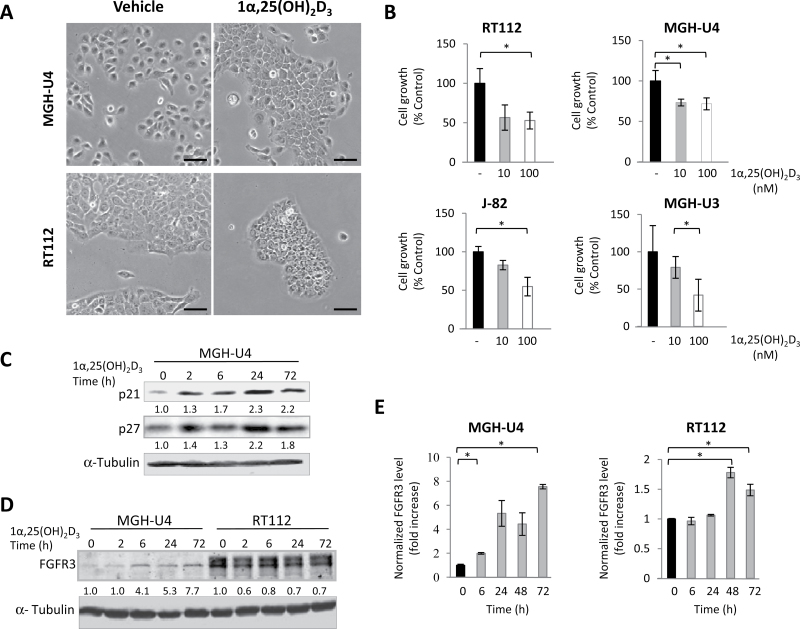
Treatment with 1α,25(OH)2D3 induced a more epithelioid phenotype and formation of more compact colonies, consistent with findings reported in other cell types (Figure 1, A). In the four UBC cell lines studied, 1α,25(OH)2D3 (10–100nM) induced a growth arrest (Figure 1, B). This was associated with the upregulation of the CDK inhibitors p21CIP1 and p27KIP1 at the protein level (Figure 1, C). As shown in Figure 1, D and E, 1α,25(OH)2D3 also induced an upregulation of FGFR3 at the messenger RNA level in MGH-U4 and RT112 cells and at the protein level in MGH-U4 cells. Altogether, these findings indicate that vitamin D treatment is associated with phenotypic changes and growth inhibition; in addition, it leads to higher FGFR3 levels in cells with low basal expression.
Figure 1.
Effects of vitamin D on proliferation and expression of p21, p27, and FGFR3 in urothelial bladder cancer (UBC) cells. Vitamin D induces growth arrest and an upregulation of p21, p27, and FGFR3 in UBC cells. A) Phase contrast microscopy of MGH-U4 and RT112 cells treated with 1α,25-dihydroxyvitamin D3 [1α,25 (OH)2D3] (100nM) or vehicle for 72 hours (scale bar = 100 µm). B) 1α,25(OH)2D3 treatment inhibits proliferation of UBC cells in vitro. C) Western blot analysis showing induction of the CDK inhibitors p21 and p27 in MGH-U4 cells treated with 1α,25(OH)2D3 at different time points. Tubulin was used as a loading control. Values indicate fold-change referred to zero time point. D) Western blot analysis showing changes in FGFR3 in MGH-U4 and RT112 cells treated with 1α,25(OH)2D3 at the indicated time points. E) Quantitative reverse-transcription polymerase chain reaction analysis showing the upregulation of FGFR3 messenger RNA levels in MGH-U4 and RT112 cells treated with 10nM 1α,25(OH)2D3 (gray bars). Values were normalized to HPRT and referred to expression at time zero (black bars). Comparisons with P less than .05 are indicated with an asterisk. Error bars represent standard deviation.
Plasma 25(OH)D3 and Risk of UBC
Based on previous epidemiological evidence and on the above in vitro findings, we analyzed the association between vitamin D levels and UBC risk in the SBC/EPICURO Study, both overall and according to molecular subphenotypes. Cases and control subjects were mostly men, with a high frequency of cigarette smokers (Table 1). Median concentrations of 25(OH)D3 were lower in cases than in control subjects (13.9 vs 15.0ng/mL; P = .001) (Table 1); 73% of all individuals (75% of cases and 71% of control subjects) had less than 20ng/mL (Supplementary Table 1, available online). The distribution of cases by 25(OH)D3 status was similar in the three tumor subphenotypes examined (P = .70) (Supplementary Table 1, available online).
Table 1.
Characteristics of study participants*
| Characteristics | Control subjects (N = 1028) | % | Cases (N = 1125) | % | P† |
|---|---|---|---|---|---|
| Age, median (range), y | 66 (20–81) | 68 (22–81) | 1.4×10−4 | ||
| Sex | |||||
| Males | 909 | 88 | 986 | 88 | .58 |
| Females | 119 | 12 | 139 | 12 | |
| Region | |||||
| Barcelona | 205 | 20 | 197 | 18 | .41 |
| Valles | 159 | 15 | 180 | 16 | |
| Elche | 81 | 8 | 88 | 8 | |
| Tenerife | 153 | 15 | 196 | 17 | |
| Asturias | 430 | 42 | 464 | 41 | |
| Smoking status | |||||
| Never smoker | 290 | 28 | 157 | 14 | 1.9×10−23 |
| Occasional smoker | 82 | 8 | 45 | 4 | |
| Former smoker | 383 | 37 | 439 | 39 | |
| Current smoker | 273 | 27 | 484 | 43 | |
| BMI (kg/m2)‡ | |||||
| <25 | 415 | 53 | 499 | 58 | .13 |
| 25–26.99 | 169 | 22 | 172 | 20 | |
| 27–29.99 | 136 | 17 | 123 | 14 | |
| >30 | 65 | 8 | 60 | 7 | |
| Tumor type§ | |||||
| Low-grade NMIBC (TaG1/G2) | –– | –– | 579 | 56 | |
| High-grade NMIBC (TaG3/T1) | –– | –– | 205 | 20 | |
| MIBC (≥T2) | –– | –– | 246 | 24 | |
| FGFR3|| | –– | –– | |||
| Wild-type | –– | –– | 496 | 59 | |
| Mutated | –– | –– | 340 | 41 | |
| FGFR3 expression¶ | –– | –– | |||
| Low | –– | –– | 396 | 59 | |
| High | –– | –– | 271 | 41 | |
| Plasma 25-hydroxyvitamin D3, median (interquartile range), ng/mL | 15.0 (10.0–21.2) | 13.9 (9.0–19.9) | 1.1×10−3 |
* BMI = body mass index; MIBC = muscle-invasive bladder cancer; NMIBC = non-muscle-invasive bladder cancer †For age and plasma 25-hydroxyvitamin D3, the P value is from the Mann–Whitney U test. For all the other variables, the P value is from the χ2 test.
‡ Two hundred forty-three control subjects and 271 cases had no information on height or weight or both.
§ Ninety-five cases could not be assigned to any tumor/grade group because the paraffin block could not be retrieved.
|| Two hundred eighty-nine cases did not yield polymerase chain reaction product.
¶ Four hundred fifty-eight cases did not have immunohistochemistry staining for FGFR3.
After adjusting for age, sex, region, smoking status, and season of blood draw, decreasing concentrations of plasma 25(OH)D3 were found to be associated with increased risk of UBC (P trend = .004) (Table 2). Individuals slightly (ORadj = 1.63; 95% CI = 1.06 to 2.51; P = .03), moderately (ORadj = 1.67; 95% CI = 1.09 to 2.56; P = .02), and severely deficient (ORadj = 1.83; 95% CI = 1.19 to 2.82; P = .006) in vitamin D presented a greater than 50% increased risk of UBC when compared with individuals with sufficient levels. The results were not substantially changed after excluding the control subjects with bone fractures or adjusting for body mass index (Supplementary Table 2, available online), intake of alcohol and calcium, occupational exposure to aromatic amines, or toenail arsenic levels (data not shown).
Table 2.
Odds ratios (ORs), 95% confidence intervals (CIs), and P values for the association between plasma 25-hydroxyvitamin D3 [25(OH)D3] and overall bladder cancer risk, risk of low- and high-grade non-muscle-invasive (NMIBC) and muscle-invasive bladder cancer (MIBC)*
| Overall | Low-grade NMIBC | High-grade NMIBC | MIBC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin D status | 25(OH) D3, ng/mL | Controls | Cases | OR (95% CI)† | P | Cases | OR (95% CI)† | P | Cases | OR (95% CI)† | P | Cases | OR (95% CI)† | P |
| Sufficient | ≥30.00 | 74 | 51 | 1.00 (referent) | 27 | 1.00 (referent) | 10 | 1.00 (referent) | 9 | 1.00 (referent) | ||||
| Insufficient | 20.00–29.99 | 227 | 229 | 1.40 (0.92 to 2.14) | .12 | 120 | 1.35 (0.81 to 2.26) | .25 | 38 | 1.14 (0.53 to 2.45) | .73 | 44 | 1.44 (0.66 to 3.14) | .37 |
| Slightly deficient | 15.00–19.99 | 212 | 219 | 1.63 (1.06 to 2.51) | .03 | 116 | 1.58 (0.94 to 2.66) | .08 | 46 | 1.68 (0.79 to 3.57) | .18 | 43 | 1.83 (0.83 to 4.02) | .14 |
| Moderately deficient | 10.00–14.99 | 255 | 280 | 1.67 (1.09 to 2.56) | .02 | 146 | 1.55 (0.92 to 2.59) | .096 | 49 | 1.40 (0.66 to 2.99) | .38 | 61 | 2.13 (0.98 to 4.65) | .06 |
| Severely deficient | <10.00 | 260 | 346 | 1.83 (1.19 to 2.82) | .006 | 170 | 1.64 (0.97 to 2.76) | .07 | 62 | 1.55 (0.72 to 3.32) | .26 | 89 | 2.81 (1.29 to 6.13) | .009 |
| P trend | .004 | .07 | .18 | .0005 | ||||||||||
* Ninety-five cases could not be assigned to any tumor/grade group because the paraffin block could not be retrieved. Likelihood-ratio test P for the pairwise comparisons between odds ratio of low-grade and high-grade NMIBC and MIBC among the severely deficient (vs sufficient) were .89, low-grade vs high-grade NMIBC; .19, low-grade NMIBC vs MIBC; and .24, high-grade NMIBC vs MIBC.
† Adjusted for age, sex, region, smoking status, and season of blood collection.
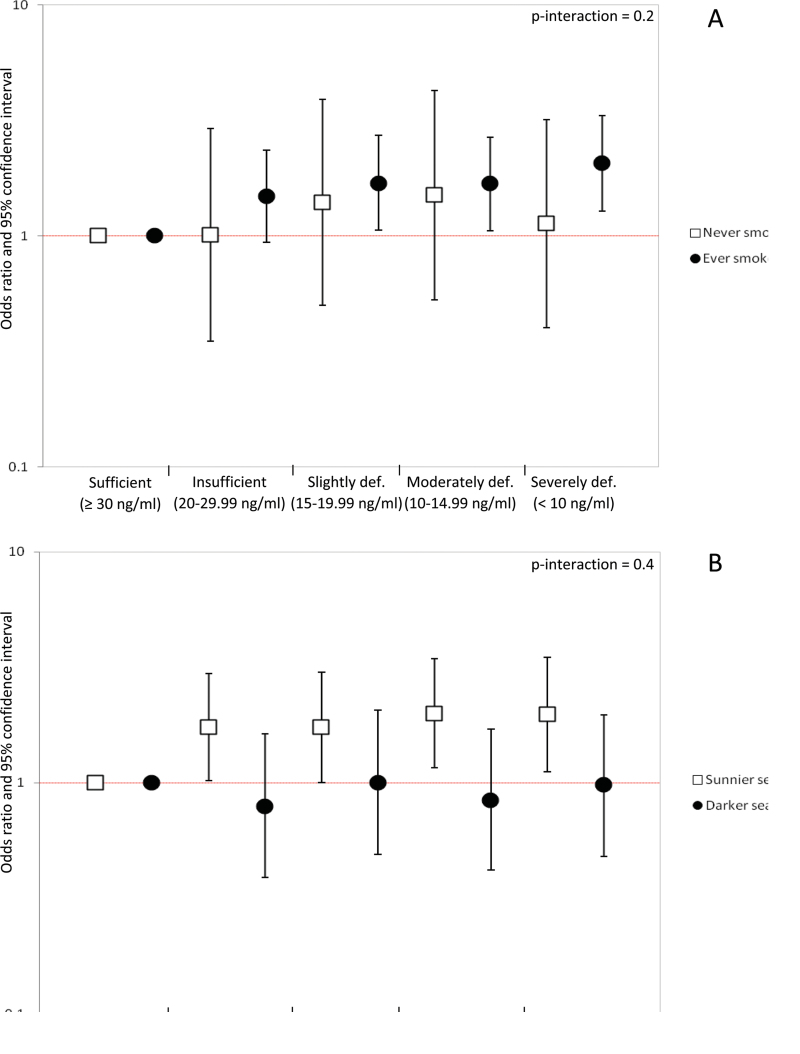
The association of plasma 25(OH)D3 levels and risk of UBC was restricted to smokers, showing a dose–response effect (P = .003) (Figure 2, A). However, no statistical interaction was observed between tobacco and 25(OH)D3 levels. Adjusting for duration of cigarette smoking, cigarettes per day, and pack-years among ever smokers did not change these results (data not shown). Stratifying by type of tobacco among ever smokers did not substantially change the results either (data not shown).
Figure 2.
Odds ratios (OR) and 95% confidence intervals for the association between plasma 25-hydroxyvitamin D3 [25(OH)D3] and bladder cancer risk by smoking status (A) and by season of blood collection (B). Estimates are adjusted for age, sex, region, smoking status, and season of blood collection, when appropriate.
The association of plasma 25(OH)D3 concentration with risk of UBC was stronger and showed a dose–response pattern among individuals whose blood was drawn in spring and summer seasons, but a statistically significant interaction was not observed (Figure 2, B).
Low concentrations of plasma 25(OH)D3 were more strongly associated with risk of MIBC. Among individuals severely deficient in vitamin D, the adjusted risk of MIBC (ORadj = 2.81; 95% CI = 1.29 to 6.13; P = .009) was 1.7 times higher than the risk of low-grade NMIBC (ORadj = 1.64; 95% CI = 0.97 to 2.76; P = .07) (Table 2). However, the differences in risk between both tumor types were not statistically significant, possibly due to low sample size.
Plasma 25(OH)D3 and FGFR3 Mutation and Protein Expression
Tumors with FGFR3 mutations were more likely to show high FGFR3 expression than those without mutations (P = 2×10−18). Plasma 25(OH)D3 levels were not associated with somatic FGFR3 mutations (P = .70) (Supplementary Table 1, available online). Risk of UBC among subjects with deficient 25(OH)D3 concentrations was slightly higher among FGFR3-mutated than wild-type tumors, although the differences were not statistically significant (Supplementary Table 3, available online). The percentage of cases with high FGFR3-expressing tumors was slightly higher in the 25(OH)D3-sufficient group than in the 25(OH)D3-deficient group (Supplementary Table 1, available online), but this difference did not reach statistical significance (P = .10).
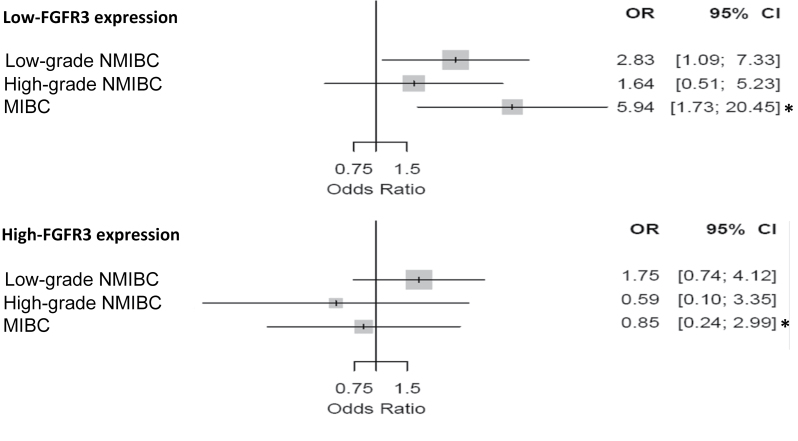
A more detailed analysis revealed that low plasma concentrations of 25(OH)D3 were associated with an increased risk of developing low FGFR3-expressing UBC but not high FGFR3-expressing UBC. This association was more notable among those severely deficient in vitamin D (ORadj = 3.03; 95% CI = 1.55 to 5.94; P = .001; P trend = .0002) (Supplementary Table 3, available online). Furthermore, in individuals with low levels of 25(OH)D3, the odds of MIBC expressing low levels of FGFR3 were almost 6-fold higher than in those with sufficient levels (ORadj = 5.94; 95% CI = 1.72 to 20.45; P = .005). This association was not statistically significantly different from that of low-grade (P = .30) and high-grade NMIBC (P = .10) (Figure 3; Supplementary Table 4, available online), also possibly due to small sample size in the subgroups.
Figure 3.
Odds ratios (ORs) and 95% confidence intervals (CIs) for the association between plasma 25-hydroxyvitamin D3 [25(OH)D3] levels and risk of low- and high-grade non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC) in relationship with tumor FGFR3 expression levels. Estimates are adjusted for age, sex, region, and smoking status. *Likelihood-ratio test P for the comparison between the odds ratio of MIBC in the low- and high-FGFR3-expression groups = .26.
Discussion
In the present study, we analyzed the association of plasma 25(OH)D3 with risk of UBC in the largest and most representative patient series tested so far. For the first time, we placed the findings in the context of the molecular taxonomy of this tumor, namely according to alterations in FGFR3, which is the most commonly mutated oncogene in UBC (18). We observed an inverse, statistically significant association between plasma 25(OH)D3 levels and risk of UBC with a dose–response effect: individuals with the lowest concentrations of plasma 25(OH)D3 presented almost twofold higher odds than individuals with concentrations greater to or equal than 30ng/mL (sufficient status). This risk pattern was mainly observed for MIBC. Furthermore, although the increased risk of low-grade NMIBC was independent of FGFR3 expression in the tumor, that of MIBC was not: individuals with deficient levels of vitamin D showed the highest risk for developing low-FGFR3-expressing MIBC. These findings suggest that vitamin D modulates tumor phenotype in specific tumor subtypes.
Tumors that express low FGFR3 protein levels or that are FGFR3 wild-type are more likely to invade muscle and display an aggressive behavior, whereas tumors that are FGFR3 mutant and express high FGFR3 levels have a lower tendency to progress (19,28). The in vitro data indicate that vitamin D regulates FGFR3 mainly in cells expressing low FGFR3 levels, and a large body of evidence indicates that tumor cells that respond to vitamin D display more differentiated properties and less aggressive in vitro behavior (29,30). Therefore, our findings are consistent with the notion that vitamin D sufficiency may support higher levels of expression of FGFR3, particularly in wild-type tumors, thus favoring a less-aggressive tumor phenotype at presentation. The recent observation that vitamin D can affect gene expression through the epigenetic modulation of histone marks suggests a possible mechanism of this effect (31). Our results support the notion that in other tumor types, such as colon and breast cancers, similar analyses should be performed to determine whether the effects of vitamin D are restricted to specific tumor subphenotypes and/or molecular pathways.
The study findings build upon data reported by the few previous epidemiologic and molecular studies suggesting that vitamin D may act as a protective factor against UBC (14,16) and extend our understanding by exploring interactions and the association of 25(OH)D3 plasma levels with UBC FGFR3 subphenotypes. None of the interactions tested were statistically significant. However, the inverse association between 25(OH)D3 and risk of UBC appeared stronger among ever smokers and among those individuals whose blood was collected during spring or summer months. Although we cannot discard that the relationship with tobacco smoking could be due to chance because of the small sample size of the nonsmoker group, it is in agreement with the findings of Mondul and colleagues, who reported an increased risk of bladder cancer in male smokers associated with low 25(OH)D3 serum concentrations (14). The stronger effects found among subjects whose blood was drawn during spring and summer confirm prior findings and suggest that assessing plasma levels of 25(OH)D3 during sunnier months provides a more sensitive biomarker of a constitutive deficiency of vitamin D and thus better discriminates those individuals with higher susceptibility to UBC (14).
These results are of relevance given that vitamin D deficiency and insufficiency are highly prevalent in Spain (32,33), where the incidence rates of UBC are among the highest worldwide (12,13), and in many other Western countries. The concentrations of 25(OH)D3 found in this study were similar to those of same-age individuals from other Southern European countries, although lower than those of individuals from the United States and Sweden. A potential explanation is that, in the latter countries, several food items are fortified with vitamin D (34–36).
This is the largest study assessing the risk of UBC in relation to 25(OH)D3 levels and the first one analyzing this association in the context of the molecular features of the tumor and the biological effects of vitamin D, supported by parallel experimental in vitro evidence providing mechanistic explanations for the epidemiological findings. Other relevant strengths of this study are the high participation rates of cases and control subjects as well as their match for area of residence and similar age distribution. In addition, detailed information on several potential confounders (eg, smoking habits, body mass index) were considered. Even though the results of the present study are based on the concentration of plasma 25(OH)D3 at a single time point, this measurement is considered a good biomarker of long-term vitamin D status because several studies have shown moderate to very high intraclass correlation coefficients (≥0.59), indicating a good concordance in 25(OH)D3 across time points (37–39).
However, our study also has some limitations. Despite its large sample size, the assessment of associations in subgroups is limited by the smaller numbers of subjects in each subphenotype, especially when considering the association with tumor FGFR3 mutation and expression. The association between 25(OH)D3 levels and FGFR3 expression and tumor subtype needs to be confirmed in adequately sized independent series. Temporality should also be taken into account because the study is inherently retrospective and we cannot discard a reverse causality due to the carcinogenesis process. Although we hypothesized that, according to the mechanistic evidence provided here, the protective effect of 25(OH)D3 should be more pronounced among patients with MIBC low-FGFR3 expressers, we cannot exclude that a cancer diagnosis may lead to a change in diet and outdoor habits, potentially influencing 25(OH)D3 concentrations. Nevertheless, all present patients with UBC were incident cases, most of whom were in good general health at the time of diagnosis and were not malnourished or cachetic. Also, blood was drawn at time of diagnosis, and plasma levels of 25(OH)D3 are considered reasonably consistent over time (37,39,40). Furthermore, the fact the association is more evident in a group molecularly defined would exclude the possibility of reverse causality. Importantly, our results are in line with previous evidence from both case–control and cohort studies at other cancer sites (5,6,14).
In summary, low plasma 25(OH)D3 concentrations were found associated with an increased risk of UBC, and the effects of vitamin D may be stronger among smokers. Our data suggest that this risk is higher among those individuals with MIBC expressing low FGFR3 levels. The in vitro findings reported here lend support to the biological plausibility of this association. Our results need to be replicated in independent populations, and the benefits of vitamin D intake have to be conclusively assessed through a clinical trial.
Funding
This work was supported by the World Cancer Research Fund (2010/250); Fondo de Investigación Sanitaria, Spain (00/0745, PI051436, PI061614, and G03/174); Red Temática de Investigación Cooperativa en Cáncer, Spain; Fundación Científica de la AECC, Spain; Consolíder ONCOBIO; Comunidad de Madrid, Spain (S2011/BMD-02344, Colomics2); and the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health.
Supplementary Material
Acknowledgments
We acknowledge the coordinators, field and administrative workers, technicians and patients of the Spanish Bladder Cancer/EPICURO Study, and the Histology and Immunohistochemistry Core Unit of the Spanish National Cancer Research Centre (CNIO).
FX Real and N Malats contributed equally to this work. The authors declare no conflicts of interest.
References
- 1. Garland CF, Gorham ED, Mohr SB, et al. Vitamin D for cancer prevention: global perspective Ann Epidemiol 2009. 19(7):468–483 [DOI] [PubMed] [Google Scholar]
- 2. Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States) Cancer Causes Control 2005. 16(2):83–95 [DOI] [PubMed] [Google Scholar]
- 3. Holick MF. Vitamin D deficiency N Engl J Med 2007. 357(3):266–281 [DOI] [PubMed] [Google Scholar]
- 4. Fedirko V, Bostick RM, Long Q, et al. Effects of supplemental vitamin D and calcium on oxidative DNA damage marker in normal colorectal mucosa: a randomized clinical trial Cancer Epidemiol Biomarkers Prev 2010. 19(1):280–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Touvier M, Chan DSM, Lau R, et al. Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms and colorectal cancer risk Cancer Epidemiol Biomarkers Prev 2011. 20(5):1003–1016 [DOI] [PubMed] [Google Scholar]
- 6. Yin L, Grandi N, Raum E, et al. Meta-analysis: serum vitamin D and breast cancer risk Eur J Cancer 2010. 46(12):2196–2205 [DOI] [PubMed] [Google Scholar]
- 7. Helzlsouer KJ. Overview of the cohort consortium vitamin D pooling project of rarer cancers Am J Epidemiol 2010. 172(1):4–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Albanes D, Mondul AM, Yu K, et al. Serum 25-hydroxy vitamin D and prostate cancer risk in a large nested case-control study Cancer Epidemiol Biomarkers Prev 2011. 20(9):1850–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yin L, Raum E, Haug U, et al. Meta-analysis of longitudinal studies: serum vitamin D and prostate cancer risk Cancer Epidemiol 2009. 33(6):435–445 [DOI] [PubMed] [Google Scholar]
- 10. Wolpin BM, Ng K, Bao Y, et al. Plasma 25-hydroxyvitamin D and risk of pancreatic cancer Cancer Epidemiol Biomarkers Prev 2012. 21(1):82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shui IM, Mucci LA, Kraft P, et al. Vitamin D-related genetic variation, plasma vitamin D, and risk of lethal prostate cancer: a prospective nested case-control study J Natl Cancer Inst 2012. 104(9):690–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Silverman D, Devesa S, Moore L, et al. Bladder cancer. In: Schottenfeld D, Fraumeni J, Jr, eds. Cancer Epidemiology and Prevention 3rd ed New York: Oxford University Press; 2006. 1101–1127 [Google Scholar]
- 13. Samanic C, Kogevinas M, Dosemeci M, et al. Smoking and bladder cancer in Spain: effects of tobacco type, timing, environmental tobacco smoke, and gender Cancer Epidemiol Biomarkers Prev 2006. 15(7):1348–1354 [DOI] [PubMed] [Google Scholar]
- 14. Mondul AM, Weinstein SJ, Mannisto S, et al. Serum vitamin D and risk of bladder cancer Cancer Res 2010. 70(22):9218–9223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mondul AM, Weinstein SJ, Horst RL, et al. Serum vitamin D and risk of bladder cancer in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial Cancer Epidemiol Biomarkers Prev 2012. 21(7):1222–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Konety BR, Lavelle JP, Pirtskalaishvili G, et al. Effects of vitamin D (calcitriol) on transitional cell carcinoma of the bladder in vitro and in vivo J Urol 2001. 165(1):253–258 [DOI] [PubMed] [Google Scholar]
- 17. Sahin MO, Canda AE, Yorukoglu K, et al. 1,25 Dihydroxyvitamin D(3) receptor expression in superficial transitional cell carcinoma of the bladder: a possible prognostic factor? Eur Urol 2005. 47(1):52–57 [DOI] [PubMed] [Google Scholar]
- 18. Luis NM, Lopez-Knowles E, Real FX. Molecular biology of bladder cancer Clin Transl Oncol 2007;9(1):5–12 [DOI] [PubMed] [Google Scholar]
- 19. Hernandez S Lopez-Knowles E Lloreta J et al. Prospective study of FGFR3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas J Clin Oncol 2006. 24(22):3664–3671 [DOI] [PubMed] [Google Scholar]
- 20. Garcia-Closas M Malats N Silverman D et al. NAT2 slow acetylation, GSTM1 null genotype, and risk of bladder cancer: results from the Spanish Bladder Cancer Study and meta-analyses Lancet 2005. 366(9486):649–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Epstein JI, Amin MB, Reuter VR, et al. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee Am J Surg Pathol 1998. 22(12):1435–1448 [DOI] [PubMed] [Google Scholar]
- 22. Mostofi F, Davis C, Sesterhenn I. Histological typing of urinary bladder tumours Berlin: Springer; 1999. [Google Scholar]
- 23. van Dam RM, Snijder MB, Dekker JM, et al. Potentially modifiable determinants of vitamin D status in an older population in the Netherlands: the Hoorn Study Am J Clin Nutr 2007. 85(3):755–761 [DOI] [PubMed] [Google Scholar]
- 24. Jaaskelainen T, Knekt P, Marniemi J, et al. Vitamin D status is associated with sociodemographic factors, lifestyle and metabolic health [published online ahead of print April 27, 2012]. Eur J Nutr 2012. doi:10.1007/s00394-012-0354-0. [DOI] [PubMed] [Google Scholar]
- 25. Kinyamu HK, Gallagher JC, Rafferty KA, et al. Dietary calcium and vitamin D intake in elderly women: effect on serum parathyroid hormone and vitamin D metabolites Am J Clin Nutr 1998. 67(2):342–348 [DOI] [PubMed] [Google Scholar]
- 26. Looker AC, Pfeiffer CM, Lacher DA, et al. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004 Am J Clin Nutr 2008. 88(6):1519–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jebar AH, Hurst CD, Tomlinson DC, et al. FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma Oncogene 2005. 24(33):5218–5225 [DOI] [PubMed] [Google Scholar]
- 28. Tomlinson DC, Baldo O, Harnden P, et al. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer J Pathol 2007. 213(1):91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palmer HG, Larriba MJ, Garcia JM, et al. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer Nat Med 2004. 10(9):917–919 [DOI] [PubMed] [Google Scholar]
- 30. Palmer HG, Gonzalez-Sancho JM, Espada J, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling J Cell Biol 2001. 154(2):369–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pereira F, Barbachano A, Silva J, et al. KDM6B/JMJD3 histone demethylase is induced by vitamin D and modulates its effects in colon cancer cells Hum Mol Genet 2011. 20(23):4655–4665 [DOI] [PubMed] [Google Scholar]
- 32. Gonzalez-Padilla E, Soria Lopez A, Gonzalez-Rodriguez E, et al. High prevalence of hypovitaminosis D in medical students in Gran Canaria. Canary Islands (Spain) Endocrinol Nutr 2011. 58(6):267–273 [DOI] [PubMed] [Google Scholar]
- 33. Gonzalez-Molero I, Morcillo S, Valdes S, et al. Vitamin D deficiency in Spain: a population-based cohort study Eur J Clin Nutr 2011. 65(3):321–328 [DOI] [PubMed] [Google Scholar]
- 34. Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications Endocr Rev 2001. 22(4):477–501 [DOI] [PubMed] [Google Scholar]
- 35. Melhus H, Snellman G, Gedeborg R, et al. Plasma 25-hydroxyvitamin D levels and fracture risk in a community-based cohort of elderly men in Sweden J Clin Endocrinol Metab 2010. 95(6):2637–2645 [DOI] [PubMed] [Google Scholar]
- 36. Looker AC, Johnson CL, Lacher DA, et al. Vitamin D status: United States, 2001–2006 NCHS Data Brief 2011;59 1–8 [PubMed] [Google Scholar]
- 37. Hofmann JN, Yu K, Horst RL, et al. Long-term variation in serum 25-hydroxyvitamin D concentration among participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Cancer Epidemiol Biomarkers Prev 2010. 19(4):927–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zeleniuch-Jacquotte A, Gallicchio L, Hartmuller V, et al. Circulating 25-hydroxyvitamin D and risk of endometrial cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers Am J Epidemiol 2010. 172(1):36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meng JE, Hovey KM, Wactawski-Wende J, et al. Intraindividual variation in plasma 25-hydroxyvitamin D measures 5 years apart among postmenopausal women Cancer Epidemiol Biomarkers Prev 2012. 21(6):916–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saliba W, Barnett O, Stein N, et al. The longitudinal variability of serum 25(OH)D levels Eur J Intern Med 2012. 23(4):e106–e111 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.